Abstract
Certain new 3H-quinazolin-4-one Schiff’s bases were synthesized and screened for their activities against ulcerative colitis “UC”. Their activity against phospholipase A2 and protease enzymes was also investigated. Some compounds possessed remarkable effect with different potentials against acetic acid-induced colitis model in rats. Compound 14 (50 mg/kg) was more effective than dexamesathone (0.01 mg/kg). It produced 79.78% protection of control colitis; however, compound 13 produced 75.80% protection and was considered as effective as dexamesathone with 75.30% protection. The observed results could be explained partially by their anti-inflammatory activities which appear as phospholipase A2 (hGIIA) and/or through protease inhibitor potentials. However, all the compounds under test showed preferential inhibition towards hG-IIA type of PLA2 rather than DrG-IB with varying degrees. Interestingly, compounds 14, 13, 12 and 11 displayed excellent inhibitory activity against phospholipase A2 accompanied by protease inhibitory profile.
Introduction
Inflammatory bowel diseases (IBD) were described as early as the 19th century and subsequently divided into two major categories, ulcerative colitis (UC) and Crohn’s disease. UC is an IBD that primarily affects the colonic mucosa and sub-mucosa. The most common symptoms of ulcerative colitis are ulcers and inflammation that lead to symptoms of diarrhea, and abdominal cramping during bowel movements. Currently, there is no effective therapy to cure the disease, only symptomatic treatment which depends on the severity of the disease; therefore treatment is adjusted for each individualCitation1.
Phospholipase A2 (PLA2) participates in the regulation of phospholipid metabolism and biosynthesis of eicosanoids. Several studies showed that serum PLA2 activity and levels are significantly increased in UCCitation2. On the other hand; a potential link between protease and protease inhibitor levels and progression of IBD were studied. Increased fecal protease activity has been observed in UC patients and was correlated with disease severityCitation3.
Over the last few decades, significant developments have been made in many areas of design and development of new molecules to meet the pressing need for new medication. However, these efforts were hampered by a number of problems which still remain and the therapeutic potential has been compromised by the developed toxicity and low safety profileCitation4,Citation5.
Quinazoline containing compounds form an important class of synthetic products and represent an attractive scaffold for designing small molecules of diverse biological effect. They have attracted interest over the past years notably as tyrosine kinase as well as carbonic anhydrse inhibitors as potential chemotherapeutic agents among many other biomedical and technologic applicationsCitation6–16.
Also, Schiff bases have sound pharmacological impact on different areas of medical applications. We decided to synthesize certain new quinazoline molecules equipped with this functionality by simple condensation of 3-amino-2-(4-nitrophenyl)-(3H)-quinazolin-4-one (5) with certain selected aromatic aldehydes for evaluation as potential anti-ulcerative colitis agents.
Experimental
Chemistry
General
Melting points (°C, uncorrected) were determined in open capillaries on a Gallenkamp melting point apparatus (Sanyo Gallenkamp, Southborough, UK) and were uncorrected. Pre-coated silica gel plates (silica gel 0.25 mm, 60G F254; Merck, Darmstadt, Germany) were used for thin layer chromatography, dichloromethane/methanol (9.5:0.5) mixture was used as a developing solvent system and the spots were visualized by ultraviolet light and/or iodine. Infra-red spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). 1H NMR spectra were recorded on Bruker AC-300 Ultra Shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 300 MHz for 1H and 75 MHz for 13C, using TMS as internal standard and peak multiplicities are designed as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Electron Impact Mass Spectra were recorded on a Shimadzu GC-MS-QP 5000 instrument (Shimadzu, Tokyo, Japan). Elemental analyses were performed, on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany), at the Microanalytical Unit, Faculty of Science, Cairo University, Cairo, Egypt, and the found results were within ± 0.4% of the theoretical values.
Synthesis of N-(4-nitrobenzoyl)-anthranilic acid (3)Citation24
4-Nitrobenzoyl chloride 2 (1.85 g, 0.05 mol) was added drop-wise to a stirred solution of anthranilic acid (1) (6.85 g, 0.05 mol) and triethylamine (2 ml) in dichloromethane (70 ml) and the reaction mixture was stirred at room temperature for 2 h. The separated solid was filtered, washed several times with water, dried and crystallized from ethanol.
