Abstract
In this preliminary study, a new series of some cerium vanadate derivatives have been investigated as new type of inhibitors of xanthine oxidase (XO; E.C 1.17.3.2). XO is a superoxide-producing enzyme found normally in serum and the lungs, and its activity is concerned with several important health problems such as gout, severe liver damage, vascular dysfunction and injury, oxidative eye injury and renal failure. In this study, we present a critical overview of the effects of these novel type agents on XO with comparing the efficacy and safety profiles of allopurinol, the efficient classical inhibitor of XO.
Introduction
Reactive oxygen species (ROS) are also capable of damaging a wide range of essential biomolecules including proteins, DNA and lipidsCitation1, and this leads to many chronic diseases, such as atherosclerosis, cancer, diabetes, ageing and other degenerative diseases in humansCitation2. One of the very important enzymes that has been reported to increase during oxidative stress is xanthine oxidase (XO), which is conventionally seen as a late enzyme of purine catabolism, catalyzing the oxidation of hypoxanthine to xanthine and of xanthine to uric acidCitation3. In the context of the inflammatory response, XO is believed to prevent infection by generating ROS and can be seen as an agent of innate immunity. Xanthine oxidoreductase (XOR) has the unusual property that it can exist in a dehydrogenase (XD) form, which uses NAD+ as electron acceptor, and an oxidase form (XO), which uses oxygen as electron acceptor. Both forms have a high affinity for hypoxanthine and xanthine as substrates. Conversion of one form to the other may occur under various conditionsCitation4. Della Corte and StirpeCitation5 established that conversion of XD into XO occurred reversibly via oxidation of sulfhydryl groups and irreversibly by proteolytic cleavage. The XO form produces ROS. XO plays an important role in various diseasesCitation6,Citation7. Therefore, the enzyme is thought to be involved in various pathological processes such as tissue injury due to ischemia followed by reperfusion, but its exact role is still a matter of debateCitation8.
One therapeutic approach for gout is the use of XO inhibitors such as allopurinolCitation9; however, allopurinol use can result in a number of adverse side effectsCitation10; thus, in this study, we aim to investigate to develop new compounds with vanadate derivatives for possible devoicing the undesirable effects of allopurinol since there is a growing interest in natural phenolic antioxidants, present in medicinal plants that might help to attenuate the oxidative damage.
A group of 15 transition metals in group III of the periodic table are called lanthanides or rare earths. Vanadium is a ubiquitous, naturally occurring, transition metal that is found in high concentrations in the earth’s crust, oceans, soil and fossil fuels. Metallic vanadium is not present in nature. Vanadium is found as vanadium compounds with the oxidation states changing from −1 to +5 (commonly +3, +4 and +5)Citation11. +4 and +5 valence states of vanadium turn between these two valence states occurring in biological systemsCitation12. Vanadium possesses a regulatory role in the biological system, influences a number of enzymes, regulates the functions of several second messengers and modulates a battery of genesCitation13,Citation14. The discovery of several pharmacological properties, such as the insulin-mimetic action, antihyperlipidemia, antihypertension, antiobesity, enhancement of oxygen affinity of hemoglobin and myoglobin, and diuretic action, opens up a number of therapeutic avenues of this trace elementCitation15. In a review, the preclinical and clinical data presented to show highlights vanadium as a unique multivalent element with versatile potential to detect, prevent and treat human cancerCitation16. In terms of anti-tumor activity, a wide range of compounds of both transition metals and main group elements have been investigated for efficacyCitation17. Cerium can act similar to calcium in organisms, so it accumulates in the bones in small amounts. However, very little cerium accumulates in the food chain. Human blood contains 0.001 ppm, human bones contain 3 ppm and human tissue contains 0.3 ppm of cerium. There is a total of 40 mg of cerium in a typical 70-kg human. Humans typically consume less than a milligram per day of cerium. Cerium (or other lanthanides) is the cofactor for the methanol dehydrogenase of the methanotrophic bacterium Methylacidiphilum fumariolicum SolVCitation18. Cerium has no known biological role, but it has been noted that cerium salts can stimulate metabolismCitation19.
