Abstract
Human serum paraoxonase (PON1, EC 3.1.8.1.) is a high-density lipid (HDL)-associated, calcium-dependent enzyme. In this study, the effects of Haloperidol, Fluoxetine hydrochloride, Diazepam and Acepromazine drugs used for the therapy of antidepressant and antipsychotic diseases, on paraoxonase enzyme activity was studied in in vitro inhibition studies on purified human serum PON1. PON1 enzyme was purified from human blood using two-step procedures, namely, ammonium sulfate precipitation and sepharose-4B-l-tyrosine-1-napthylamine hydrophobic interaction chromatography. The overall purification of human serum PON1 was obtained in a activity of 109.29 U/mL and this enzyme was purified 125-fold. The SDS–polyacrylamide gel electrophoresis of the enzyme indicates a single band with an apparent MW of 43 kDa. Inhibition studies indicated that haloperidol and fluoxetine hydrocloride were effective inhibitors on purified human serum PON1 activity with IC50 of 0.187 and 3.08 mM values, respectively. The kinetics of interaction of haloperidol and fluoxetine hydrocloride with the purified human serum PON1 indicated uncompetitive inhibiton pattern with Ki of 4.15 and 0.007 mM, respectively.
Introduction
Paraoxonase (PON) (aryldialkyl phosphatase, E.C.3.1.8.1) is a calcium dependent serum esterase. The human paraoxonase (PON) gene family consists of three members, PON1, PON2 and PON3Citation1. The three PON genes show a high similarity at the amino acid level between the mammalian species PON1 and PON3, which are expressed primarily in the liver. In contrast, PON2 is widely expressed in a number of tissues including brain, liver, kidney and testis and it may have multiple mRNA formsCitation2,Citation3.
PON1 is synthesized primarily in the liver and a portion is secreted into the plasma. PON1 is associated with high-density lipoproteins (HDL)Citation4,Citation5. Therefore, PON1’s primary physiological role is to protect low-density lipoproteins. Purified PON1 has a molecular mass of 43–45 kDa and contains as many as three carbohydrate chains accounting for 15.8% of the weightCitation6. Unlike PON2 and PON3, PON1 is an efficient esterase towards many OP (organophosphate) compounds including paraoxon, the insecticides parathion and chlorpyriphos as well as the nerve agents sarin and soman. PON1 received its name from paraoxon, the toxic metabolite of the insecticide parathion, which is one of its most studied substratesCitation1,Citation7–9. In addition, PON1 was shown to hydrolyze over 30 lactones and cyclic carbonate esters including drugs and endogenous compoundsCitation5,Citation10–12.
In addition to its important roles in a broad range of fields, PON1 has been shown to play a critical role in the metabolism of pharmaceutical drugsCitation2. PON1 activity is completely dependent upon calcium, and EDTA irreversibly abolishes its activity. Other cations, however, have been shown to have an inhibitory effect on PON1 activity. Barium, lanthanum, copper, zinc and mercurials were found to inhibit PON1 activity from rat or human liverCitation13. Most studies on the modulation of PON1 by pharmaceutical compounds have focused on lipid-lowering compoundsCitation14. Since paraoxonase enzyme (PON1) is a bioscavenger against both atherosclerosis and organophosphate toxicity, studies on paraoxonase enzyme (PON) occupy an important place in the scientific world. Due to these vital factors, we believe that further studies on PON–drug interactions are needed. In our previous study, we reported that some drugs differentially affect the PON1 enzyme activity from human serum in vitroCitation15–17. In this study, the effects of several types of antidepressant (Fluoxetine hydrochloride and Acepromazine) and antipsychotic (Haloperidol and Diazepam) drugs have been investigated on paraoxonase 1 enzyme activity purified from human blood (). This study will be the first study on the effect of antidepressant and antipsychotic drugs on paraoxonase enzyme activities.
Methods
Materials
Sepharose 4B, l-tyrosine, 1-napthylamine, protein assay reagents and chemicals for electrophoresis and all other chemicals used were of analytical grade and obtained from either Sigma (St. Louis, MO) or Merck (Darmstadt, Germany). Medical drugs were provided by the local pharmacy.