2-(4-Nitrophenyl)-4H-3,1-benzoxazin-4-one (4)Citation24
A mixture of N-(4-nitrobenzoyl)-anthranilic acid 3 (8.58 g, 0.03 mol) and acetic anhydride (7.5 g, 0.07 mol) was heated under reflux for 3 h. The solvent was removed under reduced pressure. The residue was triturated with water. The separated solid was collected by filtration, washed with water, dried and crystallized from ethanol.
3-Amino-2-(4-nitrophenyl)quinazolin-4(3H)-one (5)Citation25
Method A
A mixture of 2-(4-nitrophenyl)-4H-3,1-benzoxazin-4-one (4) (0.804 g, 0.003 mol) and 98% hydrazine hydrate (0.6 g, 0.018 mol), in ethanol (10 ml) or in n-butanol (10 ml) was heated under reflux for 10 h. The reaction mixture was cooled and the separated solid was filtered and dried. The solid obtained was separated on a column using chloroform as eluent to afford compound 5 in 15% or 30% yield, respectively.
Method B
A mixture of 2-(4-nitrophenyl)-4H-3,1-benzoxazin-4-one (4) (0.804 g, 0.003 mol) and 98% hydrazine hydrate (0.6 g, 0.018 mol) was heated under reflux for 3 h. On cooling, the separated solid was filtered, washed with water and crystallized from ethanol to give compound 5 in 71.0% yield; m.p. 175–7 °C. IR, KBr, υ cm−1: 3350, 3300 (NH2), 1680 (C = O). 1H NMR (CDCl3, δ ppm): 5.70–5.80 (bs, 2H, NH2, D2O exchanged) and 7.40–8.50 (m, 7H, Ar-Hs). Citation13C NMR: 120.6, 121.5, 123.9, 124.2, 127.9, 128.6, 132.8, 134.1, 149.0, 153.8, 160.2 (Ar-C), 164.1 (C = O). MS m/z (Rel. Int.) 282 (M+, 86.0). Anal. (C14H10N4O3, 282.25) C, H, N.
3-(Arylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one 6–16
General procedure
A mixture of 3-amino-2-(4-nitrophenyl)quinazolin-4(3H)-one (5) (2.82 g, 0.01 mol) and the appropriate aldehyde (0.01 mol) in acetic acid (20 ml) was heated under reflux for 2 h. On cooling, the separated solid was filtered, washed with water and crystallized from acetic acid to afford compounds 6–16, respectively.
3-(Benzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (6)
Yield, 87%; m.p. 159–161 °C; IR υ 1669 (C = O) cm−1. 1H NMR (DMSO-d6): δ 7.60–8.50 (m, 13H, ArHs), 9.10 (s, 1H, N = CH). Citation13C NMR: 121.62. 121.7, 123.9, 124.2, 127.9, 128.6, 128.8, 130.3, 132.8, 134.1, 138.4, 139.9, 142.6, 149.0, 149.2, 163.2, 164.7 (C = O). MS m/z (Rel. Int.) 370 (M+, 86.0). Anal. (C21H14N4O3, 370.36) C, H, N.
3-(2-Hydroxybenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (7)
Yield, 80%; m.p. 166–168 °C; IR υ 3385 (OH), 1666 (C = O) cm−1. 1H NMR (DMSO-d6): δ 5.80 (s, 1H, OH), 7.50–8.30 (m, 12H, ArHs), 9.10 (s, 1H, N = CH). Citation13C NMR: 119.6, 120.9, 123.5, 124.0, 127.1, 128.6, 128.8, 130.5, 132.4, 134.0, 138.2, 139.7, 142.0, 146.2, 148.2, 163.2, 163.9 (C = O). MS m/z (Rel. Int.) 386 (M+, 92.0). Anal. (C21H14N4O4, 386.36) C, H, N.
3-(4-Hydroxy-3-methoxybenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (8)Citation20
Yield, 85%; m.p. 222–4 °C; IR υ 3402 (OH), 1671 (C = O) cm−1. 1H NMR (DMSO-d6): δ 3.80 (s, 3H, OCH3), 5.70 (s, 1H, OH), 7.90–8.50 (m, 11H, ArHs), 8.80 (s, 1H, N = CH). Citation13C NMR: 55.6, 118.4, 119.1, 119.7, 120.3, 120.6, 120.7, 121.9, 122.7, 125.6, 128.5, 128.7, 131.9, 132.5, 140.2, 144.8, 152.4, 157.8, 164.4, 165.9 (C = O). MS m/z (Rel. Int.) 416 (M+, 69.0). Anal. (C22H16N4O5, 416.39) C, H, N.
