Abstract
A series of N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl ethane-1,2-diamines and N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl propane-1,3-diamines was designed and synthesized as 5-HT6 receptor ligands. These compounds, when screened in a functional reporter gene-based assay, displayed potent antagonistic activity with Kb values in the range of 1.8–60 nM. The lead compound 9y has shown good ADME surrogate properties, acceptable pharmacokinetic profile and is active in animal models of cognition like novel object recognition test and Morris water maze. It was selected for detailed profiling.
Introduction
World Health Organization recognizes the size and complexity of the dementia challenge and urges countries to view dementia as a critical public health priority. Dementia is a syndrome due to disease of the brain, usually chronic, characterized by a progressive, global deterioration in intellect, including memory, learning, orientation, language, comprehension and judgment. Alzheimer’s Disease International estimated that 36 million people worldwide are living with dementia in 2010 and this number is expected to increase in future with the increased life expectancy around the globe. There are 7.7 million new cases of dementia each year, implying that there is a new case of dementia somewhere in the world every 4 s. If breakthroughs are not discoveredCitation1, the number of people affected will be over 115.4 million by 2050.
The monoamine neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) mediates a wide range of physiological functionsCitation2–4 by interacting with multiple receptors, and these receptors have been implicated as playing important roles in certain pathological and psychopathological conditions. Among these receptors, 5-hydroxytryptamine6 (5-HT6) receptor is one of the most recently discovered members of serotonin receptor family (5-HT1–5-HT7). It is a G-protein-coupled receptor (GPCR) and positively coupled to adenylate cyclase secondary messenger systemCitation5,Citation6.
The 5-HT6 receptor appears to regulate several neurotransmitter systems, including acetylcholine, glutamate, dopamine, noradrenaline or aspartate. It is unique distribution in the brain and high affinity for therapeutic antipsychotics and antidepressantsCitation7,Citation8 suggests a possible role of the 5-HT6 receptor in central nervous system (CNS) disorders. Along with this, several studies have been published reporting an association of 5-HT6 receptor variants with neuropsychological and neuropsychiatric diseases such as schizophreniaCitation9–13, bipolar affective disordersCitation14, Parkinson’s diseaseCitation15 or Alzheimer’s diseaseCitation16–18 and the treatment of obesity and related metabolic disorders. Therefore, 5-HT6 receptors represent an extremely attractive target for the development of novel small molecule therapeutics for the treatment of various neurodegenerative disordersCitation19–21.
Many 5-HT6 receptor ligands have been reported and some of the clinically advancing molecules include SAM-760 from Pfizer, SB-742457 () from GSK is in Phase II clinical trials for dementia as per their product development pipeline publishedCitation22,Citation23 in February 2013. Recently, AVN-211 () from Avineuro Pharmaceuticals entered into phase IIb clinical trials for treatment of schizophreniaCitation24–26 and Lundbeck has reported that Lu AE58054 () met its primary endpoint in large placebo-controlled clinical proof of concept study in people with Alzheimer’s diseaseCitation27,Citation28. Biotie therapies have completed phase I clinical trials of the compound, SYN-120Citation29. As part of our own research program, we at Suven have designed and developed selective 5-HT6 receptor antagonist compound SUVN-502 (structure not disclosed) which has completed phase I trials for cognitive impairment in schizophrenia and Alzheimer’s diseaseCitation30.
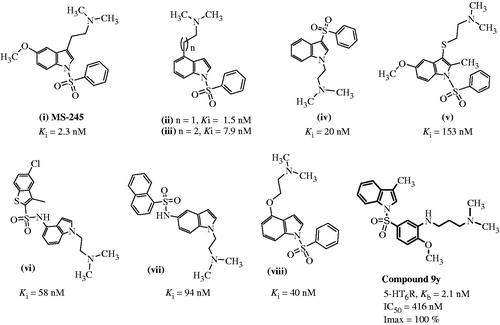
N,N-dimethyl-2-(5-methoxy-1-phenylsufonyl-1H-indole-3-yl)ethylamine (MS-245, ) is a 5-HT6R antagonist reported by Glennon et al.Citation31 in early 2000. Since then a lot of reports have been published regarding variations in N,N-dimethyl amino alkyl side chain on and around the indole nucleus and their affinityCitation21,Citation32,Citation33 (). Numerous selective antagonists of 5-HT6 receptors have been disclosed during the last decadeCitation34–42 and a pharmacophore model for this type of receptor antagonists has been developed based on known structurally diverse 5-HT6 receptor antagonistsCitation43–49. In general, the model entails the positive ionizable atom (usually a secondary or tertiary amino group), a hydrogen bond acceptor group (usually a sulfone or sulfonamide group), a hydrophobic site (HYD) and π-electron donor aromatic or heterocyclic ring (AR). Considering the reported 5-HT6 receptor ligands and an established pharmacophore model, in an attempt to identify potent and selective 5-HT6 ligands, we have designed and synthesized a series of N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl ethane-1,2-diamines and N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl propane-1,3-diamines derivatives with basic amine side chain (Compounds 9) on N-aryl sulfonyl moiety at C-3′ position.
These compounds have high selectivity over closely related receptors and activity in the animal model of cognition. The synthesis, in vitro activity, selectivity over closely related targets, pharmacokinetics and pharmacology of these series of compounds are discussed in this paper.
In vitro potencies were determined for all the synthesized compounds using functional reporter gene-based assayCitation50,Citation51. This assay uses a stable CHO cell line expressing recombinant human 5-HT6 receptor and pCRE-Luc reporter system, which allows one to determine functional activities (agonist or antagonist) and assess potency of a compound to modulate GPCR-mediated cell responses. By using this specific assay, the level of intracellular cyclic AMP which is modulated by activation or inhibition of the receptor is measured. The Kb values of N,N-dimethylamino alkyl amino aryl sulfonamides obtained from the cell-based assay of 5-HT6 receptor are given in
Table 1. 5-HT6 receptor potencies*. 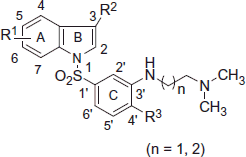 .
.
Results and discussion
Structure activity relationship
Several analogues of N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl ethane-1,2-diamines and N′-[3-(indole-1-sulfonyl) aryl]-N,N-dimethyl propane-1,3-diamines were synthesized and their Kb values are given in . In the presented series, all the compounds were found to have high potency, with Kb values in the range of 1.8–60 nM, indicating that the flexible N,N-dimethylamino alkyl amine side chain on N-aryl sulfonyl group at C-3′ position in an indolylsulfonamide amine series is well tolerated in terms of activity.
Among the compounds synthesized, 9e and 9y were the most potent compounds with Kb values of 1.8 and 2.1 nM respectively, these compounds bearing n = 1, R1 = H, R2 = CH3, R3 = OCH3 and n = 2, R1 = H, R2 = CH3, R3 = OCH3, respectively. Generally, -H, halo and smaller alkyl (namely Me) substitutions at C3 of indole ring are well tolerated and proved to have good potency as can be seen from the Kb values of the compounds 9c (Kb = 4.9 nM), 9d (Kb = 3.1 nM), 9e (Kb = 1.8 nM), 9h (Kb = 19 nM), 9n (Kb = 13 nM), 9o (Kb = 17 nM) and 9t (Kb = 11.9 nM). Compounds with electron donating and/or bulky groups (namely OCH3, OC2H5 and OCH(CH3)3) at C5 of indole were showing moderate potency toward 5-HT6 R, as it can be seen from the Kb values of the compounds 9a (Kb = 33 nM), 9b (Kb = 23.7 nM), 9f (Kb = 26.9 nM), 9p (Kb = 26 nM), 9q (Kb = 27 nM), 9v (Kb = 47 nM) and 9z (Kb = 37.7 nM). More or less a very similar trend was observed in the case of electron withdrawing and/or bulky halogen group (namely F, Cl and Br) at C5 of indole, for example, 9h (Kb = 19 nM), 9m (Kb = 40 nM), 9o (Kb = 17 nM), 9r (Kb = 33 nM), 9s (Kb = 33 nM), 9u (Kb = 43 nM), 9w (Kb = 23 nM), 9ad (Kb = 7.2 nM), 9ae (Kb = 39.4 nM) and 9af (Kb = 60 nM). Among the halo compounds with n = 1, the potencies drop down as the size of the halogen group increases, for example, 9o (Kb = 17 nM) versus 9w (Kb = 23 nM) versus 9u (Kb = 43 nM). This was also true for the compounds bearing n = 2, as can be seen by comparing 9ad (Kb = 7.2 nM) versus 9ae (Kb = 39.4 nM) versus 9af (Kb = 60 nM). This indicates that among the halo derivatives, the fluoro substitution is the most tolerated and preferred substitution at C5 position of the indole. Marginal improvement was seen in potency when the chloro group was shifted from C4 to C6 of indole ring as it can be seen from the Kb values of the compounds 9t (Kb = 11.9 nM) versus 9k (Kb = 6.6 nM). Increasing the chain length (n = 2) in the side chain gave compounds with almost equipotent activities, as it can be seen from Kb values of 9e (Kb = 1.8 nM) versus 9y (Kb = 2.1 nM), 9g (Kb = 25 nM) versus 9z (Kb = 37.7 nM), 9f (Kb = 26.9 nM) versus 9aa (Kb = 18 nM), 9x (Kb = 40 nM) versus 9ae (Kb = 39.4 nM) and 9a (Kb = 33 nM) versus 9ac (Kb = 18.9 nM).
The values of Imax and IC50 for most potent compounds 9d, 9e and 9y are given in
Table 2. Imax and IC50 for selected potent compounds.
All the lead compounds have shown full antagonistic activity with Imax 100% or close to 100% ().
Selected compounds that showed an excellent potency in the reporter gene cell-based assay were further profiled in a radioligand binding assay for specificity on a panel of closely related receptors. The compounds (9d, 9e and 9y) have an excellent selectivity (
Table 3. Compounds selectivity data*.
