Abstract
The new affinity gel reported in this study was prepared using EUPERGIT C250L as a chromatographic bed material, to which etylenediamine spacer arms were attached to prevent steric hindrance between the matrix and ligand, and to facilitate effective binding of the CA-specific ligand, of the aromatic sulfonamide type for the purification of α-carbonic anhydrases (Cas; EC 4.2.1.1). Indeed, the aminoethyl moieties of the affinity gel were derivatized by reaction with 4-isothiocyanatobenzenesulfonamide, with the formation of a thiourea-based gel, having inhibitory effects against CAs. Both bovine erythrocyte carbonic anhydrase BCA and human (h) erythrocyte CA isoforms I, II (hCA I and II) have been purified from hemolysates, by using this affinity gel. The greatest purification fold and column yields for BCA and for cytosolic (hCA I + II) enzymes were of 181-fold (21.07%) and 184-fold (9.49%), respectively. Maximum binding was achieved at 15 °C and I = 0.3 ionic strength for α-carbonic anhydrases.
Introduction
Carbonic anhydrases (CAs; EC 4.2.1.1) are ubiquitously distributed zinc containing metalloenzymes with their involvement in diverse pathological conditions, carbonic anhydrases have been the targets of drug developments for the treatments of glaucoma, epilepsy, high altitude sickness, as well as cancerCitation1. These enzymes are involved in the pH homeostasis, ion transport, water and electrolyte balance, bone resorption, calcification and tumorigenesis due to this kind of crucial physiological processes in metabolism is catalyzing the reversible hydration of CO2 to form , which is involved in a variety of biosynthetic reactions, including gluconeogenesis, synthesis of certain amino acids (via pyruvate carboxylase), lipogenesis (via acetyl-CoA carboxylase), ureagenesis (via carbamoyl phosphate synthetase I) and pyrimidine nucleotide biosynthesis (via carbamoyl phosphate synthetase-II)Citation2,Citation3.
Several inhibitors of human carbonic anhydrases have been utilized as antiglaucoma, anticonvulsant and antiepileptic, antiurolithic and anticancer agents, and a few such inhibitors have been approved as drugsCitation4–8. Most of these drugs (acetazolamide, dorzolamide, brinzolamide, etc.) are sulfonamide derivatives and they are currently used for the treatment of acute glaucomaCitation9,Citation10.
Affinity chromatography is a widely used technique for the rapid purification of proteins, provided that suitable ligands with high affinity and selectivity for the target protein based on a highly specific interaction such as that between antigen and antibody, enzyme and substrate, or receptor and ligandCitation11. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand coupled to a chromatography matrix. The technique is ideal for a capture or intermediate step in a purification protocol and can be used whenever a suitable ligand is available for the protein(s) of interest. With high selectivity, hence high resolution, and high capacity for the protein(s) of interest, purification levels in the order of several thousand-fold with high recovery of active material are achievable. The target protein is collected in a purified, concentrated formCitation12.
Biological interactions between ligand and target molecule can be a result of electrostatic or hydrophobic interactions, van der Waals’ forces and/or hydrogen bonding. To elute the target molecule from the affinity medium the interaction can be reversed, either specifically using a competitive ligand, or non-specifically, by changing the pH, ionic strength or polarityCitation13.
Carbonic anhydrases, including hCA II, are usually purified via the agarose–sulfonamide affinity column chromatographic procedureCitation14–16. However, Fierke’s group has purified the recombinant form of hCA II and its several site-specific mutations by a combination of ammonium sulfate precipitation, S-Sepharose, Sephacryl S-100 and hydroxylapatite column chromatographic methodsCitation17. In the literature, there are several methods available for purication CA isozymes with different chromatographic techniques, but most of them show different limitationsCitation18–21.
Oviya et al. used a combination of Sephadex G-75 and DEAE cellulose column chromatography, resulting in 4.64-fold purification of CACitation20. According to the results from these studies, it is seen that a multi-step purification of CA isozymes is time consuming and also having the low yields of enzyme; hence, we aim to use affinity chromatographic technique for preventing time consuming and getting the high yields purifed enzyme with a single step in mild conditions. So we synthesized an original affinity gel for purifying α-CA isozymes (e.g. hCA1, hCA II, BCA), which has a chemical structure of Eupergit C-250 L-ethylenediamine-thioureido-benzenesulfonamide.
