Abstract
In the present study, in order to evaluate antioxidant and radical scavenging properties of Pistachio gum (P-Gum), different bioanalytical methods such as DPPH• scavenging activity, DMPD•+ radical scavenging activity, total antioxidant activity determination by ferric thiocyanate, reducing ability Fe3+–Fe2+ transformation, Cuprac and FRAP assays, scavenging by riboflavin-methionine-illuminate system and ferrous ions (Fe2+) chelating activities by 2,2′-bipyridyl reagent were performed separately. P-Gum inhibited 54.2% linoleic acid peroxidation at 10 µg/ml concentration. On the other hand, BHA, BHT, α-tocopherol and trolox, pure antioxidant compounds, indicated inhibition of 80.3%, 73.5%, 36.2% and 72.0% on peroxidation of linoleic acid emulsion at the same concentration, respectively. In addition, all of sample had an effective DPPH•, DMPD•+ and
scavenging, Fe3+ reducing power by Fe3+–Fe2+ transformation and FRAP assay, Cu2+ reducing ability by Cuprac method and Fe2+ chelating activities.
Introduction
Pistachio (Pistacia vera L.), a member of Anacardiaceae family, the only 1 of the 11 species of the genus Pistacia, having an edible green kernel enclosed in a woody shell, is native to Asia minor and widely distributed in the Mediterranean region including Turkey as well as USA. Pistachio, a common desert plant, is highly tolerant of saline soil. It grows well when irrigated with water having 3–4 mm of soluble saltsCitation1. The shell of the pistachio is naturally a beige color, but it is sometimes dyed red or green in commercial pistachiosCitation2. Pistachios are rich fat source and contain essential fatty acids including oleic, linoleic and linolenic acidsCitation3.
Pistachioh as an economic value, as it is the source of a traditional medicinal agent, an oleoresin, gum, which is seeping out of some parts of this plantCitation4. Pistachio gum is obtained from Pistacia vera L., as an exudate after “hurting” the trunk and branches. This resin is custom scented, pale yellow, in the viscosity of honey while it is fresh. It hardens over time. According to Alma et al.Citation5, gum of Pistacia vera showed antibacterial activity.
Oxidation is defined as electrons transferring from one atom to another atom. It represents an essential part of aerobic life and human metabolism. Oxygen is the ultimate electron acceptor in the electron flow system that produces energy in the form of ATPCitation6. It constitutes most of the mass of living organisms, because water is their major constituent. It is very important for the continuity of vital functions and has unpaired two electrons and tends to form oxygen-centered free radicals, known as reactive oxygen species (ROS) including superoxide anion radical (), hydroxyl radical (HO·), peroxyl radical (ROO·), alkoxyl radical (RO·) and nitric oxide radical (NO·)Citation7–9. They are recognized to play a dual role as both beneficial and deleterious species. At physiological concentrations, ROS may be required for normal cell functions. ROS are important for intracellular messenger molecules. But when the antioxidant defense systems are in case of inadequate, ROS cause the cell damaging and oxidative stress occurs. As a result of damage to biomolecules such as carbohydrates, lipids, proteins, nucleic acids and DNA, free-radical chain reactions can stimulate subsequently and some disease conditions such as cancer, aging, coronary heart disease, neurodegenerative disease can be occurCitation10–12.
An antioxidant molecule has been defined as any substance that when present in low concentrations compared to that of an oxidizable substrate significantly delays or inhibits the oxidation of that substrate. Oxidizable substrate encompasses almost everything except H2O, found in foods and in living tissuesCitation10,Citation13–17. On the other hand, free radicals are the primary reason for cancer and that the risk of disease. They can be reduced by increased consumption of food-borne antioxidants has prompted an enormous growth of interest in antioxidant nutrients and other antioxidants substances in foodCitation18,Citation19. Plant foods are potential sources of natural antioxidants, such as vitamin C, α-tocopherol, carotenoids, flavonoid and phenolic acids, which prevent free radical damage. They can provide phenolic hydroxyl group to react with free radicals. The synthetic antioxidants used are phenolic compounds such as BHA, BHT, TBHQ and propyl gallate (PG), which is an ester of gallic acidCitation20,Citation21 have been very thoroughly tested for their toxicological behaviors. However, some of them are coming, after a long period of use, under heavy pressure as new toxicological data impose some caution in their use. In this context, natural products appear as healthier as and safer than synthetic antioxidantsCitation22–24. Consequently, there is a growing interest in exploring natural sources of natural and safer antioxidants for food and pharmaceutical applicationsCitation25,Citation26.