Once the in vitro activity and selectivity of compounds was achieved, our next step was to study the metabolic stability in microsomes (
Table 4. Percentage surrogate metabolism for compounds 9d, 9e and 9y*.
Since brain penetration is an important factor for molecules targeting the CNS, we determined PK parameters (
Table 5. Pharmacokinetic profile of compounds 9d, 9e and 9y in male Wister rats*.
The clearance was low (49 ± 9 mL/min/kg) for compound 9y as compared with 9d and 9e. The compounds 9d, 9e and 9y showed volume of distributions (Vz, L/kg), i.e. 22 ± 6, 17 ± 2 and 11 ± 1 at 10 mg/kg, i.v. dose.
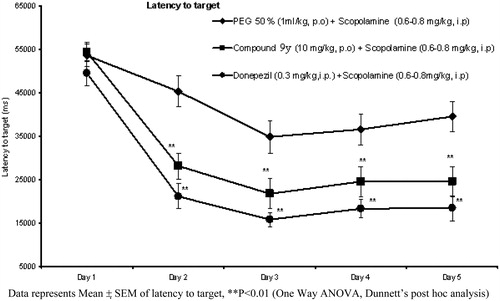
Based on overall pharmacokinetic and brain penetration data (), compound 9y was selected for further profiling in pharmacological models of cognition. Oral administration of compound 9y (10 mg/kg) has significantly improved performance of rats in novel object recognition test (NORT, ).
Figure 3. Novel object recognition test data for compound 9y in rats. Compound 9y versus Vehicle (Paired t-test), n = 9–10/group, p.o., dosing drug: 60 min prior to test (p.o.). Vehicle-PEG 400 50% v/v; 1 mL/kg, p.o. *p < 0.05 students t-test.
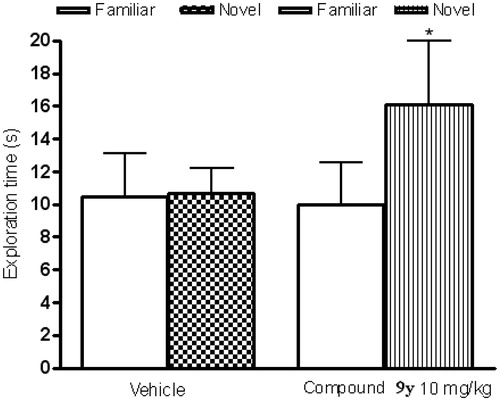
In Morris water maze test, the compound significantly reversed the scopolamine-induced memory deficit which was apparent from lesser target latency ().
Materials and methods
General considerations
Infrared spectra were recorded in KBr disk and in solid state using a Perkin-Elmer model 1600 FT-IR spectrophotometer (Perkin-Elmer, Norwalk, CT). Electrospray ionization mass spectra were recorded on API 4000 triple quadrupole instrument (MDSSCIEX, Concord, Ontario, Canada). 1H-NMR spectra were obtained on a Bruker proton NMR spectrometer (Fallanden, Switzerland) at 400 MHz. Deuterated reagents were used as solvents and were commercially procured. Tetramethylsilane was used as an internal standard. Chemical shift values are expressed in parts per million (δ) and coupling constants are expressed in hertz. Chromatography refers to column chromatography performed using 100–200 mesh silica gel and executed under nitrogen pressure (flash chromatography) conditions. All the reagents and chemicals used were of “reagent grade”.
Determination of Kb and IC50 values for 5-HT6 receptor antagonists
A stable CHO cell line expressing recombinant human 5-HT6 receptor and pCRE-Luc reporter system was used for cell-based assay. The assay offers a non-radioactive-based approach to determine potency of a compound to GPCRs. In this specific assay, the level of intracellular cyclic AMP which is modulated by activation or inhibition of the receptor is measured. The recombinant cells harbor luciferase reporter gene under the control of cAMP response element. The above cells were plated in 96-well clear bottom white plates at a density of 5 × 104 cells/well using Hams F12 medium containing 10% fetal bovine serum and incubated overnight at 37 °C and 5% CO2 followed by serum starvation for 18–20 h. Increasing concentrations of test compounds were added along with 10 µM serotonin in OptiMEM to the cells. The incubation was continued at 37 °C in CO2 incubator for 4 h. After 4 h, cells were lysed using lyses buffer and luciface assay buffer was added to each well and counts per second were recorded using luminescence counter. From CPS obtained, percentage inhibition was calculated for each well by normalizing CPS values obtained in the presence of the compounds to those with 10 µM 5-HT (0% inhibition) and with vehicle (100% inhibition). The percentage inhibition was determined for all concentrations of the antagonists (0.1 nM to 10 µM range, half-log increment) and plotted as a function of the antagonist concentration. The Kb values were calculated with Prism (GraphPad) using built-in one site competition equation, by entering EC50 values for 5-HT obtained in the same experiments (80–100 nM) and the 5-HT concentration 10 µM, as it was used in all the experiments.
Competitive binding of compound 9y and Schild analysis
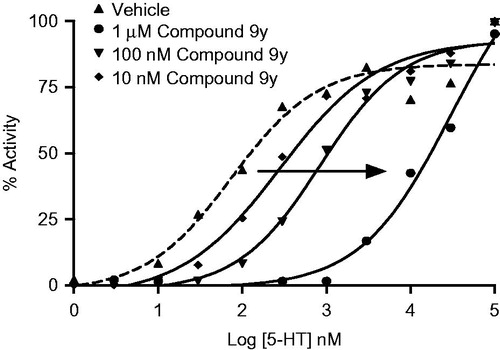
5-HT dose response curve was performed in the presence of varying concentrations of compound 9y as shown in . The compound produced a rightward shift of 5-HT dose response, with a Kb value of 5.9 nM and a slope of unity in the Schild plot analysis proving that the compound binds to 5-HT6 receptor in a competitive manner.
pEC50 values of 5-HT alone and in the presence of different concentrations of compound 9y are given in
Table 6. pEC50 values of 5-HT in the presence of different concentrations of compound 9y.
Protocol for specificity profile
The specificity of the compounds for 5-HT6 receptor was tested in 10 closely related and pharmacologically relevant receptor targets. Percentage inhibition studies were performed at single concentration of the tested compound using membrane preparations obtained from recombinant cell lines expressing relevant protein of interest, in the presence of specific radioligands. The percentage inhibition was defined as the ability of tested compound to inhibit binding of receptor-specific radioligands at tested conditions.
The radioligand used for the selectivity assay is mentioned in the following table:
Rodent pharmacokinetic study
Male Wistar rats (225 ± 25 g) were used as experimental animals. Three animals were housed in each cage. Two days prior to dosing day, male Wistar rats (225–250 g) were anesthetized with isoflurane for surgical placement of jugular vein catheter. Animals were fasted over night before oral dosing (p.o) and food pellets were allowed 2 h post-dosing, whereas during intravenous dosing food and water were provided as ad libitum. Three rats were dosed with compounds (10 mg/kg) orally and intravenously (10 mg/kg).
At each time point, blood was collected through jugular vein and immediately replenish with an equivalent volume of normal saline from freely moving rats. Collected blood was transferred into a labeled appendorf containing 10 µL of heparin as anticoagulant. Blood samples were collected at following time points: pre-dose, 0.08 (only i.v.), 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h post-dose (n = 3). Blood was centrifuged at 4000 rpm for 10 min. Plasma was prepared and stored at −20 °C until analysis. The concentrations of the compounds were quantified in plasma by qualified the LC-MS/MS method using a suitable extraction technique. The compounds were quantified in the calibration range around 2–2000 ng/mL in plasma. Study samples were analyzed using calibration samples in the batch and quality control samples spread across the batch.
Pharmacokinetic parameters Cmax, AUC0–t, t1/2 and bioavailability were calculated by the non-compartmental model using a standard non-compartmental model by using WinNonLin 5.0.1 version Software package.
Rodent brain penetration study
Male Wistar rats (225 ± 25 g) were used as experimental animals. Three animals were housed in each cage. Animals were given water and food ad libitum throughout the experiment and maintained on a 12 h light/dark cycle.
Brain penetration was determined in discrete manner in rats. At each time point (0.50, 1 and 2 h), n = 3 animals were used. The compounds were suitably preformulated and administered orally at (free-base equivalent) 10 mg/kg. Blood samples were removed via, cardiac puncture by using isoflurane anesthesia. The animals were sacrificed to collect the brain tissue. Plasma was separated and brain samples were homogenized and stored at −20 °C until analysis. The concentrations of the compound in plasma and brain were determined using the LC-MS/MS method.
The compounds were quantified in plasma and brain homogenate by the qualified LC-MS/MS method using a suitable extraction technique. The compounds were quantified in the calibration range of 1–2000 ng/mL in plasma and brain homogenate. Study samples were analyzed using calibration samples in the batch and quality control samples spread across the batch. Extent of brain–plasma ratio was calculated (Cb/Cp).
Protocol for NORT
The following protocol was followed for the experiment:
For object recognition test, Male Wistar rats 10–12 weeks old were used. Arena was 50 × 50 × 50 cm. Open field was made up of acrylic. Twenty-four hour prior to testing, rats were habituated to individual test arenas for 20 min in the absence of any objects. Twenty-four hours after the habituation, during the familiarization phase (T1), rats were placed individually in the open field for 3 min, containing two identical objects (a1 and a2). T2 trial was carried out after 24 h after the T1 trial. Rats were allowed to explore the open field for 3 min in the presence of one familiar object (a3) and one novel object (b). Discriminative index was calculated.