The commercial products EUPERGIT C and EUPERGIT C250L have been used for a wide variety of different enzymes and reactionsCitation22,Citation23. Both EUPERGIT C and EUPERGIT C 250 L are microporous, epoxy-activated acrylic beads with a diameter of 100–250 µm. The reactive oxirane groups that Eupergit C 250 L contains, provide the binding of many ligands to the matrix in mild conditions. The main reasons for selecting Eupergit C 250 L polymer as a matrix in the study is carrying out the reaction under mild conditions with high density of reactive oxirane groups. Ozensoy’s group previously had also been synthesized a new affinity gel with Eupergit C 250 L used as a matrixCitation12, but in their study, no spacer arm was used.
Materials and methods
Chemicals
Eupergit®C 250 L was kindly donated by Degussa Rohm Pharma Polymers (Wiesbaden, Germany). 4-İsocyanatobenzenesulfonamide was originally synthesized as described earlierCitation8. Protein assay reagents were obtained from Sigma-Aldrich Co (Milan, Italy). All other chemicals were of analytical grade and obtained from Merck Chem Co. (Milan, Italy). Bovine blood samples were obtained from Balikesir Slaughter House in Balikesir/Turkey and human blood samples were obtained from healthy volunteers of Balikesir University. The study protocol was approved by the local human ethics committee in Balikesir University.
Preparation of affinity gel
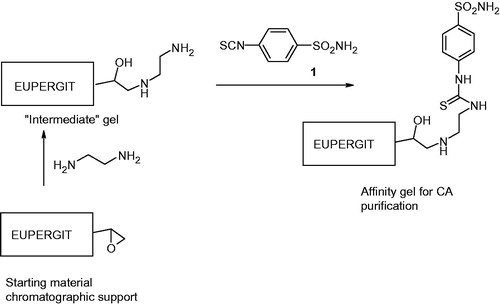
One gram of Eupergit C 250 L was suspended with 100 mL of phosphate buffer (pH 7.5) for 72 h in a shaker at 24 °C. After the incubation of the matrix, 7 mL ethylenediamine is added to the suspension for another incubation for 24 h. Then 25 mg of 4-isothiocyanatobenzenesulfonamide (1) was dissolved in 5 mL of dimetylsulphoxide at 0 °C. After the second incubation of the suspension with ethylenediamine, the sulfonamide solution was added to this suspension and the pH of the reaction was stabilized at pH 7.5. This final solution was mixed for 3 h at room temperature. The suspension was washed two times with 1 L cold distilled water and 0.05 M Tris-SO4 (pH = 7.5) buffer solution and after the washing the final suspension was stored in a buffer solution containing 0.05 M Tris-SO4 (pH = 7.5)Citation12,Citation24.
Enzyme purification protocol
Bovine blood samples were obtained from Balikesir Slaughter’s house in Balikesir/Turkey, human blood samples were ethically taken from Balikesir University healty volunteers. The tissue samples were centrifuged at 17 664g for 30 min and the plasma and precipitate were removed. The pH of the homogenate was adjusted to 7.5 with solid Tris. The homogenate was applied to the prepared Eupergit C 250 L ethylenediamine-4-isocyanatobenzenesulfonamide affinity column equilibrated with 10 mM Tris–HCl/0.1 M Na2SO4 (pH 7.5). The affinity gel was washed with 10 mM Tris–HCl/22 mM Na2SO4 (pH 7.5). The CA enzyme was eluted with 1.2 M NaCl/25 mM Na2HPO4 (pH 6.3). All procedures were performed at 4 °CCitation12.
Hydratase activity assay of CA
CA activity was assayed by following the hydration of CO2 according to the method described byWilbur and AndersonCitation25. CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation (t0 − tc/tc) where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively.
