Abstract
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma. In several studies the relationship between catalase (CAT), human cytosolic carbonic anhydrases (CA; hCA-I and hCA-II) and xanthine oxidase (XO) enzyme activities have been investigated in various types of cancers but carbonic anhydrase, catalase and xanthine oxidase activities in patients with MF have not been previously reported. Therefore, in this preliminary study we aim to investigate CAT, CA and XO activities in patients with MF. This study enrolled 32 patients with MF and 26 healthy controls. According to the results, CA and CAT activities were significantly lower in patients with mycosis fungoides than controls (p < 0.001) (p < 0.001). There was no significant difference in XO activity between patient and control group (p = 0.601). Within these findings, we believe these enzyme activity levels might be a potentially important finding as an additional diagnostic biochemical tool for MF.
Introduction
Mycosis fungoides (MF) is the most common variant of primary cutaneous T-cell lymphoma. It is characterized by slow progression, from patch to tumor. The name mycosis fungoides is very misleading; it loosely means “mushroom-like fungal disease”. The disease, however, is not a fungal infection but rather a type of non-Hodgkin's lymphoma. It was so named because a French dermatologist Jean-Louis-Marc Alibert described the skin tumors of a severe case as having a mushroom-like appearanceCitation1. Most patients present with limited skin lesions as patches or plaques in the early stage of disease. Late stage of MF is associated with involvement of lymph nodes, visceral organs and bone marrowCitation2. Therefore, the late stages have a much more negative prognosis than the early stagesCitation3. Early in the course of disease, skin lesions may be non-specific. The median duration from onset of symptoms to diagnosis is 4–6 yearsCitation4. Accurate diagnosis is thought to be very important for improving the prognosis of patients with MF in the early stages. Even with a skin biopsy, the diagnosis of MF is not always easy to establish, so additional tests are often necessary. The incidence of MF is ∼0.36 cases per 100 000 population per year. It most often presents in those aged 45–60 years but has been diagnosed in children and adolescents as well. It is 50% more common in black than in white patients and twice as frequent in men as in womenCitation5. There are multiple theories about development of MF. These include genetics, exposure to chemicals, viral infections and chronic lymphocyte stimulation. The association between MF and genetics has been confirmed by many case reports of familial aggregationCitation6.
The discovery of cancer biomarkers has become a major focus of cancer researchCitation7 which holds promising future for early detection, diagnosis, monitoring disease recurrence and therapeutic treatment efficacy to improve long-term survival of cancer patients. As cancer is a complex disease, it might require a panel of multiple biomarkers in order to achieve sufficient clinical efficacy. Researchers have noted for years, a correspondence between low levels of enzymes and cancerCitation8. Hence, we aim to investigate xanthine oxidase (XO), cytosolic carbonic anhydrases (Cas) and catalase (CAT) activities in order to identify patients at risk for the early stages of MF.
The CAs form a family of metalloenzymes that catalyze the reversible hydration of carbon dioxide to carbonic acidCitation9. It has been recently demonstrated that CA IX, a member of carbonic anhydrases, is upregulated in tumorsCitation10 and implicated in the pathogenesis of hypoxic solid tumors.
CAT is an enzyme which catalyzes the decomposition of hydrogen peroxide to water and oxygenCitation11. CAT protects the cell from oxidative damage by reactive oxygen species (ROS). CAT activity changes in oxidative stress and some diseases including cancerCitation12. XO is an enzyme that generates ROSCitation13. It catalyzes the oxidation of hypoxanthine to xanthine and xanthine to uric acid.
To the best of our knowledge in this preliminary study, XO, CA and CAT activities in patients with MF have not been investigated in the previous literature.
Material and methods
Subjects
In total, 32 patients with MF (the mean age: 52.31 ± 10.95 years) and 26 healthy controls (the mean age: 51.38 ± 11.67 years) were included in the study. Diagnosis of MF was confirmed by clinical observations, histopathological and immunocytochemical examinations of skin biopsy in Diskapi Yildirim Beyazit Training and Research Hospital in Ankara, Turkey. All patients were lifetime non-smokers and free of drug, alcohol or antioxidant supplement consumption and any metabolic disease. None of the patients had any other significant disease or malignancies except for MF. Controls were randomly selected from a group of healthy non-smoking volunteers with no history of previous disease or drug or alcohol consumption. The patients were classified into five groups according to the 2005 WHO/EORTC system (2): All patients had MF stage IA. None of the patients presented with visceral organ involvement. The study protocol was approved by the local human ethics committee (Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey) and informed consent was obtained from each patient.
