Abstract
New compounds based on the indole moiety were synthesized via the reaction of indole-3-carbinal 1 with different nucleophiles such as 6-aryl-[4-(2-methoxybenzyl)pyridazin-3-yl] hydrazones 2a–c, benzidine, 3,3′-dimethoxybenzidine 4a,b and 2,6-diaminopyridine 6 to afford hydrazine derivatives 3a–c and three different classes of bis-Schiff bases. The structures of the new compounds were elucidated on the basis of their FTIR, 1H NMR, 13C NMR spectral data, GC/MS and elemental analysis. The antimicrobial activity of the new compounds was evaluated using a broth dilution technique in terms of minimal inhibitory concentration (MIC) against four pathogenic bacteria and two pathogenic fungi strains. Compound 14b showed excellent activity against Escherichia coli and Klebsiella pneumoniae. Some of the prepared compounds were tested for anti-cancer activity against human cell lines HCT116 (colon), MCF7 (breast) and HELA (cervix). From the results of the in vitro assays, compounds 3a,b, and 18a,c presented promising anti-cancer activity.
Introduction
Hydrazones, which are also known as Schiff bases or imines, are an important class of compounds in the medicinal and pharmaceutical fields. They have made a considerable contribution to biological applications including antibacterial and antifungal diseasesCitation1–3. They can also act as ligands capable of interaction with various metal ions. Hydrazone ligands (HL) possessing potential donor sites that have coordination capability exhibit biological activities that may be due to the presence of the azomethine and the uncoordinated α-nitrogen of the hydrazone chromosphere. This coordination capability has led to research on the synthesis of metal complexes of new hydrazones with significant biological activityCitation4–6. One of the most important goals of pharmacological research is the search for new molecular structures which exhibit effective antitumor activities. This has driven inorganic and organometallic chemists to look for new metal binding compounds with strong biological activity, in particular, against tumors that are responsible for the continuing high levels of cancer induced mortality.
Spiro compounds represent an important class of naturally occurring substances characterized by their diverse biological activitiesCitation7,Citation8. Furthermore, it has been reported that sharing of the indole 3-carbon atom in the formation of spiroindoline derivatives greatly enhances biological activity against some Gram-positive bacteria (Bacillus subtilis and B. megatherium, Gram negative bacteria (Escherichia coli) and fungi (Aspergillus niger and A. oryzae) Citation9–11. The spiro-oxindole system is the core structure of many pharmacological agents and natural alkaloids such as spiro-indole-thiazolidinones which show good activity against the pathogens Rhizoctonia solani, Fusarium oxysporum and Collectotrichum capsici. Also spirotryprostatins have been found to have antimitotic properties, and as such they have become of great interest as anticancer drugs. They were also shown to be active as cell cycle inhibitors. Pteropodine and isopteropodine, which are heteroyohimbine type oxindole alkaloids, act as positive modulators of muscarinic M (1) and 5-HT (2) receptorsCitation12–16. Azetidinone and thiazolidinone derivatives have been reported to possess antibacterial, antifungalCitation17,Citation18, antituberculor activityCitation19, anti-HIVCitation20 analgesic, anti-inflammatoryCitation21 and ulcerogenic activityCitation22. As well as spiroindolines, triazole derivatives have shown strong biological activity. For example 1,2,4-triazoles exhibit pesticidal activity. Hence it was considered that combinations of indole and 1,2,4-triazole structures might provide more potent pesticidesCitation23.
In continuation of our studies on the synthesis and evaluation of biological activity of new heterocyclesCitation24–30, we report herein the efficient synthesis of new hydrazones, and Schiff bases based on the indole moiety and the evaluation of their biological activity as antimicrobial and anticancer agents.
Materials and methods
Chemistry
General remarks
All melting points are uncorrected and were determined on a Gallenkamp instrument. Infrared spectra were measured on Perkin-Elmer-1430 infrared spectrophotometer (Waltham, MA) using the potassium bromide wafer or the Nujol mull technique for metal complexes. The 1H NMR, 13C NMR were measured in DMSO-d6 measured on a Varian Genini-300, 500 MHz spectrophotometer and chemical shifts δ are in ppm. The mass spectra were measured on a HP GC MS-QPL000EX (Shimadzu, Kyoto, Japan) mass spectrophotometer at 70 eV. Microanalyses were carried out using a Perkin Elmer 2400 CHN elemental analyzer. The metal percentage was estimated using an inductively coupled argon plasma (ICP) technique on a 6500 Duo apparatus, Thermo Scientific, England. A 1000 mg/l multi-element and certified standard solution (Merck, Darmstadt, Germany) was used as the stock solution for instrument standardization. A microwave Digestion Lab Station closed system, Ethos Pro, Milestone, Italy was used to digest the organic matter in aqua regia. UV–Vis spectra were measured on UV-1600 spectrophotometer. The solid reflectance spectra were measured on a Shimadzu 3101 pc spectrophotometer. Magnetic susceptibilities of the metal complexes were measured at room temperature using a magnetic susceptibility Sherwood Scientific apparatus, Cambridge England. The molar conductance values of the chelates were calculated by measuring the conductance of the investigated metal chelates (10−3 M) in DMF using a conductivity meter ORION model 150 with a 0.6 cell constant. An Explorer Automated Microwave Synthesis Work station (CEM) was used for the synthesis of the compounds in this study. The primary evaluation of the in vitro cytotoxicity of the compounds under investigation against human tumor cells was tested at the National Cancer Institute (NCI), Cairo University, Cairo, Egypt. Four bacterial strains and two fungal strains from the Basic Science Department, Faculty of Applied Medical Science, October 6th University were employed for MIC and MBC microbial counts.
General procedure for the synthesis of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-arylpyridazin-3-yl] hydrazines 3a–c
Using thermal conditions. Each hydrazine derivative of 2a–c (0.01 mol) and indole-3-carbinal (1) (0.01 mol) were heated under reflux in absolute ethanol (30 ml) in the presence of a catalytic amount of glacial acetic acid for 5 h. The resultant solid was filtered, washed with dilute ethanol, dried and recrystallized from ethanol–chloroform to give 3a–c.
Using microwave irradiation. An equimolar mixture (0.01 mol) of indole-3-carbinal 1 and each hydrazine derivative of 2a–c in the minimum quantity of ethanol (5 ml required to form a slurry) was irradiated with microwave radiation under controlled conditions (power:150 watt, temperature:78 °C). On cooling, the crystals that separated out were found to not need further purification.
2-[(1H-Indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-phenylpyridazin-3-yl] hydrazine 3a
Orange crystals, yield 57.14%; mp 216 °C; IR: 3375 for NH(hydrazone), 3115 for NH(indole), 3075 and 3051 for CH(aromatic), 2900, 2875 and 2831 for CH(aliphatic) and 1620 for CH=N cm−1. 1H NMR (300 MHz): δ 10.20 [s, 1H, NH(indole)], 8.90 (s, 1H, CH=N), 8.62 [s, 1H, NH(hydrazone)], 8.50–6.91 (m, 13H, 3Ar–H), 6.92 [s, 1H, CH(indole)], 6.84 [s, 1H, CH(pyridazine)], 4.15 (s, 2H, CH2) and 3.84 (s, 3H, OCH3 of CH3O–Ar) ppm. 13C NMR: δ 157.91, 157.66, 150.66, 148.71, 143.38, 137.69, 136.08, 131.33, 130.89, 128.80, 127.63, 125.96, 125.10, 123.30, 123.03, 121.73, 121.03, 120.86, 113.75, 112.23, 111.39, 55.85, 30.63. Anal. C27H23N5O (433.50): Calcd: C, 74.80; H, 5.35; N, 16.16. Found: C, 74.66; H, 5.83; N, 15.90. MS: m/z 433 [M+].
2-[(1H-Indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4-methylphenyl)-pyridazin-3-yl] hydrazine 3b
Orange crystals, yield 56.70%; mp 220–222 °C; IR: 3350 for NH(hydrazone), 3150 for NH(indole), 3035 and 2950 for CH(aromatic), 2920 and 2839 for CH(aliphatic) and 1604 for CH=N cm−1 1H NMR: δ 10.20 [s, 1H, NH(indole)], 8.89 (s, 1H, CH=N), 8.60 [s,1H, NH(hydrazone)], 8.48–6.93 (m, 12H, 3Ar–H), 6.92 [s, 1H, CH(indole)], 6.82 [s, 1H CH(pyridazine)], 4.14 (s, 2H, CH2), 3.82 (s, 3H, OCH3 of CH3O–Ar) and 2.51 (s, 3H, CH3 of CH3–Ar) ppm. Anal. C28H25N5O (447.53): Calcd: C, 75.14; H, 5.63; N, 15.65. Found: C, 74.65; H, 6.05; N, 15.83. MS: m/z 448 [M+ + 1].
2-[(1H-Indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4-chlorophenyl)-pyridazin-3-yl] hydrazine 3c
Orange crystals, yield 53.30%; mp 224–226 °C; IR: 3193 for NH(hydrazone), 3109 for NH(indole), 3055 (sharp) for CH(aromatic), 2927 and 2873 for CH(aliphatic) and 1616 for HC=N cm−1. Anal. C27H22ClN5O (467.95): Calcd: C, 69.30; H, 4.74; N, 14.97; Cl, 7.58. Found: C, 69.28; H, 5.38; N, 14.84; Cl, 7.36. MS: m/z 467 [M+], 469 [M+ + 2], 470 [M+ + 3].