The aim of this study was to investigate ferric ions (Fe3+) reducing antioxidant power assay, FRAP assay, cupric ions (Cu2+) reducing antioxidant power assay, DPPH· radical scavenging, DMPD•+ radical scavenging, superoxide anion radical scavenging, ferrous ions (Fe2+) chelating activities and the inhibition of lipid peroxidation in linoleic acid system of pistachio (Pistacia vera L.) gum (P-Gum).
Materials and methods
Chemicals
2,4,6-Tripyridyl-s-triazine (TPTZ), butylated hydroxyanisole (BHA), 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), butylated hydroxytoluene (BHT), N,N-dimethyl-p-phenylenediamine (DMPD), linoleic acid, methionine, riboflavin and 3-(2-pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine) were purchased from Sigma (Sigma–Aldrich GmbH, Sternheim, Germany). All other chemicals used were analytical grade and obtained from either Sigma–Aldrich or Merck (Darmstadt, Germany).
Preparation of pistachio (Pistacia vera L.) P-gum
P-Gum of was obtained from pistachio trees from Gaziantep province of Turkey. About 10 mg P-Gum dissolved in 10 ml ethanol, is used directly, without extraction.
Determination of total phenolic contents
The total phenolics in P-Gum were determined according to a modified version of the procedure described by Slinkard and SingletonCitation27 using by Folin–Ciocalteu phenolic reagentCitation28. Gallic acid (GA) was used as a positive standard phenolic compound. The quantity of phenolic compounds in P-Gum was determined as microgram of gallic acid equivalent (GAE) using an equation that was obtained from standard gallic acid graph (r2: 0.9849).
The total phenolics in P-Gum were calculated by employing a standard above curve prepared using GA and expressed as micrograms of GAECitation26,Citation28.
Determination of total flavonoids
Total flavonoid determination of P-Gum was performed according to Gülçin et al.Citation29,Citation30. Total flavonoids quantity was calculated using quercetin as standard(r2: 0.9872):
The content of flavonoids in P-Gum was calculated from above standard curve prepared using quercetin and expressed as micrograms of quercetin equivalents (QE)Citation26,Citation29.
Ferriccyanide (Fe3+) reducing power assay
The ferric reducing antioxidant power was carried out by slight modification of method of OyaizuCitation30. Reducing power of P-Gum was calculated by the direct Fe3+(CN−)6–Fe2+(CN−)6 reduction and determined by absorbance measurement at 700 nm of the formation of the Perl’s Prussian Blue complex following the addition of excess Fe3+, as described previouslyCitation31–34. Increased absorbance of the reaction mixture indicates grater reduction capabilityCitation35.
Cupric ions (Cu2+) reducing power – Cuprac assay
Cu2+ reducing ability of P-Gum was determined by slight modification of the cupric ions (Cu2+) reducing power methodCitation36,Citation37. Cuprac assay is a chromogenic redox reaction, carried out at close to physiological pH (pH 7) and 2,9-dimethyl-1,10-phenanthroline (Neocuproine) is used as chromogenic agent. Absorbance was measured at 450 nm after 30 min against a reagent blank. Increased absorbance indicates increased reduction capability of P-Gum or standardsCitation38.
Fe3+–Fe2+ reducing capacity – FRAP assay
The FRAP assay measures the ability of antioxidants to reduce the ferric [Fe3+–(TPTZ)2]3+ to the intensely blue colored ferrous complex [Fe2+–(TPTZ)2]2+ in acidic mediumCitation39,Citation40. [Fe3+–(TPTZ)2]3+ reducing values of P-Gum are estimated by measuring the absorbance increase at 593 nm and relating it to a ferrous ions standard solution or to an antioxidant standard solution. The change in absorbance is proportional to the combined [Fe3+–(TPTZ)2]3+ reducing value of the antioxidants in P-GumCitation41,Citation42. Increased absorbance of the reaction mixture of P-Gum shows increased reduction capability.