Sample preparation
Venous peripheral blood samples (1.5 mL) were collected into Venoject tubes coated with EDTA (from 0.47MK3-EDTA) and placed on ice. The blood samples were centrifuged at 1500 rpm for 20 min, and the plasma and buff coat were removed. After washing the red cells twice with NaCl (0.9%), erythrocyte lysates were prepared by putting the cells through three freeze–thaw cycles in dry ice with the addition of five volumes of ice-cold distilled water. These hemolysates were used to determine both the CA and CAT activitiesCitation14.
Assay of CA activity
CA activity was determined by following the hydration of CO2 according to the method of Wilbur and AndersonCitation15. In this method, the rate of pH reduction is measured using an electrode as soluble CO2, in a standardized solution, is converted to by the action of CA, when compared with the uncatalyzed reaction. Assays were performed at least twice on each lysate and the mean value determined.
Assay of CAT activity
CAT activity was measured spectrophotometrically using the UV assay methodCitation16. CAT-catalyzed decomposition of H2O2 was monitored for 5 min by measuring the decrease in absorbance at 240 nm, at 25 °C. The results were expressed as units per liter (U/L).
Assay of XO activity
XO activity was assayed essentially according to the method described by RoussosCitation17. Change in absorbance was recorded at 290 nm at 15 s interval for 1 min. Suitable control was run simultaneously. RoussosCitation17 has defined 1 U of activity as change in absorbance at 290 nm in 1 min by 1 mL enzyme preparation.
Statistical analysis
All statistical calculations were performed using the Statistical Package for Social Sciences (SPSS) v15.0 for Microsoft Windows (Turkey). The results are expressed as the mean ± standard deviation. Continuous variables were compared using Student's t test and Mann–Whitney U-test. One-way ANOVA test was used for comparing CA, CAT and XO levels in patients with different stages of MF. For correlations between variables, Spearman correlation coefficients were estimated. p < 0.05 was regarded as statistically significant.
Results and discussion
The clinical data of patients with MF is available as “Supplementary material”. There were no statistically significant differences between patients with MF and healthy subjects with respect to age and gender (p > 0.05).
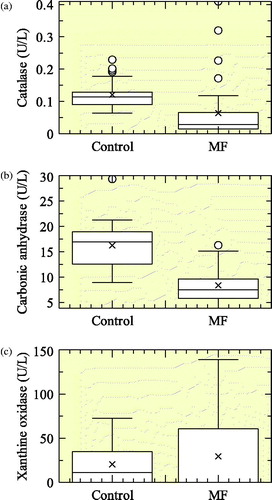
CA, CAT and XO enzyme activities were assayed in all samples. The mean activities of the enzymes are summarized in .
Table 1. The mean activities of the CA, CAT and XO.
The mean value of CA activity was significantly lower in patients with MF (8.38 ± 3.24 U/L) than those of healthy controls (16.26 ± 4.61 U/L) (p < 0.001). In addition, the mean value of CAT activity was significantly lower in patients with MF (0.064 ± 0.093 U/L) than those of healthy controls (0.121 ± 0.044 U/L) (p < 0.001). In patients with MF, there was a positive, marked, linear correlation between CA and CAT activities (p = 0.007, r = +0.353). However, statistically there was no significant differences determined between the activity of CA level, age, gender of patients, duration, stage of the disease, involvement of body area for the patients with MF, the values are also shown as; p = 0.182, p = 0.739, p = 0.821, p = 0.488, p = 0.765, respectively (). Additionally, there was also no correlation between the activity of CAT and age, gender, duration, stage of the disease, involvement of body area for the patients with MF. The values are determined as respectively, p = 0.078, p = 0.544, p = 0.623, p = 0.298, p = 0.937 (). The mean value of serum XO activity was higher in patients with MF (29.40 ± 38.14 U/L) than the mean value of serum XO activity in healthy subjects (20.40 ± 23.15 U/L). However, there was no statistically significant difference in XO activities between MF and control group (p = 0.601) (). It is apparent that tumorigenesis can elicit biochemical changes at distant sites from the origin of tumor which may have potential diagnostic or prognostic value. Because of their prognostic value, they may be utilized as peripheral markers of the disease. One of these biochemical markers that has been increasingly investigated is oxidative stress which has been implicated in the pathogenesis of solid tumors, Alzheimer's and other neurodegenerative diseasesCitation18.