General procedure for the synthesis of bis-N-[(1H-indol-3yl)-methylene]-4,4′-diamino-1,1′-biphenyl 5a and bis-N-[(1H-indol-3yl) methylene]-3,3′-dimethoxybenzidine 5b
Using thermal conditions. Indole-3-carbinal (1) (0.02 mol) and each of the 4, 4′-diamino-1,1′-biphenyl-3,3′-dimethoxybenzidines (4a,b) (0.01 mol) were dissolved in warm ethanol (20 ml) containing glacial acetic acid (0.45 ml). The reaction mixture was refluxed for 3 h. The major part of product precipitated while hot. The resulting solid on cooling was separated by filtration and vacuum dried after washing with hot ethanol to give 5a,b.
Using microwave irradiation. A mixture (0.02 mol) of indole-3-carbinal (1) and 4,4′-diamino-1,1′-biphenyl-3,3′-dimethoxybenzidine (4a,b) (0.01 mol) in the minimum quantity of ethanol (5 ml required to form a slurry) was irradiated with microwave radiation under controlled conditions (power: 150 W, temperature: 78 °C). On cooling, the crystals that separated out were found to not need further purification.
Bis-N-[(1H-indol-3yl)-methylene]-4,4′-diamino-1,1′-biphenyl 5a
Orange crystals, yield 42.55%; mp 330–332 °C; IR: 3150–3115 for 2NH(indole), 3035 for CH(aromatic), 2889 for CH(aliphatic) and 1616 for CH=N cm−1. 1H NMR: δ 11.76 [s, 2H, 2NH(indole)], 8.78 (s, 2H, 2CH=N), 8.42–7.17 (m, 16H, 4Ar–H) and 6.74 [s, 2H, 2CH(hetero)] ppm. Anal. C30H22N4 (438.52): Calcd: C, 82.16; H, 5.06; N, 12.78. Found: C, 82.00; H, 5.10; N, 12.57. MS: m/z 439 [M+ + 1], [440 M+ + 2].
Bis-N-[(1H-indol-3yl)-methylene]-3,3′-dimethoxybenzidine 5b
Red crystals, yield 36.14%; mp 216–218 °C; IR: 3170–3113 for 2NH(indole), 3105 and 3043 for CH(aromatic), 2931 and 2889 for CH(aliphatic) and 1635 for CH=N cm−1. 1H NMR: δ 12.09 [s, 2H, 2NH(indole)], 9.90 (s, 2H, 2CH=N), 8.24–6.87 (m, 14H, 4Ar–H), 6.62–6.61 [s, 2H, 2CH(hetero)] and 3.85–3.79 (s, 6H, 2OCH3 of 2CH3O–Ar) ppm. Anal. C32H26N4O2 (498.20): Calcd: N, 11.24; Found: N, 11.04. MS: m/z 498 [M+].
General procedure for synthesis of bis-N-[(1H-indol-3yl)-methylene] pyridine-2,6-diamine 7
Using thermal conditions. 2,6-Pyridinediamine (6) (0.004 mol) was added to a stirred solution of indole-3-carbinal (1) (0.008 mol) in ethanol (20 ml). The resulting mixture was refluxed at 60 °C with stirring for 3 h. The reaction mixture was cooled to room temperature and the resulting precipitate was filtered and washed with cold methanol. The solid was purified by column chromatography on alumina (eluent: 20% ethyl acetate/80% petroleum ether 60–80) to give a pure product 7.
Using microwave irradiation. A mixture of 2,6-pyridinediamine (6) (0.004 mol) and indole-3-carbinal (1) (0.008 mol) in the minimum quantity of ethanol (5 ml required to form a slurry) was irradiated with microwave radiation under controlled conditions (power: 150 W, temperature: 78 °C]. On cooling, the crystals that separated out were found to not need further purification.
Bis-N-[(1H-indol-3yl)-methylene] pyridine 2,6-diamine 7
Greenish yellow crystals, yield 77.72%; mp >350 °C; IR: 3255–3245 for 2NH(indole), 3051 for CH(aromatic), 2875 for CH(aliphatic) and 1608 for CH=N cm−1. 1H NMR: δ 10.88 [s, 2H, 2NH(indole)], 9.93 (s, 2H, 2CH=N), 8.27–6.85 (m, 11H, 3Ar–H) and 6.54 [s, 2H, 2CH(hetero)] ppm. Anal. C23H17N5 (363.42): Calcd: N, 19.28. Found: N, 19.49. MS: m/z 363 [M+].
General procedure for the synthesis of 3-[4-(2-methoxybenzyl)-6-arylpyridazin-3-ylamino]-2-(1H-indol-3-yl)thiazolidin-4-ones (8a–c)
Method A: A solution of Schiff bases 3a–c (0.001 mol) and mercaptoacetic acid. (0.002 mol) in dry dioxane (50 ml) in presence of anhydrous ZnCl2 was refluxed for 6–10 h. After completion of the reaction (tracked with TLC) excess of solvent was removed through distillation, and the sticky solid obtained was poured onto crushed ice, then filtered, dried and recrystallized from ethanol to give 8a–c.
Method B: A mixture of the Schiff bases 3a–c (0.001 mol) and mercaptoacetic acid. (0.002 mol) was placed in DMSO in a round-bottom flask fitted with a Dean-Stark apparatus. The mixture was refluxed for 6 h with removal of water azeotropically. The sticky solid that formed on evaporation of the solvent was treated with a solution of sodium bicarbonate to remove excess acid. The solid formed was filtered, washed with water, dried and recrystallized from ethanol.
3-[4-(2-Methoxybenzyl)-6-phenylpyridazin-3-ylamino]-2-(1H-indol-3-yl)-thiazolidin-4-one 8a
Pale yellow crystals, yield 85.47%; mp 278–280 °C; IR: 3393 for NH(hydrazone), 3205 for NH (indole), 3106 and 3057 for CH(aromatic), 2928 and 2834 for CH(aliphatic), 1733 for C=O(thiazolidine), 1245 for C–N and 753 for C–S–C cm−1. Anal. C29H25N5O2S (507.54): Calcd: N, 13.80. Found: N, 13.43. MS: m/z 508 [M+ + 1].
3-[4-(2-Methoxybenzyl)-6-(4-methylphenyl) pyridazin-3-ylamino]-2-(1H-indol-3-yl)-thiazolidin-4-one 8b
Pale yellow crystals, yield 67%; mp 152–154 °C; IR: 3399 for NH(hydrazone), 3214 for NH(indole), 3058 for CH(aromatic), 2919 and 2800 for CH(aliphatic), 1784 for C=O(thiazolidine), 1245 for C–N and 746 for C–S–C cm−1. Anal. C30H27N5O2S (521.57): Calcd: N, 13.43. Found: N, 12.74. MS: m/z 522, [M+ + 1], 523 [M+ + 2].
3-[4-(2-Methoxybenzyl)-6-(4-chlorophenyl)-pyridazin-3-ylamino]-2-(1H-indol-3-yl)thiazolidin-4-one 8c
Violet crystals, yield 66.5%; mp 99–100 °C; IR: 3396 for NH(hydrazone), 3214 for NH(indole), 3057 for CH(aromatic), 2928 and 2838 for CH(aliphatic) 1714 for C=O(thiazolidine), 1245 for C–N and 748 for C–S–C cm−1. 1H NMR: δ 10.92 [s, 1H, NH(indole)], 9.90 [s, 1H, 1NH(hydrazone)], 7.96–7.23 (m, 13H, 3Ar–H), 7.02 (d, 1H, CH(hetero)), 6.99–6.87 (s, 2H, 2CH(hetero)), 5.29 (s, 2H, CH2(thiazolidine ring)), 4.32 (s, 2H, CH2) and 3.90–3.78 (s, 3H, OCH3 of CH3O–Ar) ppm. 13C NMR: δ 158.78, 157.67, 152.82,147.47, 144.85, 143.81, 140.24, 139.65, 139.04, 137.53, 137.11, 136.53, 133.43, 130.36, 129.61, 128.89, 128.62, 127.74, 124.95, 121.55, 120.41, 119.36, 112.12, 110.93, 59.11, 55.62, 31.36, 30.83. Anal. C29H24N5O2SCl (541.98): Calcd.: C, 64.26; H, 4.46; N, 12.92; Cl, 6.54. Found: C, 63.94; H, 4.78; N, 12.75; Cl, 6.11. MS: m/z 542 [M+], 544 [M+ + 2].
General procedure for the synthesis of 1-[4-(2-methoxybenzyl)-6-arylpyridazin-3-yl]-1-(morpholinomethyl)-2-{[1-(morpholinomethyl)-1H-indol-3-yl] methylene} hydrazines (9a,c) and 1-[4-(2-methoxybenzyl)-6-arylpyridazin-3-yl]-1-[(piperidin-1-yl)methyl]-2-{[1-((piperidin-1-yl)methyl)-1H-indol-3-yl]methylene}hydrazines (9b,d)
A slurry was prepared consisting of the Schiff bases 3a,b (0.001 mol), absolute ethanol (2 ml) and 37% formalin (0.3 ml). To this mixture, the secondary amine (morpholine or piperidine) (0.002 mol) was added dropwise, with cooling and shaking. The reaction mixture was irradiated (microwave 150 W, temperature 78 °C] for an appropriate time until the completion of the reaction as tracked with TLC, i.e. until the reactants had disappeared. On cooling, crystals separated out and were recrystallized from ethanol to give 9a–d.
1-[4-(2-Methoxybenzyl)-6-phenylpyridazin-3-yl]-1-(morpholinomethyl)-2-{[1-(morpholino-methyl)-1H-indol-3-yl]methylene} hydrazine 9a
Yellow crystals, yield 36.7%; mp 144–146 °C; IR: 3028 for CH(aromatic), 2931 and 2831 for CH(aliphatic) and 1604 for CH=N cm−1. 1H NMR: δ 8.61 (s, 1H, CH=N), 7.86–7.17 (m, 13H, 3Ar–H), 6.95–6.94 [s, 2H, 2CH(hetero)], 4.97 (s, 4H, 2N–CH2–N), 4.14 (s, 2H, CH2), 3.83 (s, 3H, OCH3 of CH3O–Ar), 3.56–3.45 (t, 8H, 2CH2–OCH2) and 2.55–2.49 (t, 8H, 2CH2–N–CH2) ppm. Anal. C37H41N7O3 (631.77): Calcd: C, 70.34; H, 6.54; N, 15.52. Found: C, 70.09; H, 6.60; N, 15.16. MS: m/z 631 [M+].