3 -(4-(Dimethylamino)benzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (9)Citation20
Yield, 52%; m.p. 190–2 °C. IR υ 1667 (C = O) cm−1. 1H NMR (DMSO-d6): δ 2.90 (s, 6H, 2CH3), 6.66–7.87 (m, 12H, ArHs), 8.39 (s, 1H, N = CH). Citation13C NMR: 18.5, 112.7, 112.9, 119.3, 120.2, 121.7, 122.5, 128.6, 132.3, 140.2, 150.5, 151.6, 152.2, 164.3, 164.67 (C = O). MS m/z (Rel. Int.) 413 (M+, 36.0). Anal. (C23H19N5O3, 413.43) C, H, N.
3-(2-Methoxybenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (10)
Yield, 87%; m.p. 145–147 °C. IR υ 1681 (C = O) cm−1. 1H NMR (DMSO-d6): δ 3.80 (s, 3H, OCH3), 5.90–8.60 (m, 12H, ArHs), 9.00 (s, 1H, N = CH). Citation13C NMR: 55.6, 108.9, 112.9, 115.4, 119.5, 120.4, 120.5, 120.7, 122.4, 122.5, 125.1, 128.6, 128.7, 128.7, 132.4, 140.6, 148.3, 149.2, 149.7, 152.6, 164.4, 164.9 (C = O). MS m/z (Rel. Int.) 400 (M+, 36.0). Anal. (C22H16N4O4, 400.39) C, H, N.
2-(4-Nitrophenyl)-3-(3,4,5-trimethoxybenzylideneamino)quinazolin-4(3H)-one (11)
Yield, 80%; m.p. 214–216 °C. IR υ 3368 (3OH), 1675 (C = O) cm−1. 1H NMR (DMSO-d6): δ 5.30 (s, 9H, 3OCH3), 7.70–8.30 (m, 10H, ArHs), 8.39 (s, 1H, N = CH). Citation13C NMR: 55.9, 108.1, 112.9, 119.7, 120.3, 121.3, 122.5, 125.2, 128.7, 128.9, 129.7, 132.5, 139.2, 140.6, 149.9, 152.3, 153.8, 164.6, 165.1 (C = O). MS m/z (Rel. Int.) 460 (M+, 68.0). Anal. (C21H14N4O6, 460.44) C, H, N.
3-(2,4-Dichlorobenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (12)Citation25
Yield, 82%; m.p. 225–7 °C. IR υ 1662 (C = O) cm−1. 1H NMR (DMSO-d6): δ 7.19–8.08 (m, 11H, ArHs), 8.89 (s, 1H, N = CH). Citation13C NMR: 120.8, 121.5, 122.2, 128.1, 128.2, 128.5, 128.8, 129.9, 130.9, 132.8, 134.5, 135.4, 140.8, 143.7, 152.6, 164.5, 165.31 (C = O). MS m/z (Rel. Int.) 443 (M++ 4, 8.0), 441 (M++ 21, 68.0), 439 (M+, 71.0). Anal. (C21H12Cl2N4O3, 439.25) C, H, N.
3-(4-Hydroxybenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (13)
Yield, 79%; m.p. 164–166 °C. IR υ 3394 (OH), 1668 (C = O) cm−1. 1H NMR (DMSO-d6): δ 5.90 (s, 1H, OH), 7.60–7.30 (m, 12H, ArHs). 8.72 (s, 1H, N = CH). Citation13C NMR: 116.9, 119.7, 120.0, 120.3, 120.5, 122.8, 124.5, 128.4, 128.7, 129.5, 132.5, 140.4, 149.4, 152.6, 159.6, 164.3, 164.2 (C = O). MS m/z (Rel. Int.) 386 (M+, 91.0). Anal. (C21H14N4O4, 386.36) C, H, N.
3-(4-Methoxybenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (14)Citation25
Yield, 86%; m.p. 199–201 °C. IR υ 1664 (C = O) cm−1. 1H NMR (DMSO-d6): δ 3.84 (s, 3H, OCH3). 6.69–7.78 (m, 12H, Ar), 8.41 (s, 1H, N = CH). Citation13C NMR: 55.9, 114.6, 119.3, 120.8, 122.1, 126.9, 128.5, 128.7, 128.9, 131.5, 132.5, 134.7, 142.4, 148.9, 152.6, 160.3, 162.7, 165.0 (C = O). MS m/z (Rel. Int.) 400 (M+, 65.0). Anal. (C22H16N4O4, 400.39) C, H, N.