SDS polyacrylamide gel electrophoresis
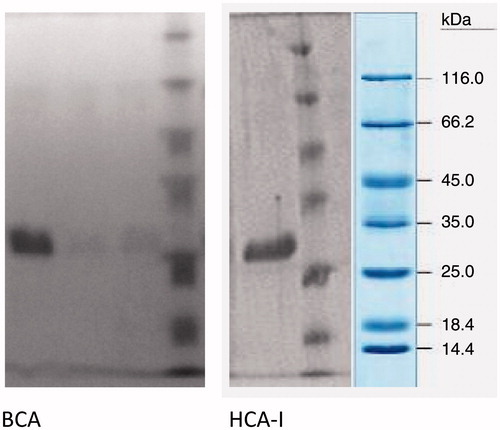
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10 and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to the Laemmli procedure. A 20 µg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dyeCitation26.
Determination of optimum pH, temperature and ionic strength of the new affinity gel
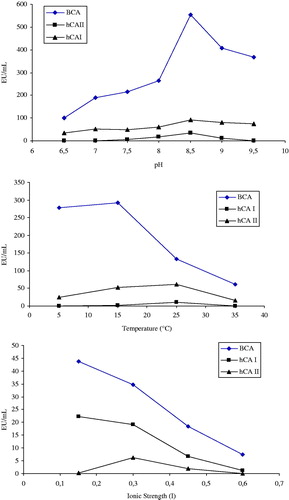
For the optimum temperature, enzyme activity was assayed at different temperatures in the range from 5 °C to 35 °C (). The desired temperature was provided by using a Grant bath. For determination of optimum ionic strength, enzyme activity was determined using different concentrations of Na-phosphate buffer, pH: 7.5, in the range from I = 0.3 to I = 0.7 and also at different pH ranges (6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5). According to the column properties, the maximum binding was achieved for CA isozymes as pH 7.5, 15 °C and the ionic strength was I = 0.3 ()Citation12.
Results and discussion
Given the rapidity in synthesis of new class of CA inhibitors, there is an emerging need for rapid production of the enzyme in high quantities for high throughput screening. Because of the rapid demanding for purifying these enzymes, in this current study, we aim to design a new affinity gel for purifying the CA enzyme from bovine and human erythrocytes by using affinity chromatograhic technique. For an even higher degree of purity, or when there is no suitable ligand for affinity purification, an efficient multi-step process must be developed using the purification strategy interest. That is why we synthesized Eupergit C 250 L ethylenediamine-4-isothiocyanatobenzenesulfonamide affinity gel by modification of washing and elution conditions for purifying the CAs (Scheme 1).
In the literature, first attempts for purifying CAs, such as hCA I and II by affinity chromatography have been described by Falkbring et al.Citation27 and WhitneyCitation28. In those procedures, the affinity gels were obtained by coupling p-aminobenzenesulfonamide and p-(aminomethyl)benzenesulfonamide, respectively to Sepharose polysaccharides by means of cyanogen bromide activation reactions. These types of matrixes were selected by the researchers because of their fluidity but using such compounds for the affinity gels involve the employment of the toxic and dangerous reagent cyanogen bromide. In our study, we did not do an activation for the matrix since the matrix of our gel Eupergit C 250 L has reactive oxirane groups that are able to react easily with nucleophiles, such as ethilenediamine (Scheme 1) without any activation being necessary. Ozensoy’s group had used this matrix also for purifying CAs without an activation stepCitation12, with good purification yieldsCitation14,Citation16,Citation27,Citation28. Eupergit C 250 L was used as a matrix in this study due to its high chemical and mechanical stability. For avoiding the steric hindrance ethylenediamine was used as a spacer arm between the matrix and the sulfonamide ligand. For affinity gel synthesis spacer arms were already reported in the literatureCitation29–31.
Various methods are used to enrich or purify a protein of interest from other proteins and components in a crude cell lysate or other sample using chromatographyCitation32. The most powerful of these methods is affinity chromatography, whereby the protein of interest is purified by virtue of its specific binding properties to an immobilized ligand. In this study, 4-isocyanatebenzenesulfonamide was used as a ligand, as this is an effective CA inhibitor (CAI) being reported earlier by one of our groupsCitation8. In this study, CAIs with enhanced water solubility and good affinity for the enzyme were obtained, which also showed efficacity for the treament of glaucoma and ocular hypertensionCitation8. Compound 1 showed Ki values of 500 nM for hCA I, of 185 nM for hCA II and of 300 nM for hCA IV, respectivelyCitation8. The thioureas obtained by reacting one with amines, amino acids, etc., were even more effective inhibitors of various α-CAs, such as hCA I, II, IV, etcCitation8.