Chelating activity on ferrous ions (Fe2+)
Ferrous ions (Fe2+) chelating activity was evaluated by following method of DinisCitation35 described previouslyCitation43. Accordingly, the inhibition of the formation of Fe2+–ferrozine complex by P-Gum screened with decreased absorbance at 562 nm as Fe2+-chelating ability of sample. The IC50 value of inhibition of ferrozine–Fe2+ complex formation was calculated by equation obtained from the graphic of P-Gum or standards. The control sample contains only FeCl2 and ferrozineCitation16,Citation44.
DPPH• free radical scavenging activity
In DPPH assay, the antioxidants were able to reduce the stable radical DPPH to the yellow colored diphenyl-picrylhydrazineCitation45. This method is based on the reduction of DPPH in alcoholic solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H in the reaction. This spectrophotometric assay uses the stable radical, DPPH·, as a reagent. The method of BloisCitation46 previously detailed described by GülçinCitation16 with slight modifications in order to assess the DPPH· free radical scavenging capacity of P-Gum. The DPPH· scavenging capacity P-Gum and standards was expressed as mM in the reaction medium and calculated from the calibration curve determined by linear regression (r2: 0.9966):
Measurement of DMPD•+scavenging ability
DMPD•+ scavenging ability of P-Gum was performed according to Fogliano et al.Citation47 and reported previouslyCitation48. The scavenging capability of ABTS•+ radical of the sample was monitored in spectrophotometer at 505 nm. The DMPD•+ concentration (mM) in the reaction medium was calculated from the below calibration curve, determined by linear regression (r2: 0.9725):
Superoxide anion radical scavenging activity
Superoxide radical scavenging activity of P-Gum was determined by spectrophotometric measurements of nitrobluetetrazolium (NBT). To this end, superoxide radicals were generated by method of Beauchamp and FridovichCitation49 with slight modificationCitation50. They are generated in riboflavin-methionine-illuminate system and may reduce NBT into formazan. This reduction was spectrophotometrically monitored at 560 nm. The un-illuminated reaction mixture was used as a blank sample. P-Gum was added to the reaction mixture, in which was scavenged, thereby inhibiting the NBT reduction.
Total antioxidant activity determination by ferric thiocyanate method
In order to measure the preventing effects of peroxidation of linoleic acid emulsion of P-Gum and reference antioxidants, the ferric thiocyanate method was performedCitation36,Citation51. This method was used to measure the peroxide level during the initial stage of linoleic acid oxidation. The peroxide levels were determined by reading the absorbance at 500 nm in a spectrophotometer (Shimadzu, UV-1208 UV-VIS Spectrophotometer, Kyoto, Japan) after reaction with FeCl2 and thiocyanate at intervals during incubation. The assay steps were repeated every 12 h until absorbance reached a maximum value. For this purpose, the percent inhibition was calculated at this point (96 h). The solution without P-Gum was used as blank samples. Linoleic acid mixture without the addition of sample was used as a control. The percent inhibition of lipid peroxidation in linoleic acid emulsion was calculated by following equation:
where ILP is inhibition of lipid peroxidation, λC is the absorbance of the control reaction, which contains only linoleic acid emulsion and sodium phosphate buffer. λS is the absorbance of sample in the presence P-Gum or other tests compoundsCitation48,Citation52.
Statistical analysis
The experimental results were performed in triplicate. The data were recorded as mean ± SD and analyzed by SPSS (version 11.5 for Windows 2000, SPSS Inc., San Francisco, CA). One-way analysis of variance (ANOVA) was performed by procedures. Significant differences between means were determined by Duncan’s Multiple Range tests, and p < 0.05 was regarded as significant and p < 0.01 was very significantCitation53.
Results and discussion
The human diet contains different compounds that possess antioxidant activities. These compounds have been suggested to scavenge ROS based on their structural properties. Phenolic compounds are a class of chemical compounds consisting of a hydroxyl group (–OH) bonded directly to an aromatic hydrocarbon group. They are secondary plant metabolites and naturally present in almost all plant materials, including food and pharmaceutical products of plant origin. Phenolic compounds are thought to be an integral part of both human and animal dietsCitation24,Citation25.