Purification of paraoxonase from human serum by hydrophobic interaction chromatography
Human serum was isolated from 35 mL fresh human blood and put into a dry tube. The blood samples were centrifuged at 1500 rpm for 15 min and the serum was removed. First, serum paraoxonase was isolated by ammonium sulfate precipitation (60–80%). The precipitate was collected by centrifugation at 15 000 rpm for 20 min, and redissolved in 100 mM Tris–HCl buffer (pH 8.0). Next, we synthesized the hydrophobic gel, including Sepharose 4B, l-tyrosine and 1-napthylamine, for the purification of human serum paraoxonase). The column was equilibrated with 0.1 M of a Na2HPO4 buffer (pH 8.00) including 1 M ammonium sulfate. The paraoxonase was eluted with an ammonium sulfate gradient using 0.1 M Na2HPO4 buffer with and without ammonium sulfate (pH 8.00). The purified PON1 enzyme was stored in the presence of 2 mM calcium chloride to maintain activity.
Total protein determination
The absorbance at 280 nm was used to monitor the protein in the column effluents and ammonium sulfate precipitation. Quantitative protein determination was achieved by absorbance measurements at 595 nm according to the Bradford method with bovine serum albumin as a standard.
SDS polyacrylamide gel electrophoresis
SDS polyacrilamide gel electrophoresis was performed after purification of the enzyme. It was carried out in 10 and 3% acrylamide concentration for the running and stacking gel, respectively, containing 0.1% SDS according to LaemmliCitation18. A 20 mg sample was applied to the electrophoresis medium. Gel was stained overnight in 0.1% Coomassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained by frequently changing the same solvent, without dye. The electrophoretic pattern was photographed with the system of produce as an image of the gel.
Paraoxonase enzyme assay
Paraoxonase enzyme activity towards paraoxon was quantified spectrophotometrically by the method described by Gan et al.Citation6. The reaction was monitored for 2 min at 37 °C by monitoring the appearance of p-nitrophenol at 412 nm in a Biotek automated recording spectrophotometer. The final substrate concentration during enzyme assay was 2 mM, and all rates were determined in duplicate and corrected for the non-enzymatic hydrolysis.
In vitro inhibition kinetic studies and determination of Ki values
For the inhibition studies of Haloperidol, Fluoxetine hydrochloride, Diazepam and Acepromazine, different concentrations of each medical drugs were added to the enzyme activity. Paraoxonase activity with medical drugs was assayed by following the hydration of paraoxon. Activity % values of paraoxonase for five different concentrations of each medical drug were determined by regression analysis using Microsoft Office 2003 Excel. Paraoxonase activity without a medical drug was accepted as 100% activity. For the drugs having an inhibition effect, the inhibitor concentration causing up to 50% inhibition (IC50 values) was determined from the graphs.
Results
To investigate the effect of these drugs on PON1 enzyme activity in vitro, human serum paraoxonase was purified by ammonium sulfate precipitation at 60–80% intervals, and subjected to hydrophobic interaction chromatography. The gel for hydrophobic interaction chromatography was synthesized using Sepharose 4B, l-tyrosine and 1-napthylamine. The overall purification of human serum PON1 was obtained in a activity of 109.29 U/mL and this enzyme was purified 125-fold (). The purity of the enzyme was confirmed by SDS-PAGE. As shown in , a single band, 43 kDa, was obtained, which corresponds to the results of previous studiesCitation16. As shown in , Fluoxetine hydrochloride and Haloperidol in vitro, inhibited the human serum PON1 activity as seen in (). However, Diazepam and Acepromazine did not show any inhibition effect on PON1 activity (). In addition, the kinetics of the interaction of well-inhibited drugs, Fluoxetine hydrochloride and Haloperidol with the purified human serum PON1 was also studied. The Lineweaver–Burk double-reciprocal graph was plotted using a range of paraoxon concentrations (0.4–8.4 mM) in the absence or presence of each drug. KM and Vmax values were determined by means of these graphs. The Ki value was calculated by the method of Dixon plots, which provides a simple way of determining the inhibition constant. The kinetic data indicate that the inhibition of paraoxonase activity by Fluoxetine hydrochloride and Haloperidol was of the uncompetitive type ( and ).