Fig. 1 Box plots comparing (a) CAT activities, (b) CA activities and (c) XO activities between MF patients and healthy volunteers, respectively.
MF is the most common type of cutaneous lymphomas in which lymphocytes become malignant and affect the skinCitation19. MF may move through patch, plaque and tumor phaseCitation2. Sometimes it may be difficult to diagnose accurately in the early stages because early lesions are frequently confused with other dermatoses. Multiple skin biopsies, peripheral blood smear, complete blood count are some of the most common tests that are used to detect and diagnose MF. In some situations, those enzyme activity tests may be inadequate to make the correct diagnosis for these patients.
In this study, we analyzed CA, CAT and XO levels in patients with MF. Additionally, we aimed to determine the relationship between these enzyme activities and stage of the disease. To the best of our knowledge, no other studies have examined cytosolic CAs, CAT and XO levels in patients with MF.
XO, which catalyzes the oxidation of xanthine to uric acid, contributes to the generation of ROS. According to the results of Samra et al., an elevated level of XO activity was observed in cancer patients. Among different cancers, comparatively high level of XO activity was measured in acute lymphoblastic lymphoma patients except cervix cancer patients, where a low level was observedCitation20. There was no other study determining XO activity in patients with MF. We didn't get any significant differences in XO activities between patient and control group. According to the results of our study, we consider that XO does not play a crucial role in the pathogenesis of MF.
CAT is usually located in peroxisomesCitation21 and protects cells against the enormous deposits of ROS. CAT prevents the accumulation of H2O2 by catalyzing the rapid decomposition of H2O2 to O2 and H2O and it is one of the most important antioxidant enzymes. Although there are several reports that CAT activity is suppressed in cancerous tissuesCitation22–25, some authors found unchanged or increased activity in some tumor tissuesCitation26,Citation27. Durak et al.Citation22 found decreased CAT activity in cancerous bladder tissues. Corrocher et al.Citation25 reported decreased CAT activity in human hepatoma. Several studies have shown decreased CAT levels in patients with lung cancerCitation23,Citation24. On the other hand, there is a study about the relationship between the antioxidant system and acute leukemiasCitation28. According to their results, CAT activity was found to be significantly decreased in acute leukemias as compared with the control group. In another study, Ozensoy et al.Citation14 also investigated whether cytosolic CAs and CAT enzyme activities were altered in patients who have carcinoma. The group was determined as the mean (±SD) CAT activities did not differ significantly,that is, 0.0035 (±0.0015) EU/mL in carcinoma patients versus 0.0031 (±0.00075) EU/mL in controls. However, mean carbonic anhydrase activities of 204 (±91) EU/mL in the carcinoma patients were significantly higher than the 158 (±35) EU/mL in controls (p value of 0.0065)Citation14.
There is a lack of studies about the levels of antioxidant enzymes on MF patients. In this study, we observed that serum CAT levels were significantly lower in patients with early stages of MF than in healthy controls. Our results suggest a possible association between reduced CAT activity, an antioxidant enzyme as a result of elevated free radicals. We consider that increased free radicals and oxidative stress may also play a key role in the pathogenesis of MF within the our study results of CAT.
CAs are involved in physiological and pathological functions such as pH and CO2 homeostasis. The primary function of the enzyme in living organisms is to interconvert carbon dioxide and bicarbonate to maintain acid–base balance in blood and other tissues and to help transport carbon dioxide out of tissues. The two major CA isozymes (hCAI and hCAII) are present at high concentrations in the cytosol of erythrocytes, while others are widely distributedCitation28. It has been recently demonstrated that carbonic anhydrase IX (CA IX), a hypoxia-inducible member of the carbonic anhydrase family, is associated with aggressive tumor phenotype and a poor prognosis. Studies have demonstrated that high CA IX expression yields in many cancer types and leads to acidification of extracellular environment surrounding the cancer cellsCitation10.