Several reports demonstrated that vanadate stimulates the oxidation of NAD(P)H by enzymatic and nonenzymatic sources of superoxide anion radicalCitation20–23 via a sequence of free-radical chain reactions with the net production of hydrogen peroxide (H)Citation24. Furthermore, several enzymes were known to generate cellular superoxide in a controlled way: one of these enzymes is a membrane-located NADPH oxidase, which is present in the endotheliumCitation25. A second and well-known superoxide generating system consists of xanthine/XO, which is found membrane-associated on endothelial and other cellsCitation25. Recently, it has been shown that incubation of mouse epidermal JB6 cells with vanadate induces generation of HO, which seems to play an essential role in the action of vanadate in apoptosisCitation26.
Materials and methods
Materials
Sepharose 4B, l-tyrosine, benzamidine, protein assay reagents, ceric sulfate and vanadium (V) oxide were obtained from Sigma Chem. Co. (Milan, Italy) All other chemicals obtained from Sigma and Merck (Istanbul, Turkey) were used without further purification.
Methods
Synthesis of the compounds
Analytically pure ceric sulfate and vanadium (V) oxide weighed an appropriate molar ratio and homogenized in an agate mortar. The mixture was placed into a porcelain crucible to heat in domestic microwave oven. After that, the material was exposed to microwave irritation for 10 min and then homogenized again. The sample was heated at 700 °C for 2 h getting the best crystallization. Then, the final product was ready for the doping process.
Cerium vanadate sample was then doped with Ca and Mg elements in the form of oxides in different concentrations. CeVO4 and CaO or MgO samples were weighed separately and ground together in an agate mortar to obtain homogenization and then transferred into an alumina crucible. The mixture was heated at 750 °C for 7 h and ground in an agate mortar. The doping procedure was based on the study performed by Özdemir et al.Citation27. The concentrations of Ca/Mg ions doped into CeVO4 are 0.1%, 0.5% and 5% by weight. The structural analyzes of samples were checked by X-ray powder diffraction. The powder X-ray diffraction (XRD) patterns were recorded using PANanalytical X’Pert PRO diffractometer (XRD) with Cu Kα (1.5406 Å, 45 kV and 30 mA) radiation.
Enzyme purification protocol
Fresh bovine milk, without added preservatives, was cooled down to 4 °C overnight. EDTA and toluene were then added to give final concentrations of 2 mM and 3% (v/v), respectively. The milk was churned with a blender at top speed for 30 min at room temperature. During this time, the temperature rose from 4 to 45 °C. After cooling the churned milk to about 4 °C, the churning process was repeated and the sample was filtered. This sample was brought to 38% saturation by addition of solid ammonium sulfateCitation28. The suspension was centrifuged at 25 155 g for 30 min, and the precipitate formed was discarded. The supernatant was brought to 50% saturation with solid ammonium sulfate. The precipitate formed was collected by centrifugation at 25 155 g for 60 min and dissolved in 0.1 M Tris-HCl, pH = 7.6. The affinity column containing sepharose-4B-l-tyrosine-p-amino benzamidine was equilibrated in 0.1 M glycine, 0.1 M NaCl, pH = 9.0Citation29. The sample was applied to the affinity gel, washed with 0.1 M glycine, pH = 9.0 XO, and then eluted with 25 mM benzamidine in 0.1 M glycine, 0.1 M NaCl, pH = 9.0Citation30. Fractions of 1.5 mL were collected, and their absorbance was measured at 280 nm.
Enzyme activity measurements
XO activity was determined at 37 °C by the modified method of Massey et al.Citation31. The conversion of xanthine to uric acid was followed by monitoring the change in absorbance at 295 nm, using a CARY 1E, UV-Visible Spectrophotometer-VARIAN spectrometer (ɛ292 = 9.5 mM−1 cm−1) (Helma GmbH & Co., Nuernberg, Germany). The reaction mixture contained 50 mM Tris-HCl, pH = 7.6, and 0.15 mM xanthine, at 37