P-Gum exhibited effective potassium ferricyanide reduction, FRAP and cupric ions (Cu2+) reducing methods when compared to the standards such as BHA, BHT, α-tocopherol and trolox. For evaluation of the reduction ability of P-Gum, the Fe3+–Fe2+ transformation was investigated by the method of OyaizuCitation31. The reducing capacity of sample was measured as direct Fe[(CN)6]3–Fe[(CN)6]2 reduction. As can be seen in , P-Gum showed potent Fe3+ reducing ability and these differences were statistically very significant (p < 0.01). Increase Fe[(CN)6]2 absorbance of the reaction mixture indicate increased reducing capacity due to an increase in the formation of the complex. So, reducing power of P-Gum and standard compounds exhibited the following order: BHA (2.098 ± 0.04) > BHT (1.591 ± 0.19) > α-tocopherol (1.052 ± 0.10) > trolox (0.567 ± 0.03) > P-Gum (0.223 ± 0.03). The results demonstrated that P-Gum changed yellow color of the test solution to green or blue depending on their ferric ions (Fe3+) reducing ability. On the other hand, according to Fe3+ to Fe2+ reducing capacity-FRAP assay results, at 30 μg/ml concentration of P-Gum and standard antioxidants were shown in , showed the following order: BHA (2.261 ± 0.02) > α-tocopherol (1.764 ± 0.05) > BHT (1.283 ± 0.09) > trolox (1.024 ± 0.07) > P-Gum (0.530 ± 0.07).
Table 1. Determination of reducing power of P-Gum by potassium ferricyanide reduction and FRAP methods, cupric ions (Cu2+) reduction capacity by Cuprac method.
The Cuprac is a reduction method based on reduction Cu2+ to Cu+ in the presence of neocuproineCitation53. Cu2+ reducing ability of P-Gum and standard compounds were shown in . Between Cu2+ reducing ability of P-Gum and its concentration (30 μg/ml) was observed a correlation. Cupric ions reducing power of P-Gum and standard compounds at the same concentration (30 μg/ml) exhibited the following order: BHA (0.598 ± 0.04) ≥ BHT (0.550 ± 0.04) ≈ α-tocopherol (0.546 ± 0.13) > trolox (0.486 ±0.11) > P-Gum (0.158 ± 0.03). Also, embedded thiols (–SH groups) of proteins release in urea buffer at pH 7 and react with reagent. So, this method can be used for measurement of activity of antioxidants, which include thiol groups such as glutathioneCitation37,Citation53.
Due to the fact that, the ionic species as ferrous (Fe2+) ions facilitate the production of ROS in organism, iron-chelating capacity is quite important. Metal chelating activity is an important antioxidant activity method, is used to prevent or delay the oxidation reactions catalyzed by metal ions. Iron is an essential mineral for organisms but more than necessary can cause cell damageCitation54. Owing to the high activity, it is known as the most important oxidizing metal among the transition metals. Ferrous ions (Fe2+) are the most significant prooxidative ions. Ferrozine can form a complex with divalent metal ions such as ferrous ions (Fe2+) in even the quantitative amount. As a result that colored ferrozine–metal complex show the maximum absorbance at 562 nm. P-Gum was assessed for its ability to compete with ferrozine for ferrous ion in the solutionCitation16.
P-Gum had effective chelating effect on ferrous ions (Fe2+). P-Gum had effective IC50 values of Fe2+ chelating activity than that of standard antioxidants (BHA, BHT, α-tocopherol and trolox). In addition, P-Gum exhibited 5.3 µg/ml as IC50 value. On the other hand, the Fe2+ chelating capacity of BHA, BHT, α-tocopherol and trolox were found to be 28.9, 8.5, 16.5 and 7.5 µg/ml, respectively. These results clearly show that the Fe2+ chelating effect of P-Gum was higher than that of BHA (28.9), BHT (8.5), α-tocopherol (16.5) and trolox (7.5). In this assay, P-Gum disrupted the formation of the f Fe2+–ferrozine complex. It suggests P-Gum have chelating activity and is able to capture Fe2+ before ferrozine.