In another study, it was shown that there was no significant difference in CA activity between esophageal cancer and control groupCitation29. In another study, it was reported that erythrocyte CA activity was significantly lower in patients with lung cancer compared with controlsCitation23. According to the results of Demir et al., erythrocyte CA activity was significantly increased in acute leukemia group compared with the control groupCitation28. However, there is no information regarding CA activity in patients with MF in the literature. CA activity increases as a result of tumor hypoxia and it indicates failure in therapy and poor prognosis. It was shown that hypoxic tumors are associated with poor prognosis, metastasisCitation30. Our patients used in this study are in the early phase of MF disease, as a consequence they have decreased levels of cytosolic CAs. MF is usually curable in the early stages of disease, there may be a possible link between non-hypoxic MF and its resistance to metastasis and better prognosis than hypoxic tumors.
We observed that CA and CAT levels were significantly lower in patients with MF than in healthy controls. Besides, there was no correlation between the activities of those enzymes and age, gender, duration, stage of the disease and involvement of body area. Decreased CA and CAT activities may play a role in the pathogenesis of MF. We did not find any statistical significant difference in patients with various stages of disease. The activities of those enzymes may be beneficial to distinguish early stage of MF from other dermatoses.
Conclusions
Cytokines are considered to be of major importance for the pathogenesis of MF. Investigations of cytokine production in skin lesions or peripheral blood lymphocytes from patient with cutaneous T-cell lymphomas led to the general concept that a shift in cytokine profile from type Th1 to Th2 accompanies disease progressionCitation31,Citation32. In the literature, Vacca et al. demonstrated a study about progression of MF, which is associated with changes in angiogenesis and expression of metalloproteinases 2 and 9 (MMP 2 and 9) and their in situ data show that angiogenesis and overexpression of MMP-2 and MMP-9 mRNAs occur simultaneously during MF clinical progression. This suggests more opportunities for MF cells to disseminate locally and to enter the circulation and spread systemically in parallel with tumor progressionCitation33. That is why this kind of studies on MF-related enzymology where it has caused alterations in circulating human proteins or enzymes such as metalloproteinases has leaded our study also. For this purpose, our study is the first attempt for investigating CA, CAT and XO enzyme activities in patients with MF. Therefore, we believe that CA and CAT levels may be an informative diagnostic marker for MF particularly in patients with early stage. Further investigations in a larger cohort of patients with MF are needed to provide definitive data about the prognostic role of CA, CAT and XO enzyme activities. Therapy with anti-angiogenic agents and/or tissue inhibitors of these enzymes can be envisaged as possible future development.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplementary material available online
SUPPLEMENTAL
Download PDF (22.1 KB)Acknowledgements
The authors thank Yildirim Beyazit University Ataturk Research and Training Hospital and Diskapi Yildirim Beyazit Training and Research Hospital Ankara/Turkey for helping us in research considerations; We also thank Balikesir University Science and Art Faculty Department of Chemistry Biochemistry division laboratory authorities for their invaluable support.