Experiments for optimization of conditions in which the new affinity gel works better, such as the pH, ionic strength and temperature, were also performed ( and ). The maximum binding conditions of the new affinity gel have been determined for each CA isozyme, working at 15 °C, pH 8.5 and I = 0.3 ionic strength. The best elution buffers were 0.1 M CH3COONa/0.5%M NaClO4 (pH 5.6) for BCA and hCA I; and 0.1 M NaCl/25 mM Na2HPO4 (pH 6.3) for hCA II. The purification of each CA has been controlled by SDS-polyacrylamide gel electrophoresis. The highest purification fold and column yields for BCA and for total cytosolic (hCAI + hCA-II) enzymes were of 181-fold (21.07%) and 184-fold (9.49%), respectively. These values are very similar to those reported by other researchersCitation12,Citation24,Citation30. The capacity of the affinity gel synthesized here was higher than that reported for other such affinity gelsCitation27,Citation28. According to the findings of this study, the Eupergit C 250 L ethylenediamine-thioureido-benzenesulfonamide affinity gel is quite useful for the purification of α-CAs, such as hCA I, II and BCA in active form.
Conclusions
In this study, we used Eupergit C 250 L, a copolymer of metacyrlamide and N-N′-bis-methylene (metacrylamide), which incorporates reactive oxirane groups, for obtaining a new affinity gel for the purtification of α-CAs. For avoiding the steric hindrance between the CA ligand and the solid matrix, etylenediamine was used to derivatize the oxirane groups present on Eupergit C 250 L. The terminal primary amine moiety of the derivatized gel was reacted in mild conditions with 4-isothiocyanatobenzenesulfonamide, leading to a gel on which the ligand for CAs recognition has been attached through thioureido functionalities. The new gel was highly efficient for purifying α-Cas, such as hCA I, II and BCA. We found out that the maximum binding of these CAs led to a 21.07% yield, 181 purification fold for BCA, and of 9.49% yield, 184 purification fold for hCA I + hCA II, respectively (). The best elution buffers were selected as 0.1 M CH3COONa/0.5 M NaClO4, pH = 5.6, and 20 mM Na2HPO4/1 M NaCl, pH = 6.3 for the elution of BCA and hCA I + II isoenzymes, respectively. Maximum binding was determined at 15 °C and the ionic strength was indicated as I = 0.3 (). These findings demonstrate that the procedure used in this study is a simple, yet quite effective and successful method for the isolation of α-CAs, by taking advantage of their good affinity for thioureido-benzenesulfonamide ligands. The column may be used for one-step purification of CA isozymes in mild conditions. Therefore, the novel affinity thioureido-benzenesulfonamide column has the potential application for further analysis and purification of different CAs, maybe belonging to other genetic classes, such as the β-, δ-, γ- and ζ-CAs.
Table 1. Purification table of CA isozymes.
Declaration of interest
The authors on this study report no declaration of interest.
Acknowledgements
The authors would like to thank to BURCAS Balikesir University, Research Center of Applied Sciences (BURCAS/Balikesir, Turkey) for providing the research facilities.