1-(4-(2-Methoxybenzyl)-6-phenylpyridazin-3-yl)-1-[(piperidin-1-yl)methyl]-2-{[1-((piperidin-1-yl)methyl)-1H-indol-3-yl]methylene} hydrazine 9b
Yellow crystals, yield 35%; mp 174–176 °C; IR: 3020 for CH(aromatic), 2931 and 2831 for CH(aliphatic) and 1604 for CH=N cm−1. 1H NMR: δ 8.57 (s, 1H, CH=N), 7.89–7.02 (m, 13H, 3Ar–H), 6.90–6.83 [s, 2H, 2CH(hetero)], 4.93 (s, 4H, 2N–CH2–N), 4.11 (s, 2H, CH2), 3.78 (s, 3H,OCH3 of CH3O–Ar), 2.46 (t, 8H, 2CH2–N–CH2) and 1.45 (m, 12H, 6CH2) ppm. Anal. C39H45N7O (627.82): Calcd: C, 74.61; H, 7.23; N, 15.62. Found: C, 74.92; H, 7.01; N, 15.90. MS: m/z 628 [M+ + 1].
1-(4-(2-Methoxybenzyl)-6-(4-methylphenyl)pyridazin-3-yl)-1-(morpholinomethyl)-2-((1-(morpholinomethyl)-1H-indol-3-yl) methylene) hydrazine 9c
Yellow crystals, yield 25.86%; mp 168–170 °C; IR: 3031 for CH(aromatic), 2920 and 2831 for CH(aliphatic) and 1627 for CH=N cm−1. 1H NMR: δ 8.62 (s, 1H, CH=N), 7.91–6.93 (m, 12H, 3Ar–H), 6.90–6.83 [s, 2H, 2CH(hetero)], 4.94 (s, 4H, 2N–CH2–N), 4.16 (s, 2H, CH2), 3.80 (s, 3H, OCH3 of CH3O–Ar), 3.55–3.54 (t, 8H, 2CH2–OCH2), 2.50–2.49 (t, 8H, 2CH2–N–CH2) and 2.32 (s, 3H, CH3 of CH3–Ar) ppm. Anal. C38H43N7O3 (645.79): Calcd: C, 70.67; H, 6.71; N, 15.19. Found: C, 70.62; H, 6.92; N, 15.00. MS: m/z 647 [M+ + 2].
1-(4-(2-Methoxybenzyl)-6-(4-methylphenyl)pyridazin-3-yl)-1-(piperidin-1-yl)methyl)-2-(1-(piperidin-1-yl)methyl)-1H-indol-3-yl)methylene)hydrazine 9d
Yellow crystals, yield 82.6%; mp 150–152 °C; IR: 3031 for CH(aromatic), 2935 and 2842 for CH(aliphatic) and 1627 for CH=N cm−1. 1HNMR: δ 8.55 (s, 1H, CH=N), 7.88–7.02 (m, 12H, 3Ar–H), 6.90–6.80 [s, 2H, 2CH(hetero)], 4.92 (s, 4H, 2N–CH2–N), 4.09 (s, 2H, CH2), 3.77 (s, 3H, OCH3 of CH3O–Ar), 2.47 (t, 8H, 2CH2–N–CH2), 2.30 (s, 3H, CH3 of CH3–Ar) and 1.44 (m, 12H, 6CH2) ppm. Anal. C40H47N7O (641.85): Calcd: C, 74.85; H, 7.38; N, 15.28. Found: C, 74.59; H, 7.12; N, 14.90. MS: m/z 642 [M+ + 1].
General procedure for synthesis of dichloro-bridged copper (II) complexes of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-arylpyridazin-3-yl] hydrazines (10a–c)
2-[(1H-indol-3-yl)-methylene]-1-[4-(2-methoxybenzyl)-6-arylpyridazin-3-yl] hydrazines (3a–c) were dissolved in the minimum amount of ethanol and heated under reflux. To the refluxing solution, copper (II) chloride was added in an equimolar amount. The refluxing was continued for 2 h. The solid products that precipitated after cooling were filtered, washed with ethanol and dried in vacuum and then over CaCl2 to give 10a–c.
Dichloro-bridged copper (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-phenylpyridazin-3-yl] hydrazine 10a
Yellowish green crystals, yield 55.70%, mp 318–320 °C. IR: 3267 for NH(hydrazone), 3125 for NH(indole), 3051 for CH(aromatic), 2931 and 2835 for CH(aliphatic), 1593 for C=N and 486 for (M–N) cm−1. UV λmax: 748, 610, 566, 518, 494, 354, 262, 220 nm. μeff: 1.5 BM. Anal. C54H48Cu2Cl3N10O3 (1118.48): Calcd: C, 57.99; H, 4.33; N, 12.52; Cu, 11.36; Cl, 9.51. Found: C, 57.55; H, 4.59; N, 12.50; Cu, 11.80; Cl, 9.82.
Dichloro-bridged copper (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4- methylphenyl) pyridazin-3-yl]hydrazine 10b
Brown crystals, yield 58.82%, mp 280–282 °C. IR: 3340 for NH(hydrazone), 3150 for NH(indole), 3050 for CH(aromatic), 2912 and 2835 for CH(aliphatic), 1596 for C=N and 482 for (M–N) cm−1. UV λmax 658, 607, 519, 346, 260, 216 nm. μeff: 1.1 BM. Anal. C56H50Cu2Cl2N10O2 (1093.06): Calcd: C, 61.53; H, 4.61; N, 12.81; Cu, 11.63; Cl, 6.49. Found: C, 61.53; H, 4.85; N, 13, 35; Cu, 11.90; Cl, 6.52.
Dichloro-bridged copper (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4-chlorophenyl) pyridazin-3-yl] hydrazine 10c
Yellowish green crystals, yield 55.60%, mp 290–292 °C. IR: 3225 for NH(hydrazone), 3182 for NH(indole), 3075 for CH(aromatic), 2925 and 2850 for CH(aliphatic), 1593 for C=N and 509 for (M–N) cm−1. UV λmax: 714, 599, 520, 348, 265, 214 nm. μeff: 1.25 BM. Anal. C54H44Cu2Cl4N10O2 (1133.90): Calcd: C, 57.20; H, 3.91; N, 12.35; Cu, 11.21; Cl, 12.51. Found: C, 57.74; H, 4.65; N, 12.53; Cu, 10.78; Cl, 13.26.
General procedure for synthesis of mononuclear Co (II) complexes of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-arylpyridazin-3-yl]hydrazines (11a–c)
2-[(1H-Indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-arylpyridazin-3-yl] hydrazines (3a–c) (0.02 mol) were dissolved in the minimum amount of ethanol and heated to reflux. To this refluxing solution, cobalt (II) chloride (0.01 mol) was added. Then refluxing was continued for 5 h. In each experiment, the solid products that separated on cooling were filtered, washed with ethanol, dried in vacuum and then over CaCl2 to give 11a–c.
Mononuclear Co (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-phenyl-pyridazin-3-yl] hydrazine 11a
Brown crystals, yield 65.01%, mp 242–244 °C. IR: 3377 for NH(hydrazone), 3139 for NH(indole), 3047 for CH(aromatic), 2920, 2835 for CH(aliphatic), 1589 for C=N and 474 for (M–N) cm−1. UV λmax 577, 545, 340, 310, 290, 243, 216 nm. μeff: 5.20 BM. Anal. C54H46CoCl2N10O2 (996.85): Calcd: C, 65.06; H, 4.65; N, 14.05; Co, 5.91; Cl, 7.11. Found: C, 65.30; H, 4.37; N, 14.32; Co, 5.70; Cl, 7.02.
Mononuclear Co (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4-methyl-phenyl) pyridazin-3-yl]hydrazine 11b
Green crystals, yield 51.20%, mp 258–260 °C. IR 3300 for NH(hydrazone), 3147 for NH(indole), 3058 for CH(aromatic), 2916 and 2835 for CH(aliphatic), 1596 for C=N and 466 for (M–N) cm−1. UV λmax: 565, 543, 318, 299, 257, 219 nm. μeff: 3.40 BM. Anal. C56H50CoCl2N10O2 (1024.9): Calcd: C, 65.63; H, 4.92; N 13.67; Co, 5.75; Cl, 6.92. Found: C, 65.08; H, 4.89; N, 12.95; Co, 5.22; Cl, 6.98.
Mononuclear Co (II) complex of 2-[(1H-indol-3-yl)-methylene)-1-(4-(2-methoxybenzyl)-6-(4-chloro-phenyl) pyridazin-3-yl]hydrazine 11c
Reddish brown crystals, yield 53.50%, mp 233–234 °C. IR: 3250 for NH(hydrazone), 3150 for NH(indole), 3058 for CH(aromatic), 2912 and 2835 for CH(aliphatic), 1593 for C=N and 478 for (M–N) cm−1. UV λmax: 601, 508, 472, 298, 225, 214 nm. μeff: 3.94 BM. Anal. C54H44CoCl4N10O2 (1065.74): Calcd: C, 60.86; H, 4.16; N, 13.14; Co,5.53; Cl,13.31. Found: C, 61.11; H, 4.35; N, 13.11; Co, 5.42; Cl, 13.23.