Morris water maze test
The following protocol was followed for the experiment:
Rats were acclimatized to the laboratory environment for seven days. Rats were housed in a group of four in a controlled environment (temp = 22 ± 2 °C; humidity = 50 ± 5%) and maintained 12 h light/dark cycle with lights were on at 07:00 with food and water provided ad libitum. Water maze consisted of a 1.8-m diameter; 0.6-m high circular water maze filled with water 24 ± 2 °C up to a platform 16-cm diameter was placed 1.0 cm below the water surface in the center of one of the eight imaginary quadrants, which remained constant for all the rats. Prominent visual cues surrounding the maze were used as spatial cues around the arena (approximate 200-lux light intensity). The experiment consisted of five days acquisition trials and one probe trial on the seventh day, i.e. one day after the completion of acquisition trial. Rats were administered with vehicle or test compounds 60 min before acquisition training and 30 min after administration of vehicle or test compounds. Scopolamine was given at a dose of 0.6 mg/kg on the first day and the dose of scopolamine was gradually increased at a rate of 0.05 mg/kg/day. Rat was lowered gently, feet first into water. Rat was allowed to swim for 60 s to find the platform. If the platform was found during the time, then trial was stopped and rat was allowed to stay on platform for 10 s before removed from the maze. If the platform was not found during 60 s trials then the rat was gently guided to the platform and allowed to stay on platform for 10 s before removed from the maze. Each rat was received four trials in a day. The maze had eight starting points. On the first day, the animals were started from 1st, 3rd, 5th and 7th starting points and on the second day the animals were started from 2nd, 4th, 6th and 8th starting points and again on the third day the animals were started from 1st, 3rd, 5th and 7th starting points, fourth day the animals were started from 2nd, 4th, 6th and 8th and on fifth day from 1st, 3rd, 5th and 7th. Retention of the task was assessed on 7th day in which each animal was received a single 30 s probe test with platform removed from the pool, and percentage of time was spent in a target quadrant (quadrant in which platform was placed during acquisition training) was calculated.
The general synthetic strategy used for the preparation of N′-[3-(indole-1-sulfonyl) phenyl]-N,N-dimethyl ethane-1,2-diamines and N′-[3-(indole-1-sulfonyl) phenyl]-N,N-dimethyl propane-1,3-diamines (compounds 9) is summarized in Scheme 1. Commercially available ortho-substituted acetanilides 1 were reacted with chlorosulfonic acid at the ambient temperature yielding the corresponding sulfonylchlorides 2, which upon treatment with the appropriately substituted indoles 3 in the presence of sodium hydride as base at room temperature gave the (3-acetylamino-4-substituted phenylsulfonyl)-1H-indole derivatives 4. The latter was treated with ethanolic hydrochloric acid under reflux to obtain (3-amino-4-substituted phenylsulfonyl)-1H-indoles 7.
Scheme 1. Reagents and conditions: (a) ClSO3H, RT, 10–12 h; (b) substituted indoles, NaH, THF, RT, 20–24 h; (c) ethanolic HCl, reflux, 2–3 h; (d) substituted indoles, Et3N, DCM, RT, 24–48 h; (e) Fe/HCl, ethanol, reflux, 2–8 h; (f) N-chlorosuccinimide, 1,4-dioxane, reflux, 5–6 h; (g) N,N-dimethylamino ethyl or propyl chloride, DMF, xylene, K2CO3, 10–12 h, 140 °C (note: compounds bearing R3 = OCH3, C2H5 were synthesized via intermediate 1, while rest of the compounds wherein R3 = H, CH3 were synthesized via intermediate 5. See ).
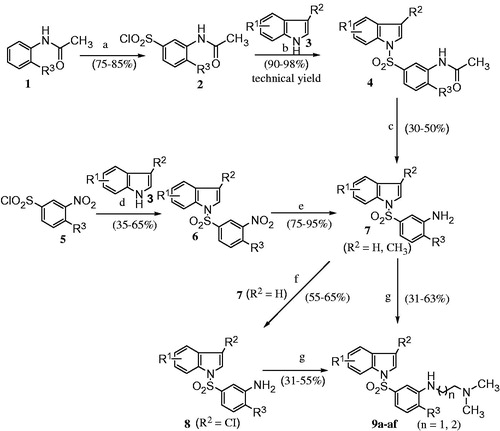
Alternatively, substituted indoles 3 were reacted with commercially available substituted 3-nitrobenzenesulfonyl chlorides 5 in the presence of triethylamine as base yielding the corresponding (3-nitro-4-substituted phenylsulfonyl)-1H-indoles 6. The latter was reduced with Fe-HCl under reflux to obtain (3-amino-4-substituted phenylsulfonyl)-1H-indoles 7.
The amine intermediate 7 was treated with N-chlorosuccinimide under reflux to afford N-substituted-3-chloro indoles 8. Finally, the amines 7 and 8 were reacted with N,N-dimethylamino ethyl or propyl chlorides in the presence of K2CO3 as base, to obtain the targeted compounds 9.
Experimental
General (representative) procedure for the synthesis of compound 9y
Experimental procedures and analytical characterization data of compounds 2a, 4a, 7a, 7b, 8b, 6g, 7g, 9a and 9b.
3-Acetamido-4-methoxy benzenesulfonyl chloride (2a)
A mixture of chlorosulfonic acid (43.5 g, 374 mmol) and o-methoxy acetanilide (23) (12.25 g, 75 mmol) was stirred at 100 °C for 2 h. The reaction mixture was brought to RT and stirred for 24 h. The progress of the reaction was monitored by TLC. The reaction mass was quenched onto ice water. The solid mass, thus obtained, was filtered under suction and washed with water (125 mL). The product was dried in a desiccators for 3 h. Above impure compound was recrystallized from hot benzene.
IR (cm−1): 3304, 1675, 1369, 1165; 1H-NMR (400 MHz, CDCl3): δ 2.25 (3H, s), 4.02 (3H, s), 6.99–7.01 (1H, d, J = 8.8 Hz), 7.73–7.76 (1H, dd, J = 2.4, 8.8 Hz), 7.83 (1H, bs), 9.13–9.14 (1H, s).
1-(3′-Acetamido-4′-methoxy benzenesulfonyl)-5-methoxy-3-methyl-1H-indole (4a)
Sodium hydride (2.15 g, 49.3 mmol) was taken into a 250 mL three-necked round bottom flask containing tetrahydrofuran (50 mL) under nitrogen atmosphere. A solution of 5-methoxy-3-methyl indole (5.3 g, 32.9 mmol) in tetrahydrofuran (25 mL) was added to the above mixture at 25° C and further stirred for 1 h. Then added a solution of 3-acetamido-4-methoxy benzenesulfonyl chloride (13.01 g, 49.3 mmol) in tetrahydrofuran (65 mL) at 0 °C. Thereafter, it was allowed to stir for 24 h at RT while monitoring the progress of the reaction by TLC. After completion of the reaction it was quenched over ice water (100 mL) under stirring and the product was extracted with ethyl acetate (2 × 250 mL). The combined organic phase was washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the technical product (10.2 g), which was used as such for the next step without purification. ESI-MS (m/z): 389.4 (M + H)+.
1-(3′-Amino-4′-methoxy benzenesulfonyl)-5-methoxy-3-methyl-1H-indole (7a)
To a stirred solution of 1-(3′-acetamido-4′-methoxy benzenesulfonyl)-5-methoxy-3-methyl-1H-indole (10 g, 25.7 mmol) (4a) in ethanol (100 mL) was added aq. HCl (10 mL, 33%) and the mixture was heated to reflux temperature and maintained under reflux for a period of 2 h. After completion of the reaction, the reaction mass was concentrated under vacuo. The residual mass, thus obtained, was diluted with chilled water (100 mL) and then pH was adjusted to 9.0–10.0 with dilute sodium hydroxide solution. The product was extracted with dichloro methane (2 × 100 mL). The combined organic phase was washed with brine, dried over anhydrous sodium sulfate and concentrated the mass under reduced pressure to obtain technical product. The obtained technical product was purified by column chromatography using silica gel (100–200 mesh), the eluent system being ethyl acetate and n-hexane (2:8) to obtain 3.6 g of the title product.
HPLC (%): 98.52; IR (cm−1): 3487, 3385, 1616, 1514, 1336, 1226, 1157, 624; ESI-MS (m/z): 347.1 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.19 (3H, s), 3.81 (3H, s), 3.82 (3H, s), 3.92 (2H, bs), 6.68–6.70 (1H, d, J = 8.52 Hz), 6.85–6.86 (1H, d, J = 2.44 Hz), 6.88–6.91 (1H, dd, J = 8.92, 2.52 Hz), 7.05–7.06 (1H, d, J = 2.32 Hz), 7.23–7.26 (2H, m), 7.83–7.85 (1H, d, J = 8.92 Hz).
1-(3′-Amino-4′-methoxy benzenesulfonyl)-5-methoxy-1H-indole (7b)
This compound was prepared by using the method described for the preparation of 7a.
HPLC (%): 98.48; IR (cm−1): 3481, 3385, 1616, 1510, 1365, 1342, 1224, 1168, 1147, 677; ESI-MS (m/z): 333.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 3.80 (3H, s), 3.82 (3H, s), 3.94 (2H, bs), 6.55–6.56 (1H, d, J = 3.6 Hz), 6.70–6.72 (1H, d, J = 8.54 Hz), 6.89–6.92 (1H, dd, J = 8.98, 2.48 Hz), 6.96–6.97 (1H, d, J = 2.41 Hz), 7.07–7.08 (1H, d, J = 2.31 Hz), 7.26–7.28 (1H, dd, 8.57, 2.31 Hz), 7.490–7.499 (1H, d, J = 3.6 Hz), 7.84–7.87 (1H, d, J = 9.0 Hz).
1-(3′-Amino-4′-methoxy benzenesulfonyl)-5-methoxy-3-chloro-1H-indole (8b)
A stirred solution of 1-(3′-Amino-4′-methoxy benzenesulfonyl)-5-methoxy-1H-indole (1.85 g, 5.57 mmol) (7a) in 20 mL of 1,4-dioxane was treated with N-chloro succinimide (0.818 g, 6.12 mmol) at 100 °C for a period of 8–10 h. After completion of the reaction, the reaction mass was cooled to room temperature, quenched over chilled water (30 mL) and the pH was adjusted to 9.0–10.0 with saturated aq.sodium carbonate. The solid compound that separated was filtered through buchner funnel and washed with 50 mL ice cold water. The wet material was dried under reduced pressure and then purified by column chromatography, eluent being ethyl acetate and n-hexane (1:5) to obtain 1.31 g of the title product.