Figure 2. SDS-PAGE of PON1 purified by ammonium sulfate precipitation and hydrophobic interaction chromatography gel serum hPON1 was purified with ammonium sulfate precipitation (60–80%) and hydrophobic interaction chromatography. Lane 1, a pooled sample obtained from a column showing paraoxonase enzyme activity. Lane 2 contains β-galactosidase (116 kDa), bovine serum albumin (66.0 kDa), ovalbumin (45 kDa), Carbonic Anhydrase (33.0 kDa), β-lactogloulin (25.0 kDa), lysozyme (19.5 kDa) protein marker. The molecular weight of PON1 was estimated to be approximately 43 kDa.
Figure 3. Inhibition of paraoxonase hydrolysis by Acepromazine (A), Diazepam (B), Fluoxetine hydrochloride (C), and Haloperidol (D). A purified paraoxonase from human serum was assayed for paraoxonase activity at pH 8 in the presence of various concentrations of Haloperidol, Fluoxetine hydrochloride, Diazepam and Acepromazine. IC50 values of these were determined from C and D. The slope of Lineweaver-Burk plots indicates uncompetitive for Fluoxetine hydrochloride (E) and Haloperidol (F).
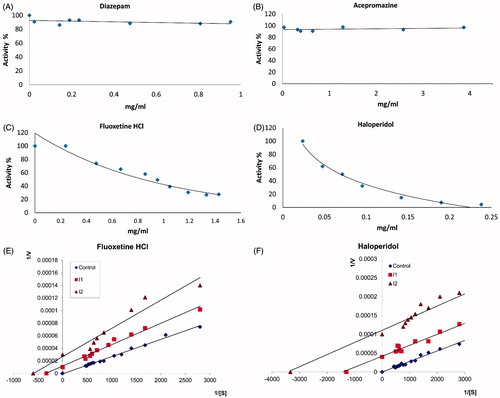
Table 1. Summary of the purification of human serum Paraoxonase.
Table 2. The effects of Fluoxetine hydrochloride and Haloperidol on the purified human serum PON1 and the inhibition type of drugs.
Discussion
Human serum paraoxonase (Arylesterase, EC 3.1.8.1, PON1) can hydrolyze organophosphate compounds and aryl esters, but its physiological substrates are still unknownCitation19. In addition, paraoxonase is one of the most important enzymes in lipid metabolism, cardiovascular disease and atherosclerosis. PON1 activity also decreases in pathophysiologic conditions such as hypercholesterolemia, smoking habit, aging, obesity, menopause and acute renal failureCitation20. For instance, excessive smokers were found to have high serum LDL and other lipid levels. Indeed, PON1 activity and HDL levels of drinkers were determined to be lower than non-drinkers. Along with other major and minor risk factors, this increases the risk of coronary artery diseaseCitation21.
Toxicology, enzyme–drug interactions and some chemical interaction studies with various enzymes are vital and the number of these studies is increasing every day worldwideCitation22–24. In general, drugs and toxic substances display their biological effects by interaction with specific enzymes.
Costa et al.Citation25 reported that modulation of PON1 have involved pharmaceutical drugs, particularly lipid-lowering compounds such as statins and fibrates, as well as some other drugsCitation26–30. A few studies have investigated the effects of some lipid-lowering agents on serum paraoxonase activityCitation31–34. The activity of serum PON1 was found to be increased in coronary patients who took low-dose aspirin (acetyl-salicylic acid)Citation35. However, administration of aspirin to healthy volunteers did not alter serum PON1 activity in another study. Aspirin also increased PON1 activity in HuH7 cellsCitation36. Various other pharmaceutical drugs have been investigated in different experimental protocols for their effects on PON1 activityand/or expression. The cholinergic muscarinic antagonist atropine was reported to inhibit human plasma paraoxonase. In our previous studies certain antibiotics, such as sodium ampicillin, ciprofloxacin and clindamycin sulphate, but notrifamycin, inhibited purified human PON1Citation37. When the same antibiotics were given in vivo to mice, however, increases or decreases of serum PON1 activities at different time-points were observed, with similar results observed in the liver. It has also been reported that mouse liver PON activity decreased with some contraceptives while the serum PON activity increasedCitation15,Citation38.