2-(4-Nitrophenyl)-3-(2,4,6-trimethoxybenzylideneamino)quinazolin-4(3H)-one (15)
Yield, 85%; m.p. 217–219 °C. IR υ 1663 (C = O) cm−1. 1H NMR (DMSO-d6): δ 3.92 (s, 9H, 3OCH3), 7.56–7.80 (m, 10H, ArHs), 8.30 (s, 1H, N = CH). Citation13C NMR: 119.6, 120.1, 122.5, 128.1, 128.6, 131.8, 133.9, 136.1, 137.3, 141.5, 144.2, 145.4, 153.1, 154.7, 155.1, 162.8, 163.5 (C = O). MS m/z (Rel. Int.) 460 (M+, 70.0). Anal. (C21H14N4O6, 460.44) C, H, N.
3-(4-Methylbenzylideneamino)-2-(4-nitrophenyl)quinazolin-4(3H)-one (16)
Yield, 87%; m.p. 133–135 °C. IR υ 1660 (C = O) cm−1. 1H NMR (DMSO-d6): δ 2.34 (s, 3H, CH3), 7.6–8.28 (m, 12H, ArHs), 8.44 (s, 1H, N = CH). Citation13C NMR: 21.3, 120.9, 121.4, 122.1, 127.8, 128.2, 128.7, 129.4, 131.6, 132.5, 134.1, 140.1, 141.7, 149.1, 152.0, 160.2, 164.4, 165.2 (C = O). MS m/z (Rel. Int.) 384 (M+, 77.0). Anal. (C22H16N4O3, 384.39) C, H, N.
Animals
Swiss albino mice of both sex (26–30 g) and male Wistar rats (180–200 g) were purchased from the animal house of King Saud University, KSA. Animals were maintained under standard conditions (temperature 23 ± 1.0 °C, humidity 55 ± 10%, 12 h light/12 h dark cycle) and housed in standard polypropylene cages with wire mesh top and They fed with a standard pellet diet with water ad libitum and were allowed to adapt to the laboratory environment for 1 week before experimentation.
Acute toxicity (LD50) test
Mice were fasted overnight (only provided water). Investigated compounds were administered orally to the groups at the dose level of 10 mg/kg and observed for 48 h for mortality. If no mortality was observed, the procedure was repeated for further higher doses such as 100, 1000 and 5000 mg/kg body weight. Toxic symptoms such as behavioral changes, motor reflexes and mortality were observed for 48 hCitation26.
Effect on ulcerative colitis
Experimental design
Thirteen groups each of six animals were used. Rats of groups 1 and 2 received the vehicle (5 ml/kg) and served as normal control and control colitis groups. Group 3 administered dexamethasone (0.1 mg/kg) and served as standard group. Rats of groups 4–13 received the investigated compounds at dose 50 mg/kg. Induction of ulcerative colitis was carried out using acetic acid-induced colitis method described by Mascolo et al.Citation27, with some modification. All medications were administered orally once daily for five consecutive days, the first dose was administrated 1 h after colitis induction.
Assessment of colonic lesions
The colon specimens were weighted and wet weight/length ratio was calculated for all the rats. The specimens were examined under a dissecting microscope and the lesion scores were quantified by scoring system (0–5). Ulcer area was measured using plain glass square. Each cell on the glass square was 1 mmCitation2 in area and the number of cells was counted and the ulcer area was determined for each colon. Ulcer index (UI) was measured by summing the lesion score and the ulcer area for each colon specimenCitation28. The curative ratio was determined according to the formula: Curative ratio = Control UI − Test UI/Control UI × 100.
Inhibition of sPLA2 activity
To evaluate the potential anti-inflammatory activity of 10 compounds investigated in this study, we tested the inhibitory effects of various compounds using two secreted phospholipases: hG-IIA involved in the inflammatory process and the DrG-IB which hydrolyzes dietary phospholipids. Our main objective was to find a compound which was able to inhibit selectively the pro-inflammatory phospholipase A2 group IIA with no or minimal inhibitory effect on the digestive phospholipase A2 group IB.