Radical scavenging assays determine the antioxidant capacities of compounds by spectrophotometricallyCitation55,Citation56. Antioxidants cause depolarization and reverse the DPPH• formation DMPD•+ and ABTS•+cation. A freshly prepared DPPH• solution exhibits a deep purple color with absorption maximum at 517 nm. This purple color of DPPH• solution generally disappears when an antioxidant compound is present in the mediumCitation24,Citation43. Thus, antioxidant molecules can scavenge free DPPH• by providing hydrogen atoms or by electron donation, via a free-radical attack on the DPPH• molecule and convert them to colorless or bleached product (DPPH-H). In this radical scavenging assay, the antioxidant was able to reduce the stable radical DPPH to the yellow colored diphenyl-picrylhydrazine (DPPH-H). In brief, this method is based on the DPPH reduction in alcoholic medium in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H in the reactionCitation16,Citation50,Citation55.
indicates an important scavenging of DPPH radical due to the scavenging ability of P-Gum (23.9 µg/ml) and the reference compounds. BHA (10.2 µg/ml), BHT (27.7 µg/ml), α-tocopherol (11.8 µg/ml) and trolox (10.9 µg/ml) were used as references for radical scavenger activity. According to the IC50 value of P-Gum in DPPH radical scavenging assay results, DPPH radical scavenging decreased as follow: BHA ≈ Trolox > α-Tocopherol > P-Gum > BHT and were shown in .
Table 2. Total antioxidant activity by ferric thiocyanate method, IC50 values of DPPH• free radical scavenging activity, DMPD radical scavenging activity, superoxide anion radical ( ) scavenging activity and metal chelating activity of P-Gum.
) scavenging activity and metal chelating activity of P-Gum.
Antioxidant compounds that can able to transfer a hydrogen atom to DMPD•+, turn off color of the solution and provide decolorization. Therefore, this study demonstrates capability of the radical hydrogen donor to scavenge a single electron from DMPD•+. Preliminary experiments have shown that the choice of oxidant solution and the ratio between the concentrations of DMPD•+ and the oxidative compound is very important for the effectiveness of the method. DMPD•+ shows a maximum absorbance at 505 nmCitation16. As shown in , P-Gum exhibited a marked DMPD•+radical scavenging activity. IC50 value of DMPD•+radical scavenging activity of P-Gum was found to be 15.4 µg/ml. On the other hand, at the same concentration this value was found as 15.4 µg/ml for α-tocopherol and was shown the same effect with P-Gum. There was a significant decrease (p < 0.05) control value and DMPD•+ scavenging capacity of P-Gum.
In superoxide anion radical scavenging method, superoxide anion reduces NBT to the yellow dye (NBT2+) to produce the blue formazan. The absorbance of formazan is spectrophotometrically measured at 560 nmCitation57. The decrease in absorbance at 560 nm with antioxidants shows superoxide anion was dissipated. P-Gum had distinctive inhibition of superoxide radical generation. As seen in , IC50 values belonging to inhibition of superoxide anion radical generation of P-Gum was found to be 16.5 µg/ml. On the other hand, at the same concentration BHA, BHT, α-tocopherol and trolox exhibited 19.8, 20.4, 17.8 and 21.0 µg/ml IC50 values in superoxide anion radical scavenging activity, respectively. According to these results, P-Gum had higher superoxide anion radical scavenging activity than that all of tested reference compounds and these differences statically were found as significant (p < 0.01).
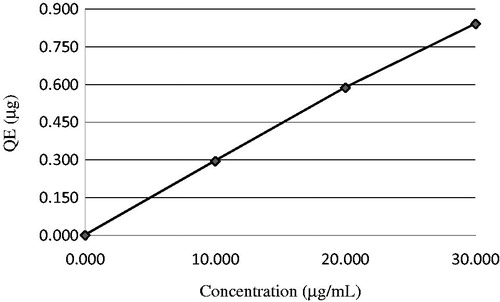
For determining total phenolic contents, standard graphic were obtained using known quantities of standard gallic acid. As can be seen in , the phenolic compound of 30 µg/ml of P-Gum exhibited 191.7 µg GAE. Flavonoids are very effective antioxidants and it has been proposed that they protect against cardiovascular disease by reducing oxidation of low-density proteinsCitation16,Citation23. At the same time, total amount of flavonoid in 30 µg/ml P-Gum was determined spectrophotometrically and found to be 0.841 µg quercetin equivalents ().
Figure 1. Total phenolic content as gallic acid equivalent (GAE/mg of extract) in P-Gum. P-Gum, pistachio (Pistacia vera L.) gum.