The effect of different pharmaceutical drugs on paraoxonase enzyme activity can be used to clarify PON1 status in the metabolism. Given the physiological importance of paraoxonase, the study of the effect of antidepressant and antipsychotic ciatric drugs on paraoxonase enzyme activity is an increasingly important issue for human health. Although there are a huge number of studies describing the critical role of PON1 in a variety of diseases specifically cardiovascular disease and atherosclerosis, there is no other information available about the effects of antidepressants and antiphysotic drugs on PON1 activity.
In our study, to investigate the effect of these drugs on PON1 enzyme activity in vitro, human serum paraoxonase was purified by ammonium sulfate precipitation at 60–80% intervals, and subjected to hydrophobic interaction chromatography. Different protocols are available for PON1 enzyme purification from serum and liver using three, four and seven stepsCitation29,Citation30. We previously reported a purification strategy designed for the human PON1 enzyme consisting of two-step procedures resulting in a shorter and more straightforward approach in contrast to other purification proceduresCitation29. Later, we investigated the in vitro effects of some antidepressant and antipsychotic drugs such as Fluoxetine hydrochloride (A), Haloperidol (B), Acepromazine (C) and Diazepam drugs (D) on PON1 activity. We found that compounds A and B inhibited enzyme activity non-competitively, compounds C and D did not show the inhibition efficiency. We can conclude from the table that Fluoxetine hydrochloride is the more efficient inhibitor than haloperidol. Fluoxetine inhibit many isozymes of the cytochrome P450 system that make drug metabolism possible. Both are potent inhibitors of CYP2D6 (the main enzyme responsible for their metabolism) and mild to moderate inhibitors of CYP1A2, CYP2B6, CYP2C9/2C19 and CYP3A4; furthermore, they inhibit the activity of P-glycoprotein, a type of membrane transport protein that plays an important role in drug transport and metabolism. This extensive effect on the body's pathways for drug metabolism creates the potential for interactions with many commonly used drugsCitation39.
However, there are still few studies on PON enzyme activity–drug interaction. In the light of the above information, it could be argued that people cannot be thought to stay away from various chemicals including drugs for their health. However, it is vital to specifically adjust drug-dosages for person and disease. In this study, we purified human serum paraoxonase enzyme using three simple purification steps and investigated the in vitro effects of the mentioned antidepressant and antipsychotic drugs on enzyme activity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was carried out in the Balikesir University Research Center of Applied Sciences (BURCAS).
References
- Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn-Schmiedeberg’s Arch Pharmacol 2004;369:78–88
- La Du BN, Aviram N, Billecke S, et al. On the physiological role(s) of the paraoxonases. Chem Biol Interact 1999;119–120:379–88
- Mochizuki H, Scherer SW, Xi T, et al. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene 1998;213:149–57
- Harel M, Aharoni A, Gaidukov L, et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 2004;11:412–19
- Teiber JF, Draganov DI, La Du BN. Lactonase and lactonizing activities human serum paraoxonase (PON1) and rabbit serum PON3. Biochem Pharmacol 2003;66:887–96
- Gan KN, Smolen A, Eckersen HW, La Du BN. Purification of human serum paraoksonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–6
- Costa LG, Furlong CE. Paraoxonase. Norwell (MA): Kluwer Academic Publishers; 2002
- Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med 2003;3:371–92
- Davies HG, Richter RJ, Keifer M, et al. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 1996;14:334–6
- Billecke S, Draganov D, Counsell R, et al. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 2000;28:1335–41
- Tougou K, Nakamura A, Watanabe S, et al. Paraoxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab Dispos 1998;26:355–9
- Biggadike K, Angell RM, Burgess CM, et al. Selective plasma hydrolysis of glucocorticoidg-lactones and cyclic carbonates by the enzyme paraoxonase: an ideal plasma inactivation mechanism. J Med Chem 2000;43:19–21