The test of inhibitory activity of PLA2 was performed as described by De Aranjo and RadvanyiCitation29. Briefly, the substrate consisted of 3.5 mM lecithin in a mixture of 3 mM NaTDC, 100 mM NaCl, 10 mM CaCl2 and 0.055 mM red phenol as colorimetric indicator in 100 ml H2O. The pH of the reaction mixture was adjusted to 7.6. The dromedary group IB phospholipase A2 (DrG-IB) or the human group IIA phospholipase A2 (hG-IIA) or were solubilized in 10% acetonitrile at a concentration of 0.02 and 0.002 μg/μl; respectively. A volume of 10 μl of these PLA2 solutions was incubated with 10 μl of each compound for 20 min at room temperature. Then, 1 ml of the PLA2 substrate was added, and the kinetic of hydrolysis was followed during 5 min by reading the optical density at 558 nm. The inhibition percentage was calculated by comparison with a control experiment (absence of compound under test).
Protease inhibitor assay
Protease inhibitor activity was assayed according to the method of KunitzCitation28 with slight modifications against trypsin. About 1 ml aliquot of trypsin (1000 units/mg)] (0.5 mg/ml prepared in 0.1 M phosphate buffer pH 7) was pre-incubated with 1 ml of a suitable dilution of the protease inhibitor at 37 °C for 15 min. To the above mixture, 2 ml of 1% casein (prepared in 0.1 M phosphate buffer) was added and incubated at 37 °C for 30 min. The reaction was terminated by the addition of 2.5 ml of 20% trichloroacetic acid (TCA) solution. The reaction mixture was transferred to a centrifuge tube and the precipitated protein was removed by centrifugation at 13 000 rpm for 15 min. The absorbance of the clear supernatant was measured at 280 nm in a UV–Visible spectrophotometer against appropriate blanks. One unit of inhibitor activity was defined as the decrease by one unit of absorbance of TCA soluble casein hydrolysis product liberated by trypsin action measured at 280 nm/min under the assay conditions. The protease inhibitor activity was expressed in terms of percent inhibition. Appropriate blanks for the enzyme, inhibitor, and the substrate were also included in the assay along with the test.
Statistical analysis
All values were expressed as mean ± SD. Comparisons between means were carried out using one-way ANOVA test followed by Tukey HSD test using SPSS, version 14 (SPSS Inc., Chicago, IL). Differences at p < 0.05 were considered statistically significant.
Results and discussion
Chemistry
Reaction of anthranilic acid (1) with 4-nitrobenzoyl chloride (2) in methylene chloride in presence of triethylamine, as acid scavenger, affords the corresponding N-benzoylanthranilic acid 3 (Scheme 1). The later compound was cyclized by boiling with acetic anhydride to produce the 3H-benzoxazin-4-one derivative 4. Boiling the later with 100% hydrazine in the absence of solvent afforded the required 3-amino derivative 5 in almost 85% yield (Scheme 1). The IR spectrum of this compound indicated the presence of doublet band corresponding to NH2 absorption band at 3287, 3251 cm−1. The NMR spectrum of 5 showed the single of NH2 protons at δ 5.7–5.8 ppm and the signal of carbonyl carbon at 164.1 ppm.
Scheme 1. Synthesis of the targeted 3-substituted benzylideneamino-2-(4-nitrophenyl)quinazolin-4(3H)-one derivatives 6–16.
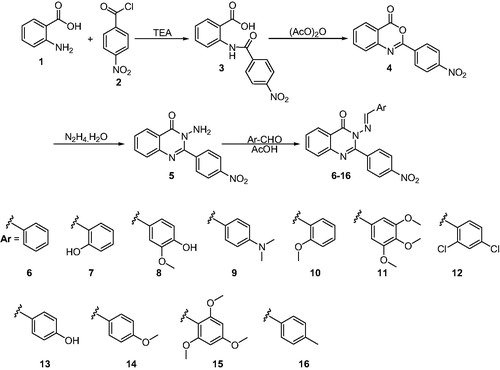
Reaction of the obtained amine 5 with certain aromatic aldehydes afforded the corresponding arylidenes derivatives 6–16 (Scheme 1) and their structure was confirmed by elemental analyses and spectral data. Generally, the spectra of these compounds showed the absence of NH2 absorption band from the IR and 1H NMR spectra. The 1H NMR spectra of compounds 6–16 showed the singlet signal of olefenic proton in the region δ 8.30–9.10 ppm. The Citation13C spectra showed the signals of carbon atoms at the expected δ values which were in accordance with the proposed structures.