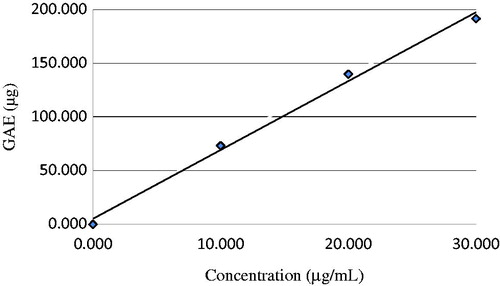
Figure 2. Total flavonoids content as quercetin equivalent (QE/mg of extract) in P-Gum. P-Gum, pistachio (Pistacia vera L.) gum.
Lipid peroxidation consists of a series of free radical mediated chain reaction and is associated with several types of biological damages. It is the process in which free radicals get electrons from the lipids in cell membranes, resulting in cell damage. In this assay, we measured the amount of peroxide produced from linoleic acid emulsion by auto-oxidation during the experiment period, indirectlyCitation58–61. P-Gum exhibited effective antioxidant activity in the linoleic acid emulsion system and is showed in graphic (). The effect of 10 µg/ml concentration of P-Gum on lipid peroxidation of linoleic acid emulsion is shown in and was found to be 54.2%. On the other hand BHA, BHT, α-tocopherol and trolox exhibited 80.3%, 73.5%, 36.2% and 72.0% on peroxidation of linoleic acid emulsion at the same concentration, respectively. The autoxidation of linoleic acid emulsion without P-Gum or standard compounds was accompanied by a rapid increase of lipid peroxides.
Figure 3. The total antioxidant activity of P-Gum [pistachio (Pistacia vera L.) gum] and standard antioxidants such as BHA, BHT, α-tocopherol and trolox at the same concentration(10 µg/ml).
![Figure 3. The total antioxidant activity of P-Gum [pistachio (Pistacia vera L.) gum] and standard antioxidants such as BHA, BHT, α-tocopherol and trolox at the same concentration(10 µg/ml).](/cms/asset/a9666ee8-ce41-410e-bb24-a0a74d1bc062/ienz_a_915395_f0003_b.jpg)
In conclusion, P-Gum was found to be an effective antioxidant in different bioanalytical assays including reducing power, DPPH•, DMPD•+ and radical scavenging, and metal-chelating activities when it is compared to standard antioxidant compounds such as popular synthetic antioxidants BHA and BHT, α-tocopherol, trolox. Based on the discussion above, P-Gum can be used for minimizing or preventing lipid oxidation in pharmaceutical products, retarding the formation of toxic oxidation products, maintaining nutritional quality and prolonging the shelf life of pharmaceuticals.
Declaration of interest
The authors declare no conflict of interest.
References
- Herrera E. Growing pistachios in New Mexico. New Mexico State University, Cooperative Extension Service, Circular; 1997:532
- Mabberley DJ. The plant book. Cambridge: Cambridge University Press; 1993:939–42
- Garcia JM, Agar IT, Streif J. Fat content and fatty acid composition in individual seeds of pistachio varieties grown in Turkey. Gartenbauwissenschaft 1992;57:130–3
- Doğan O, Baslar S, Aydın H, Mert HH. A study of the soil-plant interactions of Pistacia lentiscus L. distributed in the western Anatolian part of Turkey. Acta Botanica Croat 2003;62:73–88
- Alma MH, Nitz S, Kollmannsberger H, et al. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.). J Agric Food Chem 2004;52:3911–14
- Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios reduce serum oxidized LDL and increase serum antioxidant levels. FASEB J 2007;21:847.19
- Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 1995;61:1–31
- Gülçin İ. Determination of antioxidant activity, characterization of oxidative enzymes and investigation of some in vivo properties of nettle (Urtica dioica). PhD Thesis, Atatürk University; 2002:12
- Gülçin İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nut 2005;56:491–9
- Talaz O, Gülçin İ, Göksu S, Saraçoğlu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–9
- Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Method Enzymol 1990;186:1–85
- Gülçin İ. Antioxidant activity of L-adrenaline: an activity structure insight. Chem Biol Interact 2009;179:71–80
- Balaydın HT, Gülçin İ, Menzek A, et al. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–95
- Sies H. Strategies of antioxidant defence. Eur J Biochem 1993;215:213–9
- Halliwell B. Antioxidant characterization; methodology and mechanism. Biochem Pharmacol 1995;49:1341–8
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345–91
- Gülçin İ. Antioxidant and antiradical activities of l-carnitine. Life Sci 2006;78:803–11
- Şerbetçi Tohma H, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71
- McLarty JW. Antioxidants and cancer: the epidemiological evidence. In: Garewal HS, ed. Antioxidants and disease prevention. Boca Raton: CRC Press; 1997:45–65