References
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Bertucci A, Moya A, Tambutté S, et al. Carbonic anhydrases in anthozoan corals – a review. Bioorg Med Chem 2013;21:1437–50
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Supuran CT. Carbonic anmhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Bozdag M, Ferraroni M, Nuti E, et al. Combining the tail and the ring approaches for obtaining potent and isoform-selective carbonic anhydrase inhibitors: solution and X-ray crystallographic studies. Bioorg Med Chem 2014;22:334–40
- Zołnowska B, Sławiński J, Pogorzelska A, et al. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;71:135–47
- Ceruso M, Vullo D, Scozzafava A, Supuran CT. Inhibition of human carbonic anhydrase isoforms I-XIV with sulfonamides incorporating fluorine and 1,3,5-triazine moieties. Bioorg Med Chem 2013;21:6929–36
- Casini A, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors: water soluble 4-sulfamoylphenylthioureas as topical intraocular pressure lowering agents with long lasting effects. J Med Chem 2000;43:4884–92
- Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem 2014;75:349–59
- Carta F, Akdemir A, Scozzafava A, et al. Xanthates and trithiocarbonates strongly inhibit carbonic anhydrases and show antiglaucoma effects in vivo. J Med Chem 2013;56:4691–700
- Hage DS, Anguizola JA, Bi C, et al. Pharmaceutical and biomedical applications of affinity chromatography: recent trends and developments. J Pharm Biomed Anal 2012;69:93–105
- Ozensoy O, Arslan O, Oznur S. A new method for purification carbonic anhydrase by affinity chromatography. Biochemistry (Moscow) 2004;69:216–19
- Wilson K, Walker J. Principles and techniques of biochemistry and molecular biology. 7th ed. University of Hertfordshire, Cambridge ebook; 2010
- Bering CL, Kuhns JJ, Rowlett R. Purification of bovine carbonic anhydrase by affinity chromatography. J Chem Edu 1998;75:1021–4
- Feldstein JB, Silverman DN. Purification and characterization of carbonic anhydrase from the saliva of rat. J Biol Chem 1984;259:5447–53
- Ulmasov B, Waheed A, Shah GN, et al. Purification and kinetic analysis of recombinant CA XII, a membrane carbonic anhydrase overexpressed in certain cancers. Proc Natl Acad Sci USA 2000;97:14212–17
- Nair SK, Calderone TL, Christianson DW, Fierke CA. Altering the mouth of a hydrophobic pocket. J Biol Chem 1991;266:17320–5
- Toshiho N, Tomita Y, Yorifuji D, et al. Purification of chicken carbonic anhydrase isozyme-III (CA-III) and its measurement in White Leghorn chickens. Acta Veterinaria Scand 2011;53:63–73
- Zoë Fisher S, Tariku I, Case NM, et al. Expression, purification, kinetic, and structural characterization of an α-class carbonic anhydrase from Aedes aegypti (AaCA1). Biochim Biophys Acta 2006;1764:1413–19
- Oviya M, Sukumaran V, Giri SS. Immobilization and characterization of carbonic anhydrase purified from E. coli MO1 and its influence on CO2 sequestration. World J Microbiol Biotechnol 2013;29:1813–20
- Modak JK1, Revitt-Mills SA, Roujeinikova A. Cloning, purification and preliminary crystallographic analysis of the complex of Helicobacter pylori α-carbonic anhydrase with acetazolamide. Acta Crystallogr Sect F Struct Biol Cryst Commun 2013;69:1252–5
- Gómez de Segura A, Alcalde M, Yates M, et al. Immobilization of dextransucrase from Leuconostoc mesenteroides NRRL B-512F on Eupergit C supports. Biotechnol Prog 2004;20:1414–20
- Bezbradica DI, Ćorović JJ, Prodanović RM, et al. Covalent immobilization of lipase from Candida rugosa on Eupergit®. Acta Periodica Technologica 2005;36:179–86
- Bayram T, Arslan O, Ugras Hİ, et al. Purification of carbonic anhydrase from dog erytrocytes and investigation of inhibition kinetics. J Enzyme Inhib Med Chem 2007;22:739–44
- Wilbur KM, Anderson NG. Electrometric and colorometric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54
- Laemelli DK. Cleavege of structural ptoteins during in assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Falkbring SO, Göthe PO, Nyman L, Parath J. Affinity chromatography of carbonic anhydrase. FEBS Lett 1972;24:229–35
- Whitney PI. Affinity chromatography of carbonic anhydrase. Anal Biochem 1974;57:467–76
- Wistrand PJ. The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiol Scand 1981;113:417–26
- Demir N, Demir Y, Coşkun F. Purification and characterization of carbonic anhydrase from human erythrocyte plasma membrane. Turk J Med Sci 2001;31:477–82
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Patents 2000;10:575–600
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th ed. New York: W H Freeman Press; 2002