Biological activities
Acute toxicity (LD50) test
Oral doses of investigated compounds up to 1000 mg/kg did not produce any symptoms of acute toxicity and no mortality in mice were observed for 48 h. It was suggested that oral LD50 >1000 mg/kg. The tested compounds were generally considered safe according to the results obtained in our study, since substances possessing LD50 >50 mg/kg are non-toxic as mentioned by Buck et al.Citation14.
Effect on ulcerative colitis
The model of acetic acid induced colitis shares many of the histologic features of ulcerative colitis in human beings including mucosal ulceration and sub-mucosal edemaCitation15.
In the present study, rats of normal control group, showed no abnormal changes suggesting that handling procedure had no interference with the experimental outputs. Macroscopic damage parameters of the colon of control colitis rats revealed dark brown lesions, mucosal hyperemia, edema, erosion and ulceration.
The inflammatory changes of the intestinal tract were associated with a significant increase of wet weight/length (1.04 ± 0.09 g/cm) of the colon specimens as an indicator of inflammation. These inflammatory indices were significantly improved by oral dosing of dexamethasone (0.51 ± 0.08 g/cm) and the investigated compounds for 5 days after ulcer induction. The present results were in agreement with the results of previous workersCitation16,Citation17.
The investigated compounds possessed anti-ulcerative colitis activities with different potentials (). Compound 14 was more effective than dexamesathone; it produced percent protection of control colitis by (79.78%) followed by compound 13 which was as effective as the standard drug dexamethasone with 75.82 protection of control colitis. Other compounds were less effective than dexamesathone. They produced varying degrees of protection ranging from 60.00% to 25.82% protection and the lowest activity among the investigated compound was recorded for compound 6.
Table 1. Curative anti-ulcerative colitis effect of compounds 6–16 on acetic acid-induced colitis in rats.
Evaluation of PLA2 inhibitory effect
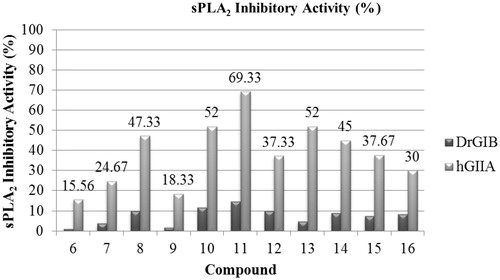
According to our study, three compounds namely, 11, 10, 13 showed significant results, it produced inhibition activity by 69.33%, 52.00%, 52.00%, respectively ( and ). It is worth noticing that these compounds showed the most promising results in inhibiting the catalytic activity of the pro-inflammatory human phospholipase A2 group IIA (hG-IIA) and the dromedary group IB (DrG-IB). However, using the same compounds, no inhibition of the phospholipase A2 activity of DrG-IB was observed. These results indicating a selective inhibition of the tested compounds against these two sPLA2, these results were in agreement with Fabia et al.Citation18 who suggested that PLA2 may play an important role in acetic acid-induced colitis and inhibition of its activity may offer an alternative mode of treatment in ulcerative colitis.
Table 2. Inhibition data of compounds 6–16 against sPLA2 (DrG-IB and hG-IIA) activity.
Phospholipase A2 participates in the regulation of phospholipid metabolism and biosynthesis of eicosanoids, serum levels of PLA2 are suggested to reflect the disease activity in patients with ulcerative colitis (UC). Several studies showed that serum PLA2 activity and levels are significantly increased in UCCitation2. Yamaguchi et al.Citation19 demonstrated that serum PLA2 group IIA levels in UC were closely correlated with histological disease activity. Immunohistochemical study showed the production of PLA2 IIA by the polymorphonuclear cells, macrophages and colonic epithelial cells. The authors suggested that serum PLA2 group IIA is a good candidate for assessing disease activity in UC as one of clinical laboratory testsCitation19,Citation20.
Evaluation of protease inhibitory effect
Anti-protease activity against commercial serine protease, trypsin were tested. The investigated compounds were evaluated for recovering protease inhibitor molecules.
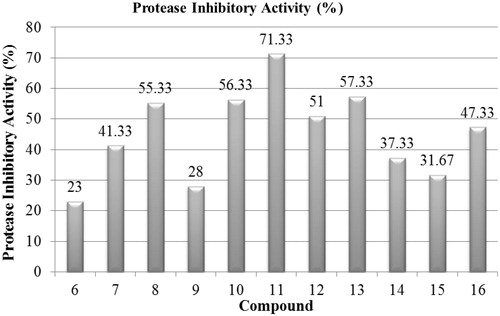
Results of protease inhibition activity are presented in and . It showed that; compound 11, exhibited maximum protease inhibitor activity (71.33% inhibition) followed by 13 (57.33% inhibition). Our findings are in line with several previous reports which have established a potential link between protease, protease inhibition levels and progression of ulcerative colitis. In fact, increased fecal protease activity has been observed in ulcerative colitis patients and was correlated with disease severity while the levels of endogenous protease inhibitors such as pancreatic secretory trypsin inhibitor and α-proteinase inhibitor are decreasedCitation3,Citation21,Citation22.