- Bursal E, Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–9
- Hudson JF. Food antioxidants. London: Elsevier Applied Science; 1990
- Göçer H, Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nut 2011;62:821–5
- Thompson D, Moldeus P. Cytotoxicity of butylated hydroxyanisole and butylated hydroxytoluene in isolated rat hepatocytes. Biochem Pharmacol 1988;37:2201–7
- Gülçin İ, Beydemir Ş. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30
- Gülçin İ, Şat İG, Beydemir Ş, Küfrevioğlu Öİ. Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital J Food Sci 2004;16:17–30
- Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–20
- Gülçin İ, Şat İG, Beydemir Ş, et al. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 2004;87:393–400
- Bursal E, Köksal E, Gülçin İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res Int 2013;51:66–74
- Elmastaş M, Gülçin İ, Beydemir Ş, et al. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) seeds extracts. Anal Lett 2006;39:47–65
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: α-Hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside F. Planta Med 2004;70:561–3
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nut 1986;44:307–15
- Büyükokuroğlu ME, Gülçin İ, Oktay M, Küfrevioğlu Öİ. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 2001;44:491–5
- Elmastaş M, Gülçin İ, Işıldak Ö, et al. Antioxidant capacity of bay (Laurus nobilis L.) leave extracts. J Iran Chem Soc 2006;3:258–66
- Gülçin İ, Topal F, Çakmakçı R, et al. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:585–93
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9
- Gülçin İ, Elmastaş M, Aboul-Enein HY. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 2007;21:354–61
- Apak R, Güçlü K, Özyürek M, Karademir SE. A novel total antioxidant capacity index for dietary polyphenols, vitamin C and E, using their cupric ion reducing capability in the presence of neocuproine: the CUPRAC method. J Agric Food Chem 2004;52:7970–81
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticul 1977;28:49–55
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 1996;239:70–6
- Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol 1999;299:15–27
- Ou B, Hampsch-Woodill M, Flanagan J, et al. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J Agric Food Chem 2002;50:2772–7
- Ou B, Huang D, Hampsch-Woodill M, et al. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem 2002;50:3122–8
- Gülçin İ, Elmastaş M, Aboul-Enein HY. Antioxidant activity of clove oil-A powerful antioxidant source. Arab J Chem 2012;5:489–99
- Gülçin İ, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthusvirginicus L.). Eur Food Res Technol 2006;223:759–67
- Çetinkaya Y, Göçer H, Menzek A, Gülçin İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Der Parm 2012;345:323–34
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;26:1199–200
- Fogliano V, Verde V, Randazzo G, Ritieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 1999;47:1035–40
- Gülçin İ, Gagua N, Beydemir Ş, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587–94
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44:276–87
- Gülçin İ, Berashvili D, Gepdiremen A. Antiradical and antioxidant activity of total anthocyanins from Perillapankinensisdecne. J Ethnopharmacol 2005;101:287–93
- Köksal E, Gülçin İ, ÖztürkSarıkaya SB, Bursal E. On the in vitro antioxidant activity of silymarine. J Enzyme Inhib Med Chem 2008;24:395–405
- Gülçin İ. In vitro prooxidant effect of caffeine. J Enzyme Inhib Med Chem 2008;23:149–52
- Apak R, Güçlü K, Özyürek M, et al. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nut 2006;57:292–304
- Gülçin İ, Topal F, ÖztürkSarikaya SB, et al. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–75
- Gülçin İ. Antioxidant activity of eugenol – a structure and activity relationship study. J Med Food 2011;14:975–85
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin. Phytother Res 2006;20:130–4
- Gülçin İ, Oktay M, Küfrevioğlu Öİ, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L). Ach J Ethnopharmacol 2002;79:325–9
- Parejo I, Viladomat F, Bastida J, et al. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem 2002;50:6882–90
- Gülçin İ, Kirecci E, Akkemik E, et al. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.). Turk J Biol 2010;34:175–88
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212