HPLC (%): 98.09; IR (cm−1): 3487, 3387, 1618, 1512, 1365, 1350, 1213, 1168, 1024, 680; ESI-MS (m/z): 367.1, 369.1 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 3.83 (3H, s), 3.84 (3H, s), 3.97 (2H, bs), 6.71–6.73 (1H, d, J = 8.55 Hz), 6.93–6.94 (1H, d, J = 2.37 Hz), 6.95–6.98 (1H, dd, J = 8.94, 2.52 Hz), 7.06–7.07 (1H, d, J = 2.35 Hz), 7.26–7.28 (1H, dd, J = 8.62, 2.35 Hz), 7.48 (1H, s), 7.85–7.87 (1H, d, J = 8.99 Hz).
1-(3′-Nitro-4′-methyl benzenesulfonyl)-5-methoxy-1H-indole (6g)
To a stirred mixture of 5-methoxy-1H-indole (3.0 g, 20.4 mmol) and triethylamine (5.15 g, 51.02 mmol) in dichloromethane (40 mL) was added a solution of 3-nitro-4-methyl benzenesulfonyl chloride (5.76 g, 24.48 mmol) in dichloromethane (65 mL) at 0 °C over a period of 10 min. Then, it was allowed to stir at room temperature for 48 h. After completion of the reaction, the organic reaction mass was washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain technical product (7.06 g). The technical product was purified by column chromatography, eluent being ethyl acetate and n-hexane (1:10) to obtain 2.60 g of the title product.
IR (cm−1): 3130, 2997, 2970 1608, 1531, 1375, 1348, 1224, 1182, 1026, 596; ESI-MS (m/z): 345 (M − H+); 1H-NMR (400 MHz, CDCl3): δ 2.59 (3H, s), 3.81 (3H, s), 6.63–6.64 (1H, d, J = 3.64 Hz), 6.94 (1H, d, J = 2.51 Hz), 6.96–6.98 (1H, dd, J = 3.5, 1.99 Hz), 7.40–7.42 (1H, d, J = 8.15 Hz), 7.49–7.50 (1H, d, 3.65 Hz), 7.86–7.88 (1H, d, J = 8.78 Hz), 7.90–7.92 (1H, dd, J = 8.14, 1.98 Hz), 8.43 (1H, d, 1.93 Hz).
1-(3′-Amino-4′-methyl benzenesulfonyl)-5-methoxy-1H-indole (7g)
Iron powder (4.2 g, 73.57 mmol) was taken in a RBF along with 40 mL of water and then activated it by adding 5 mL of conc. HCl (33%) and stirring the mass at 80 °C for 45 min. A solution of compound 6g (2.5 g, in 100 mL of ethanol) was added to the activated iron suspension at reflux temperature. Then, the mass was further stirred for a period of 8–10 h under reflux, while monitoring the progress of the reaction by thin layer chromatography. After completion of the reaction, the reaction mass was concentrated under reduced pressure, the residual mass was quenched onto chilled water and the pH adjusted to 9.0–10.0 with 40% aqueous sodium hydroxide solution. The product was extracted with dichloromethane (3 × 100 mL). The combined organic layer was washed with brine solution (100 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the technical product (2.2 g). The technical product was purified by column chromatography, the eluent being ethyl acetate and n-hexane (1:3), to obtain 1.9 g of the title compound.
HPLC (%): 95.27; IR (cm−1): 3479, 3387, 1620, 1467, 1365, 1342, 1224, 1145, 1028, 680; ESI-MS (m/z): 317.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.09 (3H, s), 3.77 (2H, bs), 3.80 (3H, s), 6.56–6.57 (1H, d, J = 3.56 Hz), 6.89–6.92 (1H, dd, J = 9.01, 2.48 Hz), 6.96–6.98 (1H, d, 2.44 Hz), 7.03–7.05 (1H, d, 7.92 Hz), 7.07 (1H, d, J = 1.83 Hz), 7.13–7.15 (1H, dd, J = 7.88, 1.86 Hz), 7.47–7.49 (1H, d, J = 7.96 Hz), 7.85–7.87 (1H, d, J = 9.02 Hz).
N′-[2-methoxy-5-(5-methoxy-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl ethane-1,2-diamine (9ae)
To a stirred solution of 1-(3′-amino-4′-methoxy benzenesulfonyl)-5-methoxy-3-methyl-1H-indole (0.6 g, 1.7 mmol) (7a) in a mixture of DMF (3 mL) and m-xylene (3 mL) was added 2-dimethylaminoethyl chloride hydrochloride (0.49 g, 3.4 mmol) and potassium carbonate (0.7 g, 5.1 mmol) at room temperature. Then, the reaction mass was heated to 135–138 °C and maintained at this temperature for a period of 10 h. After completion of the reaction, the mass was cooled to room temperature and quenched onto chilled water (25 mL). The pH of the reaction mass was adjusted to 9.0–10.0 with lye solution and the product was extracted with dichloromethane (2 × 50 mL). The combined organic layer was washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced vacuum to obtain the technical product. The latter was purified by column chromatography, eluent being ethyl acetate and triethylamine (99:1) to obtain 0.24 g of the title product.
HPLC (%): 97.67; IR (cm−1): 3404, 1597, 1359, 1166; ESI-MS (m/z): 418.5 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.2 (3H, s), 2.29 (6H, s), 2.58–2.61 (2H, t, J = 5.92 Hz), 3.14–3.16 (2H, t, J = 5.96 Hz), 3.80 (3H, s), 3.82 (3H, s), 4.8 (1H, bs), 6.62–6.64 (1H, d, J = 8.45 Hz), 6.84–6.85 (1H, d, J = 2.42 Hz), 6.86 (1H, d, J = 2.27 Hz), 6.88–6.90 (1H, dd, J = 8.93, 2.49 Hz), 7.15–7.17 (1H, dd, J = 8.39, 2.27 Hz), 7.24 (1H, s), 7.86–7.88 (1H, d, J = 8.93 Hz); HRMS: [M + H]+ C21H28N3O4S calc. 418.1801, found. 418.1804.
N′-[5-(3-chloro-5-methoxy indole-1-sulfonyl)-2-methoxy phenyl]-N, N-dimethyl ethane-1,2-diamine (9b)
This compound was prepared by using the method described for the preparation of 9a.
HPLC (%): 98.09; IR (cm−1): 3415, 1597, 1367, 1166; ESI-MS (m/z): 438.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.29 (6H, s), 2.59–2.62 (2H, t, J = 5.92 Hz), 3.15–3.18 (2H, t, J = 5.96 Hz), 3.82 (3H, s), 3.83 (3H, s), 4.91 (1H, bs), 6.66–6.68 (1H, d, J = 8.45 Hz), 6.84 (1H, d, J = 2.14 Hz), 6.92–6.93 (1H, d, J = 2.29 Hz), 6.94–6.97 (1H, dd, J = 8.98, 2.44 Hz), 7.19–7.22 (1H, dd, J = 8.4, 2.2 Hz), 7.51 (1H, s), 7.88–7.9 (1H, d, J = 8.96 Hz); HRMS: [M + H]+ C20H25ClN3O4S calc. 438.1254, found. 438.1251.
Examples 9c–9af
The compounds 9c–9af were prepared by using the method described in example 9a and 9b with some non-critical variations. The analytical data are presented below.
N′-[5-(3-chloro indole-1-sulfonyl)-2-methoxy phenyl]-N, N-dimethyl ethane-1,2-diamine (9c)
HPLC (%): 97.38; MR (°C): 96.88; IR (cm−1): 3388, 1597, 1367, 1168; ESI-MS (m/z): 408.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.35 (6H, s), 2.66–2.69 (2H, t, J = 5.96 Hz), 3.21–3.24 (2H, t, J = 6.02 Hz), 3.82 (3H, s), 4.98 (1H, bs), 6.67–6.69 (1H, d, J = 8.47 Hz), 6.86 (1H, d, J = 2.26 Hz), 7.23–7.25 (1H, dd, J = 6.24, 2.29 Hz), 7.27–7.31 (1H, dd, J = 8.05, 0.87 Hz), 7.34–7.38 (1H, dt, J = 8.28, 1.1 Hz), 7.53–7.55 (1H, d, J = 7.87 Hz), 7.56 (1H, s), 8.0–8.02 (1H, d, J = 7.62 Hz); HRMS: [M + H]+ C19H23ClN3O3S calc. 408.1149, found. 408.1145.
N′-[5-(indole-1-sulfonyl)-2-methoxy phenyl]-N, N-dimethyl ethane-1,2-diamine (9d)
HPLC (%): 98.93; MR (°C): 127.73; IR (cm−1): 3392, 1597, 1361, 1168; ESI-MS (m/z): 374.1 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.37 (6H, s), 2.68–2.71 (2H, t, J = 5.96 Hz), 3.21–3.24 (2H, t, J = 6.04 Hz), 3.81 (3H, s), 5.05 (1H, bs), 6.62–6.63 (1H, d, J = 3.6 Hz), 6.66–6.68 (1H, d, J = 8.44 Hz), 6.89–6.9 (1H, d, J = 2.25 Hz), 7.18–7.25 (2H, m), 7.29–7.31 (1H, dd, J = 8.19, 0.96 Hz), 7.51–7.53 (1H, d, J = 7.76 Hz), 7.56–7.57 (1H, d, J = 3.64 Hz), 7.99–8.01 (1H, d, J = 8.16 Hz); HRMS: [M + H]+ C19H24N3O3S calc. 374.1538, found. 374.1534.