General procedure for synthesis of bis-{[(1H-indol-3yl)-methylene)-3(4H)-thiazolidinone]}-1,1′-biphenyl 12a and bis-{[(1H-indol-3yl)methylene)-3(4H)-thiazolidinone]}-3,3′-dimethoxy-benzidine 12b
Method A: A solution of the Schiff bases 5a,b (0.001 mol) and mercaptoacetic acid (0.002 mol) in dry dioxane (50 ml) and in the presence of anhydrous ZnCl2 was refluxed for 6–10 h. After completion of the reaction as tracked with TLC until the reactants had disappeared, excess solvent was removed through distillation. Crushed ice was added to the sticky solid remaining until the solid separated which was then filtered, dried and recrystallized from appropriate solvent to give 12a,b.
Method B: A mixture of Schiff bases 5a,b (0.001 mol) and mercaptoacetic acid (0.002 mol) was placed in DMSO in a round-bottom flask fitted with a Dean–Stark apparatus. The mixture was refluxed for 6 h with removal of water azeotropically. A sticky solid was formed on evaporation of the solvent that was treated with a solution of sodium bicarbonate to remove excess acid. The solid formed was filtered, washed with water, dried and recrystallized from ethanol.
Bis-{[(1H-indol-3yl)-methylene)-3(4H)-thiazolidinone]}-1,1′-biphenyl 12a
Yellow crystals, yield 63%; mp decomposition: 300 °C; IR: 3298 and 3182 for NH(indole) and OH, 3109–3039 for CH(aromatic), 2961–2842 for CH(aliphatic) 1658 for C=O(thiazolidine), 1242 for C–N and 748 for C–S–C cm−1. 1H NMR: δ11.16, 11.07 [s, 2H, 2NH(indole)], 7.64–7.31 (m, 16H, 4Ar–H), 7.06–6.92 (s, 2H, 2CH(hetero)), 6.90–6.81 (d, 2H, 2CH(hetero)) and 5.69–5.65 [s, 4H, 2CH2(thiazolidinone)] ppm. 13C NMR: δ 167.53, 167.26, 138.52, 137.15, 135.21, 135.11, 127.79, 127.02, 126.57, 126.26, 125.87, 125.73, 120.86, 120.31, 119.33, 119.11, 118.87, 118.69, 118.44, 117.84, 113.63, 48.05, 46.78, 28.96, 28.16. Anal. C34H26N4O2S2 (586.59): Calcd: C, 69.61; H, 4.47; N, 9.55; S, 10.91; Found: C, 69.94; H, 4.78; N, 9.75; S, 11.13. MS: m/z 587 [M+ + 1].
Bis-{[(1H-indol-3yl)-methylene)-3(4H-)thiazolidinone]}-3,3′-dimethoxybenzidine 12b
Pale yellow crystals, yield 54%; mp 110–112 °C; IR: 3247 for NH(indole) and OH, 3088–3055 for CH(aromatic), 2935–2835 for CH(aliphatic) 1654 for C=O(thiazolidine), 1245 for C–N and 748 for C–S–C cm−1. Anal. C36H30N4O4S2 (646.64):Calcd: C, 66.86; H, 4.68; N, 8.67; S, 9.90. Found: C, 66.59; H, 4.52; N, 8.90; S, 10.12. MS: m/z 646 [M+], 647 [M+ + 1].
General procedure for synthesis of bis-{[(1H-indol-3yl)-methylene)-3(2H)-3-chloro-azetidinone]}-1,1′-biphenyl 13a and bis-{[(1H-indol-3yl)-methylene)-3-(2H)-3-chloro-azetidinone]}-3,3′-dimethoxybenzidine 13b
To a solution of each of the Schiff bases 5a,b (0.001 mol) in DMF (30 ml), chloroacetyl chloride (0.002 mol) and triethylamine (0.002 mol) were added at 0–5 °C with constant stirring. The reaction mixture was heated on a water bath for 7 h, and then excess of solvent was distilled off. The sticky solid was cooled, ice water was added, then filtered, and further recrystallized from ethanol to give compounds 13a,b.
Bis-{[(1H-indol-3yl)-methylene)-3(2H)-3-chloroazetidinone]}1,1′-biphenyl 13a
Brown crystals, yield 47.60%; mp 263 °C (decomp.); IR: 3166 for NH(indole), 3047 and 3000 for CH(aromatic), 2869 for CH(aliphatic), 1735 for C=O(azetidine), 1222 for C–N and 748 for C–Cl cm−1. 1H NMR: δ 9.89 [s, 2H, 2NH(indole)], 8.38–7.22 (m, 16H, 4Ar–H), 6.88 [s, 2H, 2CH(indole)], 6.75 (d, 2H, 2CH–Cl) and 4.26 (d, 2H, 2CH–N) ppm. Anal. C34H24N4O2Cl2 (591.47): Calcd: N, 9.47; Cl, 11.99. Found: N, 9.07; Cl, 12.22. MS: m/z 590 [M+], 592 [M+ + 2].
Bis-{[(1H-indol-3yl)methylene)-3-(2H)-3-chloroazetidinone]}-3,3′-dimethoxbenzidine 13b
Brown crystals, yield 57.90%; mp >300 °C; IR: 3181 for NH(indole), 3109 and 3016 for CH(aromatic), 2939 and 2842 for CH(aliphatic), 1755 for C=O(azetidine), 1222 for C–N and 756 for C–Cl cm−1. Anal. C36H28N4O4Cl2 (651.52): Calcd: N, 8.60; Cl, 10.88. Found: N, 7.88; Cl, 10.87. MS: m/z 651 [M+ + 1], 652 [M+ + 2, 653 [M+ + 3].
General procedure for the synthesis of bis-[(1H-indol-3yl)-methylene)-3-(3H-1,2,4)triazoles)-2-(1H)-one]1,1′-biphenyl 14a and bis-[(1H-indol-3yl)-methylene)-3-(3H-1,2,4)triazoles)2-(1H)-one]-3,3′-dimethoxybenzidine 14b
A mixture of each Schiff’s base 5a,b (0.01 mol) and thiosemicarbazide (0.02 mol) in enough ethanol to make a slurry was irradiated under microwave conditions (power: 200 W, temperature 80 °C]. The solid product obtained after cooling was filtered and recrystallized from ethanol to give 14a,b.
Bis-[(1H-indol-3yl)-methylene)-3-(3H-1,2,4)-triazole)-2-(1H)-one] 1,1′-biphenyl 14a
Yellow crystals, yield 97%; mp 101–102 °C; IR: 3448 for NH2, 3313 for NH(triazole), 3143 for NH(indole), 3035 for CH(aromatic), 2875 for CH(aliphatic), 1612 for C=N and 1245 for C–N cm−1. 1H NMR: δ 11.57 [s, 2H, 2NH (indole)], 11.16 [s, 2H, 2NH(triazole)], 8.28 and 8.20 (s, 4H, 2NH2), 8.00–7.09 (m, 16H, 4Ar–H) and 6.61–6.59 [s, 4H, 4CH(hetero)] ppm. 13C NMR: δ 177.10, 147.23, 142.09, 141.30, 140.88, 137.59, 136.37, 132.45, 131.79, 131.15, 130.48, 124.53, 123.80, 122.99, 122.65, 122.21, 121.82, 118.41, 115. 49, 114.35, 113.05, 111.68, 111.67, 77.01, 76.96. Anal. C32H28N10 (552.64): Calcd: N, 25.35. Found: N, 25.08. MS: m/z 553, [M+ + 1], 555 [M+ + 3].
Bis-[(1H-indol-3yl)-methylene)-3-(3H-1,2,4)triazole)-2-(1H)-one]3,3′-dimethoxy benzidine 14b
Red crystals, yield 86%; mp 230–231 °C; IR: 3389 for NH2, 3237 for NH(triazole), 3152 for NH(indole), 3039 for CH(aromatic), 2969 and 2885 for CH(aliphatic), 1615 for C=N and 1271 for C–N cm−1. 1H NMR: δ 11.53 [s, 2H, 2NH(indole)], 11.15 [s, 2H, 2NH(triazole)], 8.31 and 8.18 (s, 4H, 2NH2), 7.98–7.10 (m, 14H, 4Ar–H), 6.90–6.64 [s, 4H, 4CH(hetero)] and 3.80 (s, 6H, 2OCH3 of 2CH3O–Ar) ppm. Anal. C34H32N10O2 (612.70): Calcd: N, 22.87. Found: N, 22.90. MS: m/z 613 [M+ + 1].
General procedure for synthesis of bis-{[(1H-indol-3yl)-methylene)-3-(4H)-thiazolidinone]} pyridine-2,6-diamine (15)
Method A: A solution of the Schiff base (7) (0.001 mol) and mercaptoacetic acid (0.002 mol) in dry dioxane (50 ml) in presence of anhydrous ZnCl2 was refluxed for 6–10 h. After completion of the reaction, excess of solvent was removed through distillation, and the sticky solid obtained was poured onto crushed ice, then filtered, dried and recrystallized from ethanol to give 15.
Method B: A mixture of the Schiff base (7) (0.001 mol) and mercaptoacetic acid (0.002 mol) was placed in DMF in a round-bottom flask fitted with a Dean–Stark apparatus. The mixture was refluxed for 6 h with removal of water azeotropically. A sticky solid that formed on evaporation of the solvent was treated with a solution of sodium bicarbonate to remove excess of acid. The solid formed was filtered, washed with water, dried and recrystallized from ethanol.