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 2005;69:541–50
- Gouedard C, Koum-Besson N, Barouki R, Morel Y. Opposite regulation of the human paraoxonase-1 gene PON1 by fenofibrate and statins. Mol Pharmacol 2003;63:945–56
- Kiranoglu S, Sinan S, Gencer N, et al. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull 2007;30:1048–51
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochime 2006;88:565–74
- Sinan S, Kockar F, Gencer N, et al. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma cells (HepG2) in vitro. Biochemistry (Moscow) 2006;71:46–50
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 2013;34:313–9
- Mackness B, Mackness M. The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med 2012;54:83–90
- Eren E, Yilmaz N, Aydin O. Functionally defective high-density lipoprotein and paraoxonase: a couple for endothelial dysfunction in atherosclerosis. Cholesterol 2013;2013:792090
- Kockar F, Sinan S, Yildirim H, Arslan O. Differential effects of some antibiotics on paraoxonase enzyme activity on human hepatoma cells (HepG2) in vitro. J Enzyme Inhib Med Chem 2010;25:715–9
- Ozensoy Guler O, Arslan O, Kockar F. Differential in vitro inhibitory effects of anticancer drugs on tumor-associated carbonic anhydrase isozymes CA IX and CA XII. Methods Find Exp Clin Pharmacol 2008;30:335–40
- Ozensoy O, Arslan O, Kockar F. Differential in vitro inhibition effects of some antibiotics on tumor associated carbonic anhydrase isozymes of hCA-IX and hCA-XII. J Enzyme Inhib Med Chem 2008;23:579–85
- Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol 2011;81:337–44
- Mirdamadi HZ, Sztanek F, Derdak Z, et al. The human paraoxonase-1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. Br J Clin Pharmacol 2008;66:366–73
- Kassai A, Illyes L, Mirdamadi HZ, et al. The effect of atorvastatin therapy on lecithin: cholesterol acyltransferase, cholesteryl ester transfer protein and the antioxidant paraoxonase. Clin Biochem 2007;40:1–5
- Paragh G, Seres I, Harangi M, et al. Ciprofibrate increases paraoxonase activity in patients with metabolic syn-drome. Br J Clin Pharmacol 2006;61:694–701
- Kurban S, Mehmetoglu I. Effects of acetylsalicylic acid on serum paraoxonase activity, ox-LDL, coenzyme Q 10 and other oxidative stress markers in human volunteers. Clin Biochem 2010;43:287–90
- Van Wijk J, Coll B, Castro Cabezas M, et al. Rosiglitazone modulates fasting and post-prandial paraoxonase 1 activity in type 2 diabetic patients. Clin Exp Pharmacol Physiol 2006;33:1134–7
- Deakin S, Leviev I, Guernier S, James RW. Statin modulates expression of the PON1 gene and increases serum paraoxonase. A role for sterol regulatory element binding protein 2. Arterioscler Thromb Vasc Biol 2003;23:2083–9
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS. Atorvastatin and gemfibrozilmetabolites, but not the parent drugs, are potent antioxidants againstlipoprotein oxidation. Atherosclerosis 1998;138:271–80
- Turay J, Grniakova V, Valka J. Changes in paraoxonase and apolipoprotein A-I, B, C-III and E in subjects with combined familiar hyperlipoproteinemia treated with ciprofibrate. Drugs Exp Clin Res 2000;26:83–8
- Durrington PN, Mackness MI, Bhatnagar D, et al. Effects of two different fibric acid derivatives on lipoproteins, cholesteryl ester transfer, fibrinogen, plasminogen activator inhibitor and paraoxonase activity in type IIb hyperlipoproteinaemia. Atherosclerosis 1998;138:217–25
- Blatter-Garin MC, Kalix B, De Pree S, James RW. Aspirin use is associated with higher serum concentrations of the antioxidant enzyme, paraoxonase-1. Diabetologia 2003;46:593–4
- Ahmad S, Carter JJ, Scott JE. A homogenous cell-based assay for measurement of endogenous paraoxonase activity. Anal Biochem 2010;400:1–9
- Wojcicka G, Jamroz-Wisniewska A, Marciniak A, et al. The differentiating effect of glimepiride and glibenclamide on paraoxonase-1 and platelet-activating factor acetylohydrolase. Life Sci 2010;87:126–32
- Sinan S, Kockar F, Gencer N, et al. Effects of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull 2006;29:1559–63
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug ınteractions. Psychosomatics 2005;46:464–94


![Figure 1. Molecular structure of antidepressant and antipsychotic drugs: [A] Fluoxoetine hydrochloride, [B] Haloperidol, [C] Acepromazine and [D] Diazepam.](/cms/asset/f687810b-5bcd-46b8-a489-c6d67e1d3958/ienz_a_913038_f0001_b.jpg)