N′-[2-methoxy-5-(3-methyl-indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9e)
HPLC (%): 99.04; MR (°C): 110.42; IR (cm−1): 3396, 1597, 1361, 1166; ESI-MS (m/z): 388.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.2 (3H, s), 2.29 (6H, s), 2.59–2.62 (2H, t, J = 5.92 Hz), 3.15–3.18 (2H, t, J = 5.96 Hz), 3.80 (3H, s), 4.86 (1H, bs), 6.65–6.67 (1H, d, J = 8.44 Hz), 6.90 (1H, d, J = 2.26 Hz), 7.20 (1H, d, J = 2.24 Hz), 7.22–7.23 (1H, m), 7.27–7.31 (2H, m), 7.43–7.45 (1H, d, J = 7.61 Hz), 7.98–8.0 (1H, d, J = 8.24 Hz); HRMS: [M + H]+ C20H26N3O3S calc. 388.1695, found. 388.1692.
N′-[5-(5-methoxy-3-methyl indole-1-sulfonyl)-2-methyl phenyl]-N,N-dimethyl ethane-1,2-diamine (9f)
HPLC (%): 97.19; IR (cm−1): 3390, 1598, 1363, 1168; ESI-MS (m/z): 402.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.07 (3H, s), 2.19–2.20 (3H, s), 2.23 (6H, s), 2.55–2.58 (2H, t, J = 6.0 Hz), 3.10 (2H, t), 3.82 (3H, s), 4.48 (1H, bs), 6.85–6.86 (1H, d, J = 2.43 Hz), 6.88–6.91 (2H, m), 6.99–7.01 (1H, d, J = 7.98 Hz), 7.05–7.07 (1H, dd, J = 7.78, 1.84 Hz), 7.26 (1H, s), 7.87–7.89 (1H, d, J = 8.94 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1855.
N′-[5-(5-methoxy indole-1-sulfonyl)-2-methyl phenyl]-N,N-dimethyl ethane -1,2-diamine (9g)
HPLC (%): 98.21; MR (°C): 99.7–101.2; IR (cm−1): 3336, 1604, 1350, 1138; ESI-MS (m/z): 388.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.08 (3H, s), 2.25 (6H, s), 2.57–2.60 (2H, t, J = 6.0 Hz), 3.10–3.13 (2H, t, J = 5.72 Hz), 3.80 (3H, s), 4.53 (1H, bs), 6.55–6.56 (1H, d, J = 3.55 Hz), 6.89 (1H, d, J = 2.52 Hz), 6.90–6.92 (1H, d, J = 2.74 Hz), 6.95–6.96 (1H, d, J = 2.45 Hz), 7.01–7.03 (1H, d, J = 7.89 Hz), 7.07–7.10 (1H, dd, J = 7.78, 1.87 Hz), 7.51–7.52 (1H, d, J = 3.62 Hz), 7.88–7.90 (1H, d, J = 9.0 Hz); HRMS: [M + H]+ C20H26N3O3S calc. 388.1695, found. 388.1693.
N′-[2-ethyl-5-(5-fluoro indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9h)
HPLC (%): 99.50; MR (°C): 81.21; IR (cm−1): 3412, 1595, 1367, 1168; ESI-MS (m/z): 390.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.16–1.20 (3H, t, J = 7.45 Hz), 2.22 (6H, s), 2.39–2.45 (2H, q, J = 7.48 Hz), 2.54–2.57 (2H, t, J = 5.67 Hz), 3.06–3.10 (2H, q, J = 5.08 Hz), 4.66 (1H, bs), 6.58–6.59 (1H, d, J = 3.6 Hz), 6.91–6.92 (1H, d, J = 1.90 Hz), 6.99–7.04 (1H, dt, J = 9.0, 2.54 Hz), 7.05–7.07 (1H, d, J = 7.92 Hz), 7.12–7.18 (2H, m), 7.59–7.60 (1H, d, J = 3.64 Hz), 7.93–7.96 (1H, d, J = 4.40 Hz); HRMS: [M + H]+ C20H25FN3O2S calc. 390.1651, found. 390.1653.
N′-[2-ethyl-5-(5-methoxy-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9i)
HPLC (%): 98.89; IR (cm−1): 2964, 1597, 1363, 1168; ESI-MS (m/z): 416.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.15–1.19 (3H, t, J = 7.48 Hz), 2.2 (3H, s), 2.22 (6H, s), 2.37–2.43 (2H, q, J = 7.48 Hz), 2.53–2.56 (2H, t, J = 5.68 Hz), 3.06–3.10 (2H, q, J = 5.08 Hz), 3.82 (3H, s), 4.60 (1H, bs), 6.86 (1H, d, J = 2.38 Hz), 6.89–6.92 (1H, dd, J = 2.47, 8.94 Hz), 6.92–6.93 (1H, d, J = 1.72 Hz), 7.02–7.03 (1H, d, J = 7.94 Hz), 7.09–7.11 (1H, dd, J = 7.84, 1.80 Hz), 7.27 (1H, s), 7.88–7.90 (1H, d, J = 8.9 Hz); HRMS: [M + H]+ C22H30N3O3S calc. 416.2008, found. 416.2005.
N′-[5-(6-chloro indole-1-sulfonyl)-2-methyl phenyl]-N, N-dimethyl ethane-1,2-diamine (9j)
HPLC (%): 99.48; MR (°C): 84.8–87.5; IR (cm−1): 3412, 1598, 1365, 1134; ESI-MS (m/z): 392.3 (M + H)+;. 1H-NMR (400 MHz, CDCl3): δ 2.1 (3H, s), 2.25 (6H, s), 2.59–2.62 (2H, t, J = 5.92 Hz), 3.12–3.16 (2H, t, J = 5.88 Hz), 4.59 (1H, bs), 6.58–6.60 (1H, d, J = 3.64 Hz), 6.96 (1H, d, J = 1.8 Hz), 7.05–7.10 (2H, m), 7.16–7.19 (1H, dd, J = 8.4, 1.84 Hz), 7.41–7.43 (1H, d, J = 8.36 Hz), 7.54–7.55 (1H, d, J = 3.68 Hz), 8.03–8.04 (1H, d, J = 1.84 Hz); HRMS: [M + H]+ C19H23ClN3O2S calc. 392.1199, found. 392.1202.
N′-[3-(6-chloro indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9k)
HPLC (%): 97.69; IR (cm−1): 2933, 1602, 1369, 1170; ESI-MS (m/z): 378.7, 380.7(M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.28 (6H, s), 2.60–2.63 (2H, t, J = 5.63 Hz), 3.13–3.16 (2H, t, J = 5.55 Hz), 5.0 (1H, bs), 6.60–6.61 (1H, d, J = 3.70 Hz), 6.72–6.75 (1H, m), 7.00–7.01 (1H, dt, J = 2.1 Hz), 7.10–7.12 (1H, m), 7.17–7.21 (2H, m), 7.42–7.44 (1H, d, J = 8.36 Hz), 7.53–7.54 (1H, d, J = 3.68 Hz), 8.02 (1H, d, J = 1.0 Hz); HRMS: [M + H]+ C18H21ClN3O2S calc. 378.1043, found. 378.1046.
N′-[2-ethyl-5-(5-methoxy indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9l)
HPLC (%): 98.06; IR (cm−1): 3377, 2966, 1597, 1365, 1147; ESI-MS (m/z): 402.6 (M+H)+; 1H-NMR (400 MHz, CDCl3): δ 1.15–1.19 (3H, t, J = 7.48 Hz), 2.31 (6H, s), 2.39–2.44 (2H, q, J = 7.48 Hz), 2.65–2.68 (2H, t, J = 5.96 Hz), 3.15–3.18 (2H, t, J = 5.6 Hz), 3.80 (3H, s), 5.3 (1H, bs), 6.55–6.56 (1H, d, J = 3.56 Hz), 6.89–6.90 (1H, d, J = 2.23 Hz), 6.91–6.92 (1H, d, J = 2.51 Hz), 6.96–6.97 (1H, d, J = 2.44 Hz), 7.04–7.06 (1H, d, J = 7.91 Hz), 7.12–7.15 (1H, dd, J = 7.90, 1.89 Hz), 7.51–7.52 (1H, d, J = 3.61 Hz), 7.89–7.91 (1H, d, J = 9.0 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1854.
N′-[5-(5-bromo indole-1-sulfonyl)-2-ethyl phenyl]-N, N-dimethyl ethane-1,2-diamine (9m)
HPLC (%): 98.66; IR (cm−1): 3388, 1571, 1369, 1170; ESI-MS (m/z): 450.6, 452.6 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.15–1.18 (3H, t, J = 7.48 Hz), 2.23 (6H, s), 2.39–2.45 (2H, q, J = 7.48 Hz), 2.55–2.58 (2H, t, J = 5.64 Hz), 3.07–3.11 (2H, q, t = 5.16 Hz), 4.69 (1H, bs), 6.56–6.57 (1H, d, J = 3.69 Hz), 6.90–6.91 (1H, d, J = 1.9 Hz), 7.05–7.07 (1H, d, J = 7.91 Hz), 7.12–7.15 (1H, dd, J = 7.88, 1.92 Hz), 7.37–7.39 (1H, dd, J = 8.80, 1.92 Hz), 7.56–7.57 (1H, d, J = 3.36 Hz), 7.65 (1H, d, J = 1.83 Hz), 7.87–7.89 (1H, d, J = 8.82 Hz); HRMS: [M + H]+ C20H25BrN3O2S calc. 450.0851, found. 450.0849.