Bis-{[(1H-indol-3yl)-methylene)-3-(4H)-thiazolidinone]} pyridine-2,6-diamine (15)
Orange crystals, yield 53.5%; mp 138–140 °C; IR: 3344 and 3224 for NH(indole) and OH(enolic), 3055 for CH(aromatic), 2916 for CH(aliphatic), 1658 for C=O(thiazolidine), 1226 for C–N and 744 for C–S–C cm−1. 1H NMR: δ 11.01 and 0.96 [s, 2H, 2NH(indole)], 7.64–7.10 (m, 11H, 3Ar–H), 7.04–6.98 (s, 2H, 2CH(hetero)), 6.90–6.82 (d, 2H, 2CH(indole)) and 5.74 [s, 4H, 2CH2(thiazolidinone)] ppm, 13C NMR (500 MHz): δ 171.69, 137.32, 131.04, 129.43, 128.74, 126.90, 126.57, 125.84, 124.58, 123.30, 121.86, 121.60, 119.73, 119.19, 56.61, 19.09. Anal. C27H21N5S2O2 (511.49): Calcd: N, 13.70; S, 12.51. Found: N, 13.58; S, 12.55. MS: m/z 511 [M+].
General procedure for synthesis of bis-[((1H-indol-3yl)-methylene)-3-(2H)-3-chloroazetidinone)] pyridine-2,6-diamine (16)
To a solution of the Schiff base (7) (0.001 mol) in DMF (30 ml), chloroacetyl chloride (0.002 mol) and triethylamine (0.002 mol) were added at 0–5 °C with constant stirring. The reaction mixture heated on a water bath for 7 h, and then excess of solvent was distilled off. The sticky solid was cooled, poured in ice water then filtered, further recrystallized from ethanol to give 16.
Bis-[((1H-indol-3yl)-methylene)-3-(2H)-3-chloroazetidinone)] pyridine-2,6-diamine (16)
Violet crystals, yield 65.50%; mp >300 °C; IR: 3185 for NH(indole), 3100 and 3028 for CH(aromatic), 2900 for CH(aliphatic), 1760 for C=O(azetidine), 1228 for C–N and 748 for C–Cl cm−1. 1H NMR: δ 11.36 [s, 2H, 2NH(indole)], 8.67–6.91 (m, 11H, 3Ar–H), 6.59 [s, 2H, 2CH(indole)], 4.90 (d, 2H, 2CH–Cl) and 4.23 (d, 2H, 2CH–N) ppm. Anal. C27H19N5O2Cl2 (516.37): Calcd: N, 13.57; Cl, 13,73; Found: N, 13.49; Cl, 13.81. MS: m/z 517 [M+ + 2].
General procedure for the synthesis of bis-N-[1-(morpholinomethyl)-1H-indol-3-yl)-methylene] pyridine-2,6-diamine 17a and bis-N-[1-(piperidinomethyl)-1H-indol-3-yl)-methylene] pyridine-2,6-diamine (17b)
A slurry consisting of the Schiff base (7) (0.001 mol), absolute ethanol (2 ml) and 37% formalin (0.3 ml) was prepared. To this slurry, the secondary amine, morpholine or piperidine, (0.002 mol) was added dropwise, with cooling and shaking. The reaction mixture was microwave irradiated (power: 150 W, temperature, 78 °C] for an appropriate time until the completion of the reaction was noted from TLC tracking of the reaction. On cooling, crystals separated out which were recrystallized from DMF to give 17a,b.
Bis-N-[1-(morpholinomethyl)-1H-indol-3-yl)-methylene] pyridine-2,6-diamine 17a
Green crystals, yield 70%; mp 148–150 °C; IR: 3075 for CH(aromatic), 2931, 2854 and 2815 for CH(aliphatic) and 1608 for CH=N cm−1. 1H NMR: δ 9.94 (s, 2H, 2CH=N), 8.32–7.23 (m, 11H, 3Ar–H), 5.62 [s, 2H, 2CH(indole)], 5.06 (s, 4H, 2N–CH2–N), 4.02 (t, 8H, 2CH2–O–CH2) and 2.54–2.49 (t, 8H, 2CH2–N–CH2) ppm. Anal. C33H35N7O2 (561.68): Calcd: C, 70.57; H, 6.28; N, 17.46. Found: C, 70.59; H, 6.12; N, 17.90. MS: m/z 562 [M+ + 1].
Bis-N-[1-(piperidinomethyl)-1H-indol-3-yl)-methylene] pyridine-2,6-diamine (17b)
Yellow crystals, yield 68%; mp >300 °C; IR: 3047 for CH(aromatic), 2931 and 2850 for CH(aliphatic) and 1608 for CH=N cm−1. 1HN MR: δ 9.90 (s, 2H, 2CH=N), 8.26–7.02 (m, 11H, 3Ar–H), 5.23 [s, 2H, 2CH(indole)], 4.62–4.39 (s, 4H, 2N–CH2–N), 2.70–2.46 (t, 8H, 2CH2–N–CH2) and 1.43 (m, 12H, 6CH2) ppm. Anal. C35H39N7 (557.73): Calcd: C, 75.37; H, 7.05; N, 17.58. Found: C, 75.59; H, 7.12; N, 17.90. MS: m/z 558 [M+ ].
General procedure for the synthesis of mononuclear Cu (II), Co(II) and Ni(II) complexes of bis-N-[(1H-indol-3yl)-methylene] pyridine-2,6-diamine with acetylacetone (18a–c)
To a stirred solution of metal chloride (0.01 mol) in methanol (20 ml), acetylacetone (1 ml, 0.01 mol) was added. The reaction mixture was refluxed for 30 min. Then a hot solution of the ligand (7) (0.01 mol) in methanol was added, and refluxing continued for 2 h with stirring. On cooling and concentration, the solid product was separated, washed with methanol and diethyl ether to give 18a–c.
Cu (II) complexes of bis-N-[(1H-indol-3yl)-methylene] pyridine-2,6-diamine with acetylacetone 18a
Brown crystals, yield 50.78%, mp 300–301 °C: IR: 3305 for OH, 3201 for NH(indole), 3058 for CH(aromatic), 2923 and 2850 for CH(aliphatic),1643 for C=O, 1581 for C=N and 505 for (M–N) cm−1. UV λmax: 756, 708, 604, 570, 414, 386, 355, 326, 259, 241 nm. μeff: 1.79 BM. Anal. C28H25CuCl2N5O2 (597.98): Calcd: N, 11.71; Cu, 10.63; Cl, 11.86. Found: N, 11.88; Cu, 10.55; Cl, 12.01.
Co (II) complexes of bis-N-[(1H-indol-3yl)-methylene]pyridine-2,6-diamine with acetylacetone 18b
Brown crystals, yield 52.87%, mp 176–178 °C; IR 3300 for OH, 3174 for NH(indole), 3047 for CH(aromatic), 2927 and 2819 for CH(aliphatic), 1635 for C=O, 1600 for C=N and 497 for (M–N) cm−1. UV λmax: 615, 573, 482, 388, 351, 321, 273 nm. μeff: 4.12 BM. Anal. C28H25CoCl2N5O2 (593.37): Calcd: N, 11.80; Co, 9.93; Cl, 11.95. Found: N, 11.61; Co, 9.40; Cl, 11.97.
Ni (II) complexes of bis-N-[(1H-indol-3yl)-methylene]pyridine-2,6-diamine with acetylacetone (18c)
Reddish brown crystals, yield 50.90%, mp 256–258 °C; IR: 3301 for OH, 3174 for NH(indole), 3000 for CH(aromatic), 2920 and 2831 for CH(aliphatic), 1643 for C=O, 1573 for C=N and 486 for (M–N) cm−1. UV λmax 758, 499, 412, 354, 314, 271, 242 nm. μeff: 4.48 BM. Anal. C28H25NiCl2N5O2 (593.13): Calcd: N, 11.81; Ni, 9.90; Cl, 11.95. Found: N, 11.20; Ni, 9.42; Cl, 11.92.
Biological activity evaluation
In vitro antimicrobial measurement
The compounds were tested for their in vitro antimicrobial activity by the broth-dilution technique in terms of minimum inhibitory concentrations (MIC). Experimentally for potent drugsCitation31. The antimicrobial activities of the compounds in this study were evaluated against six pathogenic microbial species: Gram +ve bacteria S. aureus and S. epidermidis, Gram −ve bacteria E. coli and K. pneumonia and fungi A. fumigatu, and C. albicans. Reference drugs used were sulfamethoxazole as an antibacterial standard and fluconazole as an antifungal standard.
In vitro cytotoxicity evaluation
The primary evaluation of in vitro cytotoxicity of the selected new compounds against human tumor cells was carried out at the National Cancer Institute (NCI), Cairo University using the using the method of Skehan and StorengCitation32. The cytotoxicity evaluation also involved the use of vinblastine sulphate or doxorubicinCitation33 as antitumor drug reference standards. The procedure used was as follows:
Cells were plated in 96-multiwell plate (105 cells/well) for 24 h before treatment with the compound to allow attachment of the cell to the wall of the plate.
Different concentrations of the compound under test (0, 1, 2.5, 5 and 10 μg/mL) were added to the cell monolayer triplicate walls that were prepared for each individual dose.
Monolayer cells were incubated with the compound for 48 h at 37 °C and in atmosphere of 5% CO2.
After 48 h, the cells were fixed, washed and stained with sulphorhodamine-B.
Excess stain was washed away with acetic acid and attached stain was recovered with Tris–EDTA buffer.
Color intensity was measured with an ELISA reader.
The relationship between the surviving fraction and drug concentration is plotted to give the survival curve of cancer breast cell line.
The results of the in vitro cytotoxicity activity on human tumor cell lines HCT116 (colon), MCF7 (breast) and HELA (cervix) were determined according to the dose values of the drug exposure required to reduce survival in the cell lines to 50%.