Table 3. Inhibition data of compounds 6–16 against protease activity.
Lower protease inhibitor levels could enhance tissue destruction by inflammatory proteases in the vicinity of active colonic inflammation. Most of the activity was derived from serine proteases of pancreatic and granulocytic cell origin and was inhibited by diisopropyl fluorophosphates (DFP), a potent serine protease inhibitor. Further, the amount of pancreatic secretory trypsin inhibitor (PSTI), a 56-amino acid serine protease inhibitor present in the intestinal lumen, is lower in colonic tissue from ulcerative colitis and Crohn’s disease patientsCitation23. A reduction in PSTI levels could render the intestinal mucosa less resistant to proteolytic attack and more susceptible to inflammationCitation24.
Structure-activity correlation
One compound showed interesting anti-ulcerative colitis activities. Compound 14 substituted with methoxy group at position 4 of the phenyl ring. This compound was followed by compound 13 which bears two chloro-substitutions at positions 2 and 4. The lowest activities were recorded to compound 6 with phenyl moiety; it produced 25.82% curative activity. In addition, compound 16 with toluyl moiety showed low activity (29.70%). The other compounds showed moderate activity ranged from 33.40% to 52.75% in acetic acid-ulcerative colitis rats.
Among the 10 investigated compounds, three of them namely 11 (3,4,5 tri-methoxy), 13 (4-hydroxy), 10 (2-methoxy) showed significant PLA2 inhibitory effect results, it produced inhibition activity by 69.33%, 52.00%, 52.00%, respectively. However, compounds 9 (N,N-dimethyl amino) and compound 6 (phenyl) showed the lowest activity towards inhibition of PLA2 (18.33% and 15.56%).
In evaluating the protease inhibitor activity of the investigated compounds; compound 11 (3,4,5 tri-methoxy), showed maximum protease inhibitory activity (71.33% inhibition) followed by 13 (4-hydroxy), 10 (2-methoxy) and 8 (4-hydroxy,3-methoxy), (57.33%, 56.33% and 55.33% inhibition, respectively).
Conclusion
In the present study, among the investigated compounds; the most effective agents for protection against UC were compounds 14 and 13 with percent protection of 79.78% and 75.80%. Both compounds moderately inhibited PLA2 enzyme type hG-IIA [45.00%, 52.00%] and minimally inhibited protease enzyme [9.00%, 4.67%], respectively. Compounds 12, 15, 11, which exhibited [60.00%, 52.75%, 50.00%], protection against UC, moderately inhibited hG-IIA [37.33%, 37.67%, 69.33%] and protease [51.00%, 31.67%, 71.33%], respectively. The least active one was compound 6, it minimally inhibited both hG-IIA and protease [15.00%, 23.00%]. Compounds 14, 13, 12 and 11 showed the best protection against UC in a mechanism based on their moderate differential inhibition of hG-IIA and protease enzymes. However, all the compounds under test selectively inhibited hG-IIA type of PLA2 rather than DrG-IB with varying degrees.
Declaration of interest
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for the work through the research group project NO RGP-VPP-060.