N′-[3-(4-chloro-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9n)
HPLC (%): 95.06; IR (cm−1): 3390, 1600, 1367, 1174; ESI-MS (m/z): 392.3, 394.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.24 (6H, s), 2.46 (3H, s), 2.53–2.55 (2H, t, J = 5.64 Hz), 3.06–3.10 (2H, t, J = 5.63 Hz), 4.71 (1H, bs), 6.72 (1H, m), 6.97–6.98 (1H, dt, J = 2.1 Hz), 7.08–7.11 (1H, m), 7.13–7.19 (3H, m), 7.30 (1H, d, J = 1.22 Hz), 7.86–7.89 (1H, dd, J = 9.17, 2.53 Hz); HRMS: [M + H]+ C19H23ClN3O2S calc. 392.1199, found. 392.1202.
N′-[2-ethyl-5-(5-fluoro-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9o)
HPLC (%): 98.52; MR (°C): 105.4–108.2; IR (cm−1): 3404, 1595, 1359, 1178; ESI-MS (m/z): 404.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.16–1.19 (3H, t, J = 7.45 Hz), 2.19–2.20 (3H, s), 2.22 (6H, s), 2.39–2.44 (2H, q, J = 7.49 Hz), 2.54–2.57 (2H, t, J = 5.67 Hz), 3.06–3.10 (2H, q, J = 4.89 Hz), 4.64 (1H, bs), 6.92 (1H, d, J = 1.85 Hz), 7.01–7.12 (4H, m), 7.33 (1H, d, J = 0.92 Hz), 7.92–7.95 (1H, dd, J = 4.38 Hz); HRMS: [M + H]+ C21H27FN3O2S calc. 404.1808, found. 404.1806.
N′-[3-(5-isopropoxy-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9p)
HPLC (%): 96.13; MR (°C): 86.24; IR (cm−1): 3387, 2978, 1602, 1359, 1176; ESI-MS (m/z): 416.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.32–1.33 (6H, d, J = 6.0 Hz), 2.18 (3H, s), 2.23 (6H, s), 2.51–2.54 (2H, t, J = 5.65 Hz), 3.05–3.07 (2H, q, J = 4.98 Hz), 4.50–4.56 (1H, sept, J = 6.0Hz), 4.64 (1H, bs), 6.66–6.69 (1H, m), 6.88–6.90 (2H, m), 6.98–6.99 (1H, t, J = 3.04 Hz), 7.07–7.09 (1H, m), 7.12–7.16 (1H, t, J = 7.84 Hz), 7.23 (1H, s), 7.84–7.86 (1H, dd, J = 7.8, 1.8 Hz); HRMS: [M + H]+ C22H30N3O3S calc. 416.2008, found. 416.2010.
N′-[3-(5-isopropoxy indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9q)
HPLC (%): 97.46; IR (cm−1): 3387, 2974, 1600, 1367, 1174; ESI-MS (m/z): 402.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.31–1.33 (6H, d, J = 6.0 Hz), 2.22 (6H, s), 2.50–2.53 (2H, t, J = 5.64 Hz), 3.03–3.07 (2H, t, J = 5.04 Hz), 4.47–4.53 (1H, m), 4.61(1H, bs), 6.54–6.55 (1H, d, J = 3.6 Hz), 6.70 (1H, m), 6.88–6.91 (1H, dd, J = 8.96, 2.44 Hz), 6.97 (1H, d, J = 2.36 Hz), 6.98–6.99 (1H, t, J = 1.96 Hz), 7.11 (1H, m), 7.14–7.16 (1H, dt, J = 7.92 Hz), 7.48–7.49 (1H, d, J = 3.64 Hz), 7.85–7.87 (1H, d, J = 9.0 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1849.
N′-[2-ethyl-5-(5-chloro indole-1-sulfonyl) phenyl]-N,N-dimethyl ethane-1,2-diamine (9r)
HPLC (%): 99.34; IR (cm−1): 3390, 1597, 1369, 1168; ESI-MS (m/z): 406.3, 408.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.16–1.20 (3H, t, J = 7.45 Hz), 2.22 (6H, s), 2.39–2.45 (2H, q, J = 7.49 Hz), 2.55–2.57 (2H, t, J = 5.67 Hz), 3.06–3.10 (2H, q, J = 5.08 Hz), 4.67 (1H, bs), 6.56–6.57 (1H, d, J = 3.69 Hz), 6.91 (1H, d, J = 1.89 Hz), 7.05–7.07 (1H, d, J = 7.90 Hz), 7.12–7.15 (1H, dd, J = 7.9, 1.96 Hz), 7.23–7.24 (1H, d, J = 2.04 Hz), 7.49–7.52 (1H, dd, J = 9.89, 1.99 Hz), 7.58–7.59 (1H, d, J = 3.66 Hz), 7.92–7.94 (1H, d, J = 8.8 Hz); HRMS: [M + H]+ C20H25ClN3O2S calc. 406.1356, found. 406.1354.
N′-[3-(5-bromo indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9s)
HPLC (%): 97.54; IR (cm−1): 3311, 1600, 1369, 1172; ESI-MS (m/z): 422.5, 424.5 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.28 (6H, s), 2.58–2.61 (2H, t, J = 5.87 Hz), 3.10–3.13 (2H, t, J = 5.57 Hz), 4.96 (1H, bs), 6.58–6.59 (1H, d, J = 3.60 Hz), 6.70–6.73 (1H, m), 6.96–6.97 (1H, t, J = 2.08 Hz), 7.08–7.11 (1H, m), 7.15–7.19 (1H, dt, J = 7.88 Hz), 7.38–7.40 (1H, dd, J = 8.81, 1.9 Hz), 7.55 (1H, d, J = 3.64 Hz), 7.66 (1H, d, J = 1.8 Hz), 7.85–7.87 (1H, d, J = 8.81 Hz); HRMS: [M + H]+ C18H21BrN3O2S calc. 422.0538, found. 422.0536.
N′-[3-(4-chloro indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9t)
HPLC (%): 96.30; IR (cm−1): 3371, 1600, 1371, 1166; ESI-MS (m/z): 378.5, 380.5 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.29 (6H, s), 2.60–2.63 (2H, t, J = 5.64 Hz), 3.11–3.14 (2H, t, J = 5.56 Hz), 5.29 (1H, bs), 6.69–6.72 (1H, m), 6.75–6.76 (1H, d, J = 3.72 Hz), 6.95–6.96 (1H, t, J = 2.09 Hz), 7.10–7.12 (1H, m), 7.15–7.19 (1H, t, J = 7.84 Hz), 7.20–7.22 (2H, m), 7.58–7.59 (1H, d, J = 3.7 Hz), 7.86–7.90 (1H, m); HRMS: [M + H]+ C18H21ClN3O2S calc. 378.1043, found. 378.1045.
N′-[5-(5-bromo-3-methyl indole-1-sulfonyl)-2-ethyl phenyl]-N, N-dimethyl ethane-1,2-diamine (9u)
HPLC (%): 99.25; MR (°C): 105.4–108.2; IR (cm−1): 3373, 1597, 1367, 1166; ESI-MS (m/z): 464.7, 466.7 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.15–1.19 (3H, t, J = 7.44 Hz), 2.20 (3H, s), 2.28 (6H, s), 2.61–2.64 (2H, t, J = 5.97 Hz), 2.96–3.02 (2H, q, J = 7.3 Hz), 3.13–3.16 (2H, t, J = 5.65 Hz), 4.75 (1H, bs), 6.89–6.90 (1H, d, J = 1.85 Hz), 7.04–7.06 (1H, d, J = 7.93 Hz), 7.10–7.12 (1H, dd, J = 7.88, 1.89 Hz), 7.37–7.39 (1H, dd, J = 8.78, 1.91 Hz), 7.57 (1H, s), 7.76–7.78 (1H, d, J = 8.3 Hz), 7.87–7.89 (1H, d, J = 8.82 Hz); HRMS: [M + H]+ C21H27BrN3O2S calc. 464.1007, found. 464.1009.
N′-[3-(5-ethoxy-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl ethane-1,2-diamine (9v)
HPLC (%): 97.12; IR (cm−1): 3390, 1602, 1363, 1172; ESI-MS (m/z): 402.5 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.40–1.43 (3H, t, J = 6.99 Hz), 2.19 (3H, s), 2.22 (6H, s), 2.49–2.52 (2H, t, J = 5.98 Hz), 3.03–3.07 (2H, q, J = 5.01 Hz), 4.02–4.07 (2H, q, J = 6.98 Hz), 4.56–4.58 (1H, bs), 6.66–6.68 (1H, m), 6.86 (1H, d, J = 2.38 Hz), 6.89–6.92 (1H, dd, J = 8.92, 2.46 Hz), 6.98–6.99 (1H, t, J = 2.07 Hz), 7.06–7.09 (1H, m), 7.12–7.16 (1H, dt, J = 7.84 Hz), 7.24 (1H, s), 7.85–7.87 (1H, d, J = 8.95 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1848.
N′-[3-(5-chloro-3-methyl indole-1-sulfonyl)-2-ethyl phenyl]-N, N-dimethyl ethane-1,2-diamine (9w)
HPLC (%): 98.28; IR (cm−1): 3390, 1597, 1367, 1168; ESI-MS (m/z): 420.2, 422.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.16–1.19 (3H, t, J = 7.48 Hz), 2.20 (3H, s), 2.23 (6H, s), 2.39–2.44 (2H, q, J = 7.48 Hz), 2.55–2.57 (2H, t, J = 5.66 Hz), 3.07–3.11 (2H, q, J = 5.14 Hz), 5.3 (1H, bs), 6.91–6.92 (1H, d, J = 1.86 Hz), 7.04–7.06 (1H, d, J = 7.91 Hz), 7.09–7.12 (1H, dd, J = 7.9, 1.9 Hz), 7.23–7.24 (1H, d, J = 2.06 Hz), 7.32 (1H, d, J = 1.14 Hz), 7.40–7.41 (1H, d, J = 1.98 Hz), 7.91–7.93 (1H, d, J = 8.82 Hz); HRMS: [M + H]+ C21H27ClN3O2S calc. 420.1512, found. 420.1515.