Results and discussion
Chemistry
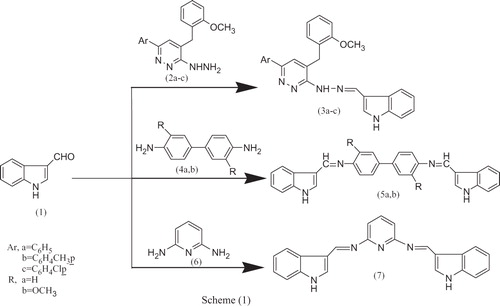
The development of new methods for synthesis of libraries of heterocyclic compounds is an important topic in combinatorial chemistry. Microwave technology is particularly suitable for the rapid and automated production of heterocyclic librariesCitation24–30. In this study, we have synthesized hydrazine derivatives and three different classes of bis-Schiff bases. Thus condensation of indole-3-carbinal 1 with 6-aryl-[4-(2-methoxybenzyl) pyridazin-3-yl] hydrazones 2a–cCitation24,Citation34 and diamines such as benzidine (4,4′-diamino-1,1′-biphenyl), 3,3′-dimethoxybenzidine 4a,b and 2,6-diaminopyridine 6 in refluxing absolute ethanol yielded the corresponding Schiff bases 2-[(1H-indol-3-yl)-methylene]-1-[4-(2-methoxybenzyl)-6-aryl-pyridazin-3-yl] hydrazines 3a–c, bis-N-[(1H-indol-3yl)-methylene] 4,4′-diamino-1,1′-biphenyl 5a, bis-N-[(1H-indol-3yl)-methylene] 3,3′-dimethoxy-benzidine 5b and bis-Schiff base bis-N-[(1H-indol-3yl)-methylene] pyridine- 2,6-diamine 7, respectively, as illustrated in Scheme 1.
The structure of these compounds was confirmed by elemental analysis, FTIR, 1HNMR, 13CNMR and MS. 1H NMR of compound 3a showed bands at 10.20 [s, 1H, NH(indole)], 8.90 (s, 1H, CH=N), 8.62 [s, 1H NH(hydrazone)], 8.50–6.91 (m,13H, 3ArH), 6.92(s,1H, CH(indole)), 6.84 (s,1H CH(pyridazine)), 4.15 (s, 2H,CH2) and 3.84 (s, 3H, OCH3 of CH3O–Ar) ppm. 13C NMR of compound 3a showed bands at 157.91, 157.66, 150.66, 148.71, 143.38, 137.69, 136.08, 131.33, 130.89, 128.80, 127.63, 125.96, 125.10, 123.30, 123.03,121.73, 121.03,120.86, 113.75, 112.23, 111.39, 55.85, 30.63.
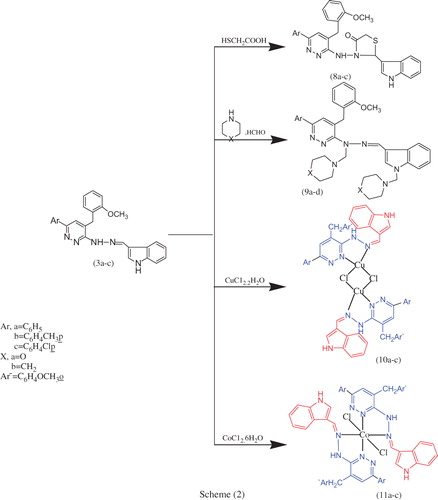
This study was extended to prepare new heterocycles bearing nitrogen, sulphur and oxygen atoms in their structure with the anticipation of biological activity. Schiff base derived products were utilized as key starting materials for the synthesis of the thiazolidin-4-one derivatives and N-Mannich bases. Attempts to cyclize the Schiff products 3a–c to the corresponding thiazolidin-4-one derivatives 8a–c and N-Mannich bases 9a–d named by 3-(4-(2-methoxybenzyl)-6-arylpyridazin-3-ylamino)-2-(1H-indol-3-yl)-thiazolidin-4-ones 8a–c, 1-[4-(2-methoxybenzyl)-6-arylpyridazin-3-yl]-1-(morpholinomethyl)-2-{[1-(morpholinomethyl)-1H-indol-3-yl]methylene} hydrazines 9a,c and 1-[4-(2-methoxybenzyl)-6-arylpyridazin-3-yl]-1-[(piperidin-1-yl)-methyl]-2-{[1-((piperidin-1-yl)-methyl)-1H-indol-3-yl]-methylene}hydrazines 9b,d, respectively, were carried out by reaction of the Schiff bases 3a–c with mercaptoacetic acid and formaldehyde in the presence of secondary amine such as morpholine and piperidine. Further support for the structure of these compounds was obtained from FTIR, 1H NMR and MS spectra. The synthetic routes used to synthesize these compounds are outlined in Scheme 2. The 1H NMR spectrum of 3-[4-(2-methoxybenzyl)-6-(4-chlorophenyl)-pyridazin-3-ylamino]-2-(1H-indol-3-yl)-thiazolidin-4-one 8c showed bands at δ 10.92 (s, 1H, NH(indole), D2O exchangeable), 9.90 (s, 1H, 1NH(hydrazone), D2O exchangeable), 7.96–7.23 (m, 13H, 3Ar–H), 7.02 (d, 1H, CH(hetero)), 6.99–6.87 (s, 2H, 2CH(hetero)), 5.29 (s, 2H, CH2(thiazolidine ring)), 4.32 (s, 2H, CH2) and 3.78 (s, 3H, OCH3 of CH3O–Ar) ppm.
The hydrazone derivatives 3a–c were reacted with Cu(II) chloride and Co(II) chloride to give the corresponding Cu(II) and Co(II) complexes 10a–c and 11a–c, respectively. The complexes 10a–c were designed with the aim of exploring their potential as anticancer agents. The structure of metal complexes 10a–c and 11a–c was confirmed by elemental analysis and spectral studies. The elemental analyses showed 1:1 for Cu(II) and 1:2 for Co(II) (metal:ligand) stoichiometry for all complexes as shown in Scheme 2. The analytical data of ligands and complexes agreed well with the general formula [M2(μCL)2(HL)2·yCl] (where M = Cu (II) and y = 0–1) and [M(HL)2Cl2] [where M = Co (II)], respectively.
IR spectral studies and mode of coordination of complexes 10a–c and 11a–c
In the IR spectra of the complexes 10a–c and 11a–c, the band located at 1620–1604 cm−1 in all ligands is attributed to the HC=N group of the indole moiety that has moved to lower frequencies by values up to 27 and 31 cm−1, i.e. to 1596–1593 and 1596–1589 in the new copper and cobalt complexes. In addition, the band due to the (C=N) of pyridazine moiety at 1519–1434 cm−1 was lowered by values of 54 and 61 cm−1 to 1492–1419 (Cu) and 1465–1423 (Co) due to the coordination of these sites to the metal. New bands in the 509–482 (Cu) and 478–466 (Co) cm−1 regions are assigned to the stretching frequencies of (M–N) bonds. Thus the IR spectral results provide strong evidence for the complexation of the hydrazones with the metal ions in the bidentate mode.
Electronic spectra and magnetic moment studies complexes 10a–c and 11a–c
The copper and cobalt complexes show two bands at 354–346, 265–214 and 340–290, 257–214 nm, respectively. These bands can be attributed to π–π* and n–π* transition states within the hydrazone ligand. Bands at 748–494 and 601–472 nm can be attributed to d–d transition states of the metal and ligand to metal charge transfer. The copper and cobalt complexes have magnetic moment values in the range 1.1–1.5 BM and 3.94–5.20 BM, respectively. These magnetic measurements for the solid Co (II) complexes are indicative of three unpaired electrons per Co (II) ion suggesting consistency with their octahedral environment. The observed values are less than the spin value (1.73–2.20 BM) for Cu (II) 3dCitation9 mono-nuclear complexes having no interactions between two copper moieties. The lower value of magnetic moment at room temperature which ranged between 1.10–1.50 BM is consistent with square planar geometry around the metal ions. In general, the low magnetic moment values of binuclear copper complexes are attributed to the anti-ferromagnetic moment interaction between two central metal ions; this is an indication of the formation of binuclear complex. These values along with elemental analysis data may be accounted for by assuming a dimeric dichloro-bridged structure.
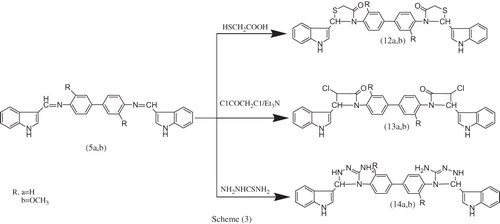
In the same manner, the reaction of bis-Schiff bases 5a,b with mercaptoacetic acid in the presence of anhydrous ZnCl2, chloroacetyl chloride in presence of triethylamine at temperature 80 °C and thiosemicarbazide yielded thiazolidinone derivatives 12a,b, the new bis-spiro-azetidinone derivatives 13a,b and bis-spiro-1,2,4-triazole derivatives 14a,b, respectively as illustrated in Scheme 3. The structure of these compounds was confirmed by FTIR, 1H NMR 13C NMR, and MS spectra and elemental analysis. The 1H NMR spectrum of bis-{[(1H-indol-3yl)-methylene)-3-(4H)-thiazolidinone]} 1,1′-biphenyl 12a showed bands at δ 11.16, 11.07 [s, 2H, 2NH (indole)], 7.64–7.31 (m, 16H, 4Ar–H), 7.06–6.92 (s, 2H, 2CH(hetero)), 6.90–6.81 (d, 2H, 2CH(hetero)) and 5.69–5.65 [s, 4H, 2CH2(thiazolidinone)] ppm. The 13C NMR spectrum of bis-{[(1H-indol-3yl)-methylene)-3(4H)-thiazolidinone]} 1,1′-biphenyl 12a showed bands at δ 167.53, 167.26, 138.52, 137.15, 135.21, 135.11, 127.79, 127.02, 126.57, 126.26, 125.87, 125.73, 120,86, 120.31, 119.33, 119.11, 118.87, 118.69, 118.44, 117.84, 113.63, 48.05, 46.78, 28.96, 28.16.