References
- Cerroni L, Gatter K, Helmut K. An illustrated guide to Skin Lymphomas. Massachusetts: Blackwell Publishing Malden; 2005: 13
- Edelson RL. Cutaneous T cell lymphoma. The helping hand of dendritic cells. Ann NY Acad Sci 2001;941:1–11
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768–85
- van Doorn R, van Kester MS, Dijkman R, et al. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood 2009;113:127–36
- Morales-Suarez-Varela MM, Olsen J, Johansen P, et al. Occupational risk factors for mycosis fungoides: a European multicenter case-control study. J Occup Environ Med 2004;46:205–11
- Shelley Walter B. Familial mycosis fungoides revisited. Arch Dermatol 1980;116:1177–8
- Liang SL, Chan DW. Enzymes and related proteins as cancer biomarkers: a proteomic approach. Clin Chim Acta 2007;381:93–7
- Sakalova A, Bock PR, Dedik L, et al. Retrolective cohort study of an additive therapy with an oral enzyme preparation in patients with multiple myeloma. Cancer Chemother Pharmacol 2001;47:38–44
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators Nat Rev Drug Discov 2008;7:168–81
- Ozensoy Guler O, Arslan O, Kockar F. Differential in vitro inhibitory effects of anticancer drugs on tumor-associated carbonic anhydrase isozymes CA IX and CA XII Methods Find. Exp Clin Pharmacol 2008;30:335–40
- Zelen I, Djurdjevic P, Popovic S, et al. Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. J Buon 2010;15:330–6
- Haliwell B, Gutteridge J. Oxygen is poisonous: an introduction to oxygen toxicity and free radicals. In: Halliwell B, Gutteridge J, eds. Free radicals in biology and medicine. Oxford: Clarendon Press; 1989:1–21
- Ardan T, Kovaceva J, Cejková J. Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium. Acta Histochem 2004;106:69–75
- Ozensoy O, Kockar F, Arslan O, et al. An evaluation of cytosolic erythrocyte carbonic anhydrase and catalase in carcinoma patients: an elevation of carbonic anhydrase activity. Clin Biochem 2006;39:804–9
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1976;176:147–51
- Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of enzymatic analysis. 3rd ed. Weinheim, Germany: Verlag Chemie; 1983:273–86
- Roussos GG. Xanthine oxidase from bovine small intestine. In: Grossman L, Moldave K, eds. Methods in enzymology. New York (NY): Academic Press; 1967: XII A: 5–16
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med 1997;23:134–47
- Hallermann C, Niermann C, Fischer RJ, Schulze HJ. Survival data for 299 patients with primary cutaneous lymphomas: a monocentre study. Acta Derm Venereol 2011;91:521–5
- Samra ZQ, Pervaiz S, Shaheen S, et al. Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase 1) enzymes and lipid parameters in different cancer patients. Clin Lab 2011;57:741–7
- Alberts B, Johnson A, Lewis J, et al. Peroxisomes. In: Molecular biology of the cell. 4th ed. New York: Garland Science; 2002: Chapter 12. Available from: http://www.ncbi.nlm.nih.gov/books/NBK26858/
- Durak I, Perk H, Kavutcu M, et al. Adenosine deaminase, 5′nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human bladder tissues. Free Radic Biol Med 1994;16:825–31
- Cobanoglu U, Demir H, Duran M, et al. Erythrocyte catalase and carbonic anhydrase activities in lung cancer. Asian Pac J Cancer Prev 2010;11:1377–82
- Korotkina RN, Matskevich GN, Devlikanova A, et al. Activity of glutathione-metabolizing and antioxidant enzymes in malignant and benign tumors of human lungs. Bull Exp Biol Med 2002;133:606–8
- Corrocher R, Casaril M, Bellisola G, et al. Severe impairment of antioxidant system in human hepatoma. Cancer 1986;58:1658–62
- Nakada T, Akiya T, Koike H, Katayama T. Superoxide dismutase activity in renal cell carcinoma. Eur Urol 1988;14:50–5
- Bayraktar N, Kilic S, Bayraktar MR, Aksoy N. Lipid peroxidation and antioxidant enzyme activities in cancerous bladder tissue and their relation with bacterial infection: a controlled clinical study. J Clin Lab Anal 2010;24:25–30
- Demir C, Demir H, Esen R, et al. Erythrocyte catalase and carbonic anhydrase activities in acute leukemias. Asian Pac J Cancer Prev 2010;11:247–50
- Demir H, Akkus ZA, Cebi A, et al. Catalase, carbonic anhydrase and other biochemical parameters in esophageal cancers in Turkey. Asian Pac J Cancer Prev 2010;11:1029–32
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Vowels BR, Cassin M, Vonderheid EC, et al. Aberrant cytokin produktion by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol 1992;99:90–4
- Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T cell lymphoma. J Invest Dermatol 1994;103:669–71
- Vacca A, Moretti S, Ribatti D, et al. Progression of mycosis fungoides is associated with changes in angiogenesis and expression of the matrix metalloproteinases 2 and 9. Eur J Cancer 1997;33:1685–92