N′-[2-methoxy-5-(5-chloro-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl ethane-1,2-diamine (9x)
HPLC (%): 98.90; IR (cm−1): 3408, 2941, 1596, 1361, 1167; ESI-MS (m/z): 422.2, 424.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 2.2 (3H, s), 2.24 (6H, s), 2.53–2.56 (2H, t), 3.09–3.10 (2H, t), 3.82 (3H, s), 4.85 (1H, bs), 6.65–6.69 (1H, d, J = 8.44 Hz), 6.85–6.86 (1H, d, J = 2.28 Hz), 7.17–7.19 (1H, dd, J = 8.4, 2.28 Hz), 7.23–7.25 (1H, dd, J = 8.8, 2.08 Hz), 7.31 (1H, d, J = 1.08 Hz), 7.40 (1H, d, J = 2.0 Hz), 7.90–7.92 (1H, d, J = 8.76); HRMS: [M + H]+ C20H25ClN3O3S calc. 422.1305, found. 422.1303.
N′-[2-methoxy-5-(3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl propane-1,3-diamine (9y)
HPLC (%): 98.20; IR (cm−1): 3407, 2962, 1595, 1361, 1166; ESI-MS (m/z): 402.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.74–1.81 (2H, m), 2.23 (3H, s), 2.28 (6H, s), 2.41–2.44 (2H, t, 6.88 Hz), 3.11–3.14 (2H, t, 6.72 Hz), 3.81 (3H, s), 4.8 (1H, bs), 6.64–6.66 (1H, d, J = 8.44 Hz), 6.90–6.91 (1H, d, J = 2.28 Hz), 7.17–7.23 (2H, m), 7.27–7.31 (2H, m), 7.43–7.45 (1H, d, J = 7.76 Hz), 7.98–8.0 (1H, d, J = 8.16 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1854.
N′-[5-(5-methoxy indole-1-sulfonyl)-2-methyl phenyl]-N, N-dimethyl propane-1,3-diamine (9z).
HPLC (%): 98.21; MR (°C): 139.5–141; IR (cm−1): 3226, 1602, 1355, 1139; ESI-MS (m/z): 402.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.77–1.83 (2H, m), 2.02 (3H, s), 2.25 (6H, s), 2.43–2.46 (2H, t, J = 6.0 Hz), 3.14–3.17 (2H, t, J = 6.0 Hz), 3.80 (3H, s), 5.80 (1H, bs), 6.54–6.55 (1H, d, J = 3.54 Hz), 6.86–6.87 (1H, d, J = 1.8 Hz), 6.88–6.91 (1H, dd, J = 9.0, 2.52 Hz), 6.95–6.96 (1H, d, J = 2.46 Hz), 6.99–7.01 (1H, d, J = 7.88 Hz), 7.04–7.06 (1H, dd, J = 7.77, 1.86 Hz), 7.51–7.52 (1H, d, J = 3.62 Hz), 7.87–7.90 (1H, d, J = 8.98 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1854.
N′-[5-(5-methoxy-3-methyl indole-1-sulfonyl)-2-methyl phenyl]-N, N-dimethyl propane-1,3-diamine (9aa)
HPLC (%): 98.64; MR (°C): 122.3–125.3; IR (cm−1): 3221, 1595, 1355, 1166; ESI-MS (m/z): 416.6 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.77–1.83 (2H, m), 2.02 (3H, s), 2.19 (3H, s), 2.25 (6H, s), 2.44–2.47 (2H, t, J = 6.0 Hz), 3.14–3.17 (2H, t, J = 6.0 Hz), 3.82 (3H, s), 5.29 (1H, bs), 6.85 (1H, d, J = 2.44 Hz), 6.87–6.89 (1H, dd, J = 6.98, 2.51 Hz), 6.90–6.91 (1H, d, J = 2.51 Hz), 6.97–6.99 (1H, d, J = 7.85 Hz), 7.01–7.04 (1H, dd, J = 7.78, 1.81 Hz), 7.26 (1H, s), 7.87–7.89 (1H, d, J = 8.92 Hz); HRMS: [M + H]+ C22H30N3O3S calc. 416.2008, found. 416.2010.
N′-[3-(5-methoxy-3-methyl indole-1-sulfonyl) phenyl]-N, N-dimethyl propane-1,3-diamine (9ab)
HPLC (%): 97.62; IR (cm−1): 3402, 1601, 1364, 1174; ESI-MS (m/z): 402.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.73–1.76 (2H, m), 2.04 (3H, s), 2.26 (6H, s), 2.41–2.44 (2H, t, 6.6 Hz), 3.10–3.13 (2H, t, 6.4 Hz), 3.83 (3H, s), 4.8 (1H, bs), 6.65 (1H, m), 6.86–6.89 (1H, d, J = 2.44 Hz), 6.89–6.92 (1H, dd, J = 8.92, 2.52 Hz), 6.94–6.95 (1H, t, 2.08 Hz), 7.05 (1H, m), 7.10–7.12 (1H, t, 7.96 Hz), 7.25 (1H, m), 7.86–7.88 (1H, d, J = 8.88 Hz); HRMS: [M + H]+ C21H28N3O3S calc. 402.1851, found. 402.1848.
N′-[2-methoxy-5-(5-methoxy-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl propane-1,3-diamine (9ac)
HPLC (%): 97.33; IR (cm−1): 3418, 2941, 1597, 1519, 1359, 1166; ESI-MS (m/z): 432.4 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.75–1.80 (2H, m), 2.19 (3H, s), 2.27 (6H, s), 2.4–2.44 (2H, t, J = 6.96 Hz), 3.10–3.13 (2H, t, J = 6.72 Hz), 3.80 (3H, s), 3.82 (3H, s), 4.8 (1H, bs), 6.63–6.65 (1H, d, J = 8.44 Hz), 6.85–6.91 (3H, m), 7.14–7.16 (1H, dd, J = 8.40, 2.28 Hz), 7.26 (1H, s), 7.87–7.89 (1H, d, J = 8.92 Hz); HRMS: [M + H]+ C22H30N3O4S calc. 432.1957, found. 432.1954.
N′-[2-methoxy-5-(5-fluoro-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl propane-1,3-diamine (9ad)
HPLC (%): 98.69; IR (cm−1): 3426, 2942, 1596, 1363, 1166; ESI-MS (m/z): 420.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.76–1.79 (2H, m), 2.19 (3H, s), 2.27 (6H, s), 2.41–2.44 (2H, t, 6.92 Hz), 3.10–3.13 (2H, t, 6.72 Hz), 3.82 (3H, s), 4.8 (1H, bs), 6.65–6.67 (1H, d, J = 8.48 Hz), 6.86–6.87 (1H, d, J = 2.28 Hz), 7.0–7.01 (1H, m), 7.06–7.09 (1H, dd, J = 8.76, 2.48 Hz), 7.15–7.17 (1H, dd, J = 8.44, 2.32 Hz), 7.32 (1H, s), 7.91–7.94 (1H, m); HRMS: [M + H]+ C21H27FN3O3S calc. 420.1757, found. 420.1755.
N′-[2-methoxy-5-(5-chloro-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl propane-1,3-diamine (9ae)
HPLC (%): 98.20; IR (cm−1): 3425, 2942, 1596, 1364, 1167; ESI-MS (m/z): 436.3, 438.3 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.74–1.80 (2H, m), 2.19 (3H, s), 2.27 (6H, s), 2.40–2.44 (2H, t), 3.10–3.13 (2H, t), 3.82 (3H, s), 4.85 (1H, bs), 6.64–6.67 (1H, d, J = 8.84 Hz), 6.86–6.87 (1H, d, J = 2.28 Hz), 7.14–7.17 (1H, dd, J = 8.4, 2.28 Hz), 7.22–7.25 (1H, dd, J = 8.8, 2.04 Hz), 7.31 (1H, d, J = 1.12 Hz), 7.40 (1H, d, J = 2.0 Hz), 7.90–7.92 (1H, d, J = 8.56 Hz); HRMS: [M + H]+ C21H27ClN3O3S calc. 436.1462, found. 436.1465.
N′-[2-methoxy-5-(5-bromo-3-methyl indole-1-sulfonyl) phenyl]-N,N-dimethyl propane-1,3-diamine (9af)
HPLC (%): 96.08; IR (cm−1): 3411, 2924, 1596, 1364, 1167; ESI-MS (m/z): 480.2, 482.2 (M + H)+; 1H-NMR (400 MHz, CDCl3): δ 1.73–1.82 (2H, m), 2.19 (3H, s), 2.33 (6H, s), 2.49–2.53 (2H, t, 7.08 Hz), 3.11–3.14 (2H, t, 6.72 Hz), 3.82 (3H, s), 4.8 (1H, bs), 6.65–6.67 (1H, d, J = 8.48 Hz), 6.85 (1H, d, J = 2.4 Hz), 7.15–7.18 (1H, dd, J = 8.4, 2.28 Hz), 7.29 (1H, dd, J = 1.2 Hz), 7.36–7.39 (1H, dd, J = 8.76, 1.92 Hz), 7.56 (1H, d, J = 2.0 Hz), 7.88–7.85 (1H, d, J = 8.76 Hz); HRMS: [M + H]+ C21H27BrN3O3S calc. 480.0956, found. 480.0959.
Conclusion
In summary, more flexible N,N-dimethylamino alkyl amino group was well tolerated on N-benzene sulfonyl ring at C-3′ position in an indolylsulfonamide amines series in terms of 5-HT6 receptor activity. The compounds have shown selectivity over other closely related receptors.
The lead compound 9y has shown adequate steady-state brain penetration (Cb/Cp), and active in cognition models like NORT and Water maze. Metabolic instability of these compounds could be the reason for suboptimal pharmacokinetic profile. Efforts are underway toward identifying the metabolic profile, so that it can be further optimized by a suitable choice of substitutions while maintaining the desired 5-HT6 potency. The results of these studies will be reported in due course in a future publication.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The support received from Discovery Analytical, DMPK, In vitro, In vivo departments and Mr Venkateswarlu Jasti, CEO, Suven Life Sciences Ltd., Hyderabad, India is gratefully acknowledged.