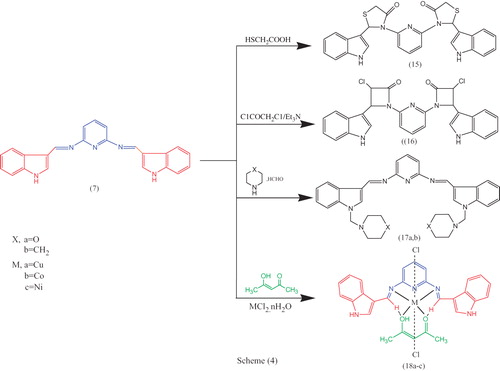
The reaction of bis-Schiff base 7 with mercaptoacetic acid in the presence of anhydrous ZnCl2, chloroacetyl chloride and triethylamine at 80 °C and then with formaldehyde in the presence of a secondary amine such as morpholine and piperidine yielded the thiazolidinone derivative 15, the new bis-spiro-azetidinone derivative 16 and the N-Mannich bases 17a,b respectively, as illustrated in Scheme 4. The structure of these compounds was established from FTIR, 1H NMR, 13C NMR and MS spectra and elemental analysis. The 1H NMR spectrum of bis-{[(1H-indol-3yl)-methylene)-3-(4H)-thiazolidinone]} pyridine-2,6-diamine 15 showed bands at δ 11.01, 10.96 [s, 2H, 2NH(indole) D2O exchangeable], 7.64–7.10 (m, 11H, 3Ar–H), 7.04–6.98 (s, 2H, 2CH(hetero)), 6.90–6.82 (d, 2H, 2CH(indole)) and 5.74–5.32 (s, 4H, 2CH2(thiazolidinone)) ppm 13C NMR spectrum of bis-{[(1H-indol-3yl)-methylene)-3-(4H)-thiazolidinone]} pyridine-2,6-diamine 15 showed bands at δ 171.69, 137.32, 131.04, 129.43, 128.74, 126.90, 126.57, 125.84, 124.58, 123.30, 121.86, 121.60, 119.73, 119.19, 56.61, 19.09.
The complexes 18a–c were prepared by a two steps procedure. The first step involved mixing equal amounts of a hot saturated methanolic solution of the first ligand in acetylacetone with the same ratio of MCl2·nH2O salts (where M = Cu, Co or Ni). The mixture was refluxed for 30 min, and then the second ligand, the bis-Schiff base 7 was added in the same ratio to the previous mixture and refluxed for 3 h. The structures of metal complexes 18a–c were confirmed by elemental analysis and spectral studies. The elemental analysis showed a ratio of 1:1:1[M: L1:L2] as shown in Scheme 4. The theoretical values were in a good agreement with the found values. The presence of chlorine confirmed from element analysis and the low molar conductance values (30.60–24.30 S cm2 mol−1) for the complexes 18a–c supports the non-electrolytic nature of the metal complexes.
IR spectral studies and mode of coordination of complexes 18a–c
In the IR spectra of the complexes 18a–c, the band located at 1608 cm−1 in all ligands is attributed to the HC=N group of the indole moiety that has moved to the lower frequency with up to 35 cm−1 to 1600–1573 in the new metal complexes. Further the band due to (C=O), (C=O→OH enolic) of the acetylacetone moiety at 1643–1635 cm−1 and 3305–3300 cm−1 involved in new metal complexes confirm the coordination of one molecule of acetylacetone to the metal. New bands in the 505–439 cm−1 region are assigned to the stretching frequencies of (M–N) bonds. Thus the IR spectral results provide strong evidence for the complexation of both bis-Schiff base 7 and acetylacetone with metal ions in the bidentate mode.
Electronic spectra and magnetic moment studies 18a–c
The magnetic susceptibility measurement for the solids Cu (II), Co (II), Ni (II) complexes (1.79 BM, 4.12 BM and 4.48 BM) are indicative of octahedral environment, respectively. The diffuse reflectance spectra of the copper, cobalt, nickel complexes show two bands at 386–241, 388–273, 354–242 nm, respectively. These bands can be attributed to π–π* and n–π* transition states within the Schiff base ligand. Bands at 756–414, 615–482, 758–412 nm, respectively, can be attributed to d–d transition states of the metal and ligand to metal charge transfer.
Biological activity evaluation
In vitro antimicrobial measurement
Most of the synthesized compounds were tested for their in vitro antimicrobial activity by the broth-dilution technique in terms of minimum inhibitory concentrations (MIC). The antimicrobial activities of the compounds against six pathogenic microbial species are presented in and . The study also included the reference compounds sulfamethoxazol as an antibacterial agent and fluconazole as an antifungal agent. From the data obtained, the following conclusion can be drawn:
Schiff bases and bis-Schiff bases based on the indole moiety have low antimicrobial activity compared to the synthesized heterocyclic compounds obtained by formation of thiazolodine, azetidinone, 1,2-triazole and Mannich base derivatives.
Bis 1,2,4-triazole 14b containing the thiosemicarbazide entity according to MICs count gave the highest antimicrobial activity of the 1,2,4-triazole derivatives.
All the azetidinone derivatives gave similar activities.
Bis-spiro-1,2,4-triazole derivatives showed remarkable activity relative to the reference drugs and should serve as useful lead compounds in search for potent antimicrobial agents.
Mannich bases with morpholine moiety (9a) greater activities when compared to those with piperidine moiety (9b).
Table 1. Inhibition zone diameter in (mm) as a criterion of antibacterial and antifungal activities of the newly synthesized compounds.
Table 2. Minimum inhibitory concentration (MIC) in µg/ml of the newly synthesized compounds.
In vitro cytotoxicity evaluation of Cu (II) and Co (II) complexes 10a, 11a
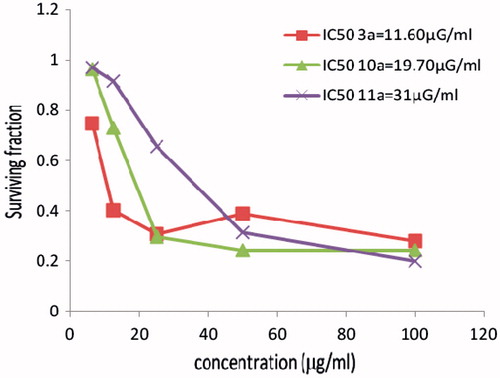
The new hydrazones containing the indole moiety and having different geometries for the copper complex 10a and cobalt complex 11a and were also screened against a panel of three human cancer cell lines HCT116 (colon), MCF7 (breast) and HELA (cervix). The cytotoxicity of the ligand (3a) with the indole moiety was also evaluated. All the results are recorded and as shown in . The copper complex 10a showed that the highest activity toward the three tumor cell lines comparable to data obtained with cobalt complex 11a. Cobalt complex 11a showed higher activity than ligand 3a only towards the HCT116 and MCF7 cell lines and for the cervix tumor cell line (HELA). The ligand 3a showed that the highest activity comparable to the two based indole moiety complexes 10a, 11a. The cobalt complex 11a did not show any significant activity towards the HCT116 cell and MCF7 cell lines and exhibited only moderate activity toward the cervix tumor cell line (HELA; ).
Figure 1. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against colon (HCT116) tumor cell line compared to Vinblastine sulphate IC50:9.8.
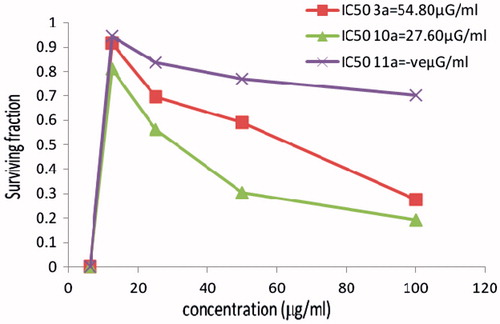
Figure 2. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against breast (MCF7) tumor cell line compared to Vinblastine sulphate IC50:11.6.
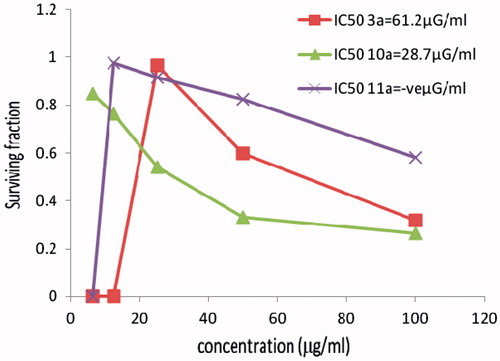
Figure 3. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against cervix (HELA) tumor cell line compared to Vinblastine sulphate IC50:10.9.
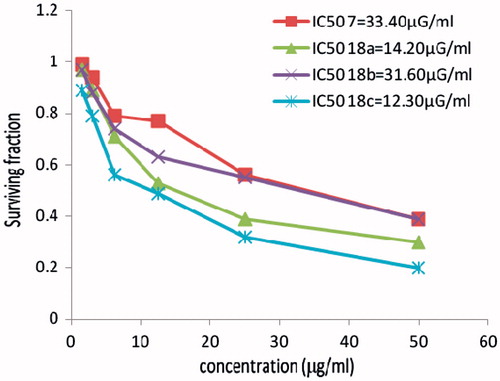
In vitro cytotoxicity evaluation of Cu(II), Co(II) and Ni(II) complexes 18a–c
Comparison of the activities of three different mixed metals complexed with the new bis-Schiff bases with acetylacetone fragment 18a–c were performed against the cervix human cancer cell line (HELA) alongside the examination of the cytotoxicity of the ligand 7. The nickel complex 18c gave the highest activity comparable to the reference drug followed by the copper complex 18a, while the cobalt complex 18b gave the lowest activity. The three complexes were more potent than the parent ligand 7. The results are recorded in .