References
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369:1641–7
- Minami T, Tojo H, Shinomura Y, et al. Raised serum activity of phospholipase A2 immunochemically related group II enzyme in inflammatory bowel disease: its correlation with disease activity of Crohn’s disease and ulcerative colitis. Gut 1992;33:914–21
- Bohe M. Pancreatic and granulocytic endoproteases in faecal extracts from patients with active ulcerative colitis. Scand J Gastroenterol 1987;22:59–64
- Hargreaves KR, Kropinski AM, Clokie MR. What does the talking? Quorum sensing signalling genes discovered in a bacteriophage genome. PLoS One 2014;24;9:e85131
- Cooksley CM, Davis IJ, Winzer K, et al. Regulation of neurotoxin production and sporulation by a Putative agrBD signaling system in proteolytic Clostridium botulinum. Appl Environ Microbiol 2010;76:4448–60
- Sleebs BE, Czabotar PE, Fairbrother WJ, et al. Quinazoline sulfonamides as dual binders of the proteins B-cell lymphoma 2 and B-cell lymphoma extra-long with potent proapoptotic cell-based activity. J Med Chem 2011;54:1914–26
- Koller M, Lingenhoehl K, Schmutz M, et al. Quinazolinedione sulfonamides: a novel class of competitive AMPA receptor antagonists with oral activity. Bioorg Med Chem Lett 2011;21:3358–61
- Paick JS, Cho MC, Song SH, et al. Impacts of the quinazoline-based alpha1-antagonist, terazosin, and of the sulfonamide derivative, tamsulosin, on serum prostate-specific antigen and prostate volume. J Korean Med Sci 2008;23:509–13
- Stutzmann JM, Vuilhorgne M, Mignani S. Synthesis and structure-activity relationships of 4,10-dihydro-4-oxo-4H-imidazo[1,2-a]indeno[1,2-e]pyrazine derivatives: highly potent and selective AMPA receptor antagonists with in vivo activity. Mini Rev Med Chem 2004;4:123–40
- Spanò V, Montalbano A, Carbone A, et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur J Med Chem 2014;74:340–57
- Li Z, Tan JH, He JH, et al. Disubstituted quinazoline derivatives as a new type of highly selective ligands for telomeric G-quadruplex DNA. Eur J Med Chem 2012;47:299–311
- Barraja P, Caracausi L, Diana P, et al. Pyrrolo[3,2-h]quinazolines as photochemotherapeutic agents. Chem Med Chem 2011;6:1238–48
- Traquandi G, Ciomei M, Ballinari D, et al. Identification of potent pyrazolo[4,3-h]quinazoline-3-carboxamides as multi-cyclin-dependent kinase inhibitors. J Med Chem 2010;53:2171–87
- Buck WB, Osweiter GD, VanGelder AG. In clinical and diagnostic veterinary toxicology. 2nd ed. Dubuque (IA): Kendall, Hunt Publishing Co.; 1976
- Sharon P, Stenson WF. Metabolism of arachidonic acid in acetic acid induced colitis in rats. Gastroenterology 1985;88:55–63
- Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res 2006;53:324–30
- Thippeswamy BS, Mahendran S, Biradar MI, et al. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol 2011;654:100–5
- Fabia R, Ar’Rajab A, Willén R, et al. Effect of putative phospholipase A2 inhibitors on acetic acid-induced acute colitis in the rat. Br J Surg 1993;80:1199–204
- Yamaguchi O, Sugimura K, Ishizuka K, et al. Correlation between serum phospholipase A(2) IIA levels and histological activity in patients with ulcerative colitis. Int J Colorectal Dis 2002;17:311–16
- Bohe M, Genell S, Ohlsson K. Protease inhibitors in plasma and faecal extracts from patients with active inflamatory bowel disease. Scand J Gastroenterol 1986;21:598–604
- Hawkins JV, Emmel EL, Feuer JJ, et al. Protease activity in a hapten-induced model of ulcerative colitis in rats. Dig Dis Sci 1997;42:1969–80
- Playford RJ, Hanby AM, Patel K, Calam J. Infuence of inflammatory bowel disease on the distribution and concentration of pancreatic secretory trypsin inhibitor within the colon. Am J Pathol 1995;146:310–16
- Senda SY, Fujiyama Y, Bamba T, Hosoda S. Treatment of ulcerative colitis with camostat mesilate, a serine protease inhibitor. Intern Med 1993;32:350–4
- Bogert MT, Gortner RA, Amend CG. Researches on quinazolines. XXVII. Synthesis of 3-aminoaryl-4-quinazolones from acylanthranils and aromatic diamines. J Am Chem Soc 1911;33:949–62
- Verma M, Singh S, Singh, KN. Synthesis of some new benzoxazine derivatives of biological interest. Heterocyclic Comm 2003;9:499–502
- Ecobichon DJ. The basis of toxicity testing. 3rd ed. New York: CRC Press;1984:43–86
- Mascolo N, Izzo A, Autore G, et al. Acetic acid-incluced colitis in in normal and essential fatty acid deficient rats. J Pharmacol 1995;272:469–75
- Kunitz, M. Crystalline soyabean trypsin inhibitor II. General properties. J Gen Physiol 1947;30:291–310
- De Araújo AL, Radvanyi F. Determination of phospholypase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987;25:1181–8