References
- ADI/WHO. World Health Organization and Alzheimer’s Disease International say dementia must be a global health priority, Geneva. News Release/11-Apr-2012. Available from: http://www.alz.co.uk [last accessed 21 Jun 2013]
- Roth BL, Lopez E, Patel S, Kroeze WK. Neuronal signal transduction pathways. Neuroscientist 2000;6:252–62
- Hoyer D, Hannon JP. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 2002;71:533–54
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav 2002;71:555–68
- Bikker JA, Trump-Kallmeyer S, Humblet S. G-protein coupled receptors: models, mutagenesis, and drug design. J Med Chem 1998;41:2911–27
- Sebben M, Ansanay H, Bockaert J, Dumuis A. 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport 1994;5:2553–7
- Arnt J, Olsen CK. 5-HT6 receptor ligands and their antipsychotic potential. Int Rev Neurobiol 2011;96:141–61
- Roth BL, Craigo SC, Choudhary MS, et al. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther 1994;268:1403–10
- Shinkai T, Ohmori O, Kojima H, et al. Association study of the 5-HT6 receptor gene in schizophrenia. Am J Med Genet 1999;88:120–2
- Tsai SJ, Chiu HJ, Wang YC, Hong CJ. Association study of serotonin-6 receptor variant (C267T) with schizophrenia and aggressive behavior. Neurosci Lett 1999;271:135–7
- Vogt IR, Shimron-Abarbanell D, Neidt H, et al. Investigation of the human serotonin 6 [5-HT6] receptor gene in bipolar affective disorder and schizophrenia. Am J Med Genet 2000;96:217–21
- Ohmori O, Shinkai T, Hori H, Nakamura J. Novel polymorphism in the 50-upstream region of the human 5-HT6 receptor gene and schizophrenia. Neurosci Lett 2001;310:17–20
- East SZ, Burnet PW, Leslie RA, et al. 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: autoradiographic studies with [125I]SB-258585. Synapse 2002;45:191–9
- Wu WH, Huo SJ, Cheng CY, et al. Association study of the 5-HT6 receptor polymorphism (C267T) and symptomatology and antidepressant response in major depressive disorders. Neuropsychobiology 2001;44:172–5
- Messina D, Annesi G, Serra P, et al. Association of the 5-HT6 receptor gene polymorphism C267T with Parkinson’s disease. Neurology 2002;58:828–9
- Thome J, Retz W, Baader M, et al. Association analysis of HTR6 and HTR2A polymorphisms in sporadic Alzheimer’s disease. J Neural Transm 2001;108:1175–80
- Liu HC, Hong CJ, Liu CY, et al. Association analysis of the 5-HT6 receptor polymorphism C267T with depression in patients with Alzheimer’s disease. Psychiatry Clin Neurosci 2001;55:427–9
- Garcia-Alloza M, Hirst WD, Chen CP, et al. Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology 2004;29:410–16
- Glennon RA. Higher-end serotonin receptors: 5-HT(5), 5-HT(6), and 5-HT(7). J Med Chem 2003;46:2795–812
- Holenz J, Pauwels PJ, Díaz JL, et al. Medicinal chemistry strategies to 5-HT(6) receptor ligands as potential cognitive enhancers and antiobesity agents. Drug Discov Today 2006;1:283–99
- Liu KG, Robichaud AJ. 5-HT6 antagonists as potential treatment for cognitive dysfunction. Drug Develop Res 2009;70:145–68
- Available from: http://www.pfizer.com/sites/default/files/product-pipeline/pipeline_2013_0509.pdf/Neuroscience & pain [last accessed 21 Jun 2013]
- Available from: http://www.gsk.com/research/our-product-pipeline.html [last accessed 21 Jun 2013]
- Available from: http://www.biocentury.com/products/avn-211 [last accessed 21 Jun 2013]
- Ivachtchenko AV, Savchuk NP, Ivachtchenko AA. Substituted 4-sulphonyl-pyrazoles and 3-sulphonyl-pyrazolo[1,5-a]pyrimidines-antagonists of serotonin 5-HT6 receptors, active component, pharmaceutical composition, medicinal agent and method of obtaining them. RU Patent 2369600; 2009
- Ivachtchenko AV, Savchuk NP, Ivachtchenko AA. 3-Sulfonyl-pyrazolo[1,5-a] pyrimidines/antagonists of serotonin 5-ht6 receptors, methods for the production and the use thereof. WO Patent 093206; 2009
- Lundbeck. Lundbeck’s Lu AE58054 meets primary endpoint in large placebo-controlled clinical proof of concept study in people with Alzheimer’s disease. Corporate Releases/29-May-2012. Available from: http://www.lundbeck.com/global [last accessed 02 Apr 2013]
- Jorn A, Benny B, Ben G, et al. Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol 2010;13:1021–33
- Available from: http://www.biotie.com/en/product_and_development/development_pipeline/syn120 [last accessed 21 Jun 2013]
- Nirogi R, Kambhampati R, Shinde A, et al. 12th International Conference on Alzheimer’s disease; 2009; Vienna
- Glennon RA, Lee M, Rangisetty JB, et al. 2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors. J Med Chem 2000;43:1011–18
- Kambhampati R, Konda J, Reballi V, et al. Design, synthesis and preliminary screening of novel 3-(2-N,N-dimethylaminoethylthio) indole derivatives as potential 5-HT6 receptor ligands. J Enz Inhib Med Chem 2008;23:302–12
- Nirogi RVS, Kambhampati R, Daulatabad AV, et al. Design, synthesis and pharmacological evaluation of conformationally restricted N-arylsulfonyl-3-aminoalkoxy indoles as a potential 5-HT6 receptor ligands. J Enz Inhib Med Chem 2011;26:341–9
- Davies SL, Silvestre JS, Guitart X. Drug discovery targets: 5-HT6 receptor. Drugs of the Future 2005;30:479–95
- Kim HJ, Doddareddy MR, Choo H, et al. New serotonin 5-HT6 ligands from common feature pharmacophore. Hypotheses. J Chem Inf Model 2008;48:197–206
- Nirogi RVS, Kambhampati R, Kothmirkar P, et al. Synthesis and structure–activity relationship of novel conformationally restricted analogues of serotonin as 5-HT6 receptor ligands. J Enz Inhib Med Chem 2012;27:443–50
- Ivachtchenko A, Golovina E, Kadieva M, et al. Synthesis of substituted diphenyl sulfones and their structure–activity relationship with the antagonism of 5-HT6 receptors. Bioorg Med Chem 2013;21:4614–27
- Ivachtchenko AV, Golovina ES, Kadieva MG, et al. Synthesis and structure–activity relationship (SAR) of (5,7-disubstituted 3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidin-2-yl)-methylamines as potent serotonin 5-HT6 receptor (5-HT6R) antagonists. J Med Chem 2011;54:8161–73
- Ivachtchenko AV, Dmitriev DE, Golovina ES, et al. (3-Phenylsulfonylcycloalkano[e and d]pyrazolo[1,5-a]pyrimidin-2-yl)amines: potent and selective antagonists of the serotonin 5-HT6 receptor. J Med Chem 2010;53:5186–96
- Ivachtchenko AV, Golovina ES, Kadieva MG, et al. Antagonists of 5-HT6 receptors. Substituted 3-(phenylsulfonyl) pyrazolo[1,5-a]pyrido[3,4-e]pyrimidines and 3-(phenylsulfonyl) pyrazolo[1,5-a]pyrido[4,3-d]pyrimidines – synthesis and ‘structure–activity’ relationship. Bioorg Med Chem Lett 2012;22:4273–80
- Ivachtchenko AV, Golovina ES, Kadieva MG, et al. Synthesis and SAR of 3-arylsulfonyl-pyrazolo[1,5-a]pyrimidines as potent serotonin 5-HT6 receptor antagonists. Bioorg Med Chem 2011;19:1482–91
- Ivachtchenko AV, Golovina ES, Kadieva MG, et al. 2-Substituted 5,6-dimethyl-3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidines: new series of highly potent and specific serotonin 5-HT6 receptor antagonists. Eur J Med Chem 2011;46:1189–97
- Lopez-Rodriguez ML, Benhamu B, Fuente T, et al. A three-dimensional pharmacophore model for 5-hydroxytryptamine6 (5-HT6) receptor antagonists. J Med Chem 2005;48:4216–19
- Bromidge SM. Potent and selective 5-HT6 receptor antagonists. Spec Publ – R Soc Chem 2001;264:101–19
- Hirst WD, Minton JA, Bromidge SM, et al. Characterization of [125I]-SB-258585 binding to human recombinant and native 5-HT6 receptors in rat, pig and human brain tissue. Br J Pharmacol 2000;130:1597–605
- Pullagurla MR, Westkaemper RB, Glennon RA. Possible differences in modes of binding of agonist and antagonist binding at human 5-HT6 receptors. Bioorg Med Chem Lett 2004;14:4569–73
- Dukat M, Mosier PD, Kolanos R, et al. Binding of serotonin and N1-benzenesulfonyltryptamine-related analogs at human 5-HT6 serotonin receptors: receptor modeling studies. J Med Chem 2008;51:603–11
- Sikazwe D, Bondarev ML, Dukat M, et al. Binding of sulfonyl-containing arylalkylamines at human 5-HT6 serotonin receptors. J Med Chem 2006;49:5217–25
- Heal DJ, Smith SL, Fisas A, et al. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Therapeutics 2008;17:207–31
- Kohen R, Fashingbauer LA, Heidmann DE, et al. Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Brain Res Mol Brain Res 2001;90:110–17
- Gonzalo R, Elisabeth S, Marta P, et al. Efficacy of selective 5-HT6 receptor ligands determined by monitoring 5-HT6 receptor-mediated cAMP signaling pathways. Br J Pharmacol 2006;148:1133–43