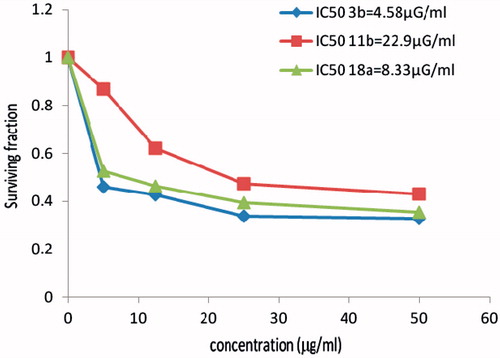
In vitro cytotoxicity evaluation of Cu (II), Co(II) complexes 11b, 18a and ligand 3b
Comparison between activities of two different mixed metal complexes 11b and 18a were performed against the cervix human cancer cell line (MCF7) alongside the examination of the cytotoxicity of the ligand 3b. The ligand 3b showed that the highest activity comparable to the two based indole complexes 11b and 18a and also the highest activity comparable to the reference drug, while the cobalt complex 11b gave the lowest activity ().
Conclusion
In this study, we report a convenient route for the synthesis of some novel heterocycles incorporating indole moiety in order to investigate their antimicrobial and antifungal activity. The in vitro evaluation of their antimicrobial against several pathogenic bacterial and fungal strains revealed that compound 14b showed the highest activity against E. coli and K. pneumoniae. Then the evaluation of the anti-cancer activity of selected compounds against panel of three human cancer cell lines HCT116 (colon), MCF7 (breast) and HELA (cervix) was performed in order to study the impact of metal complexation of compounds 10a and 11a and compare the results with those obtained with ligand 3a (which contains the indole moiety). The copper complex 10a showed that the highest activity toward the three tumor cell lines and was comparable to the data obtained with the cobalt complex 11a. The cobalt complex 11a showed higher activity than ligand 3a towards the HCT116 cell line and MCF7 and for the cervix tumor cell line (HELA). Ligand 3a showed the highest activity comparable to the two based indole moiety complexes 10a and11a. The cobalt complex 11a did not show any significant activity towards the HCT116 and MCF7 cell lines and showed only moderate activity toward the cervix tumor cell line (HELA). Comparison between the activities of three different mixed metal complexes of the new bis-Schiff bases with the acetylacetone fragment 18a–c were performed against the cervix human cancer cell line (HELA) also the examination of the cytotoxicity of the ligand 7. The nickel complex 18c gave the highest activity comparable to the reference drug followed by the copper complex 18a, while the cobalt complex 18b gave the lowest activity. The three complexes were more potent than the parent ligand 7. Comparison between activities of the two different mixed metal complexes 10a and 11a were performed against the cervix human cancer cell line (MCF7) as well as the cytotoxicity of the ligand 3b. The ligand 3b showed the highest activity compared to the two based indole complexes 10a and11a and also the highest activity comparable to the reference drug, while the cobalt complex 11b gave the lowest activity.
Acknowledgements
The authors are indebted to Dr Nashwa A. Ahmed, Basic Science Department, Faculty of Applied Medical Science, October 6th University City, Egypt for help with antimicrobial activity measurements.
Declaration of interest
The authors confirm that this article’s contents have no conflict of interest.
References
- Karthikeyan MS, Prasal DJ, Poojary B, et al. Synthesis and spectroscopic studies on ternary bis-Schiff base complexes having oxygen and/or nitrogen donors. J Coord Chem 2006;59:157–76
- Panneerselvam P, Nair RR, Vijayalakshmi G, et al. Synthesis of Schiff bases of 4-(4-aminophenyl) morpholine as potential antimicrobial agents. Eur J Med Chem 2005;40:225–9
- Mladenova R, Ignatova M, Manolova N, et al. Preparation, characterization and biological activity of Schiff base compounds derived from 8-hydroxyquinoline-2-carboxaldehyde and Jeffamines. Eur J Polym 2002;38:989–99
- Kumar S, Dhar DN, Saxena PN. Application of metal complexes of Schiff bases – a review. J Sci Ind Res 2009;68:181–7
- Ajaykumar D, Patil AS, Badam SP. Electrochemical properties of some transition metal complexes. Synthesis, characterization and in vitro antimicrobial studies of Co(II), Ni(II), Cu(II), Mn(II) and Fe(III) complexes. Int J Electrochem Sci 2009;4:717–29
- Sandras K, Blaga CR, Zivojin C, Vensa V. Synthesis and characterization of Co(II), Ni(II), Cu(II) and Zn(II) complexes with 3-salicylidenehydrazono-2-indolinone. J Serb Chem Soc 2003;8–9:641–7
- Kobayashi J, Tsuda M, Agemi K, et al. Purealidins B and C new bromotyrosine alkaloids from the Okinawan marine sponge Psammaplysilla purea. Tetrahedron 1991;47:6617–22
- James DM, Kunze HB, Faulkner DJ. Two new brominated tyrosine derivatives from the sponge Druinella (Psammaplysilla) purpurea. J Nat Prod 1991;54:1137–40
- Da-Silva JFM, Garden SJ, Pinto AC. The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc 2001;12:273–324
- Joshi KC, Chand P. Biologically active indole derivatives. Pharmazie 1982;37:1–12
- Abdel-Rahman AH, Keshk EM, Hanna MA, El-Bady Sh M. Synthesis and evaluation of some new spiro-indoline-based heterocycles as potentially active antimicrobial agents. Bioorg Med Chem 2004;12:2483–8
- Dandia A, Singh R, Khaturia S, et al. Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro[indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3,2′-thiazolidine]-3′(1,2,4-triazol-3-yl)-2,4′(1H)-dione. Bioorg Med Chem 2006;4:2409–17
- Khafagy MM, El-Wahas AHFA, Eid FA, El-Agrody AM. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 2002;57:715–22
- Sebahar PR, Williams RM. The asymmetric total synthesis of (+) and (−)-spirotryprostatin B. J Am Chem Soc 2000;122:5666–7
- Ma J, Hecht SM. Javaniside, a novel DNA cleavage agent from Alangium javanicum having an unusual oxindole skeleton. Chem Commun 2004;20:1190–1
- Edmondson S, Danishefsky SJ, Sepp-lorenzinol L, Rosen N. Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J Am Chem Soc 1999;121:2147–55
- Singh GS, Mmolotsi BJ. Synthesis of 2-azetidinones from 2-diazo-1,2-diaryl ethanones and N-(2-thienylidene) imines as possible antimicrobial agents. Il Farmaco 2005;60:727–30
- Rahman VPM, Mukhtar S, Ansari WH, Lemiere G. Synthesis, stereochemistry and biological activity of some novel long alkyl chain substituted thiazolidin-4-ones and thiazan-4-one from 10-undecenoic acid hydrazide. Eur J Med Chem 2005;40:173–84
- Kucukguzel SG, Oruc EE, Rollas S, et al. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur J Med Chem 2002;37:197–206
- Rawal RK, Prabhakar YS, Katti SB, De Clercq E. 2-(Aryl)-3-furan-2-yl-methyl-thiazolidin-4-ones as selective HIV-RT inhibitors. Bioorg Med Chem 2005;13:6771–6
- Vigorita MG, Ottana R, Monforte F, et al. Synthesis and antiinflammatory, analgesic activity of 3,3′-(1-2-ethanediyl)-bis-[2-aryl-4-thiazolidinone] chiral compounds part 10. Bioorg Med Chem Lett 2001;11:2791–4
- Goel B, Ram T, Tyagi R, et al. 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. Eur J Med Chem 1999;34:265–9
- Dandia A, Singh R, Sachdeva H, Arya K. Microwave assisted one pot synthesis of a series of trifluoromethyl substituted spiro[indoletriazoles. J Fluorine Chem 2001;111:61–7
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Synthesis and antibacterial activity of some pyridazine derivatives. J Chin Chem Soc 2004;51:963–8
- Mohamed MI. Synthesis and antimicrobial activity of new pyridazinyl sulfonamide derivatives. Bulg Chem Commun 2007;39:152–8
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Synthesis and antibacterial activity of some pyridazine derivatives. Eur J Med Chem 2009;44:1989–96
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Silver nanoparticles effect on antimicrobial and antifungal activity of new heterocycles. Bull Korean Chem Soc 2010;31:3530–8
- Mohamed MI. Synthesis and antibacterial activity of some novel heterocycles. Bulg Chem Commun 2004;36:241–8
- Kandile NG, Mohamed MI, Ismaeel HM. Antiproliferative effects of metal complexes of new isatin hydrazones against HCT116, MCF7 and HELA tumour cell lines. J Enzyme Inhib Med Chem 2012;27:330–8
- Kandile NG, Zaky HT, Mohamed MI, et al. Synthesis, characterization and in vitro antimicrobial evaluation of new compounds incorporating oxindole nucleus. J Enzyme Inhib Med Chem 2012;27:599–608
- Ahmad S, Rathish IG, Bano S, et al. Synthesis and biological evaluation of some novel 6-aryl-2-(psulfamylphenyl)-4,5-dihydropyridazin-3(2H)-ones as anti-cancer, antimicrobial, and anti- Inflammatory agents. J Enzyme Inhib Med Chem 2010;25:266–71
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–12
- Roth I, Brakovic D, Pomer S, Ponstingl H. Synergistic antitumor effects of vinblastine sulfate, its photoactivatable derivative Napavin and laser irradiation in murine renal carcinoma. J Cancer Res Clin Oncol 1995;121:A58
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Novel heterocyclic systems of pyridazines. Afinidad 2005;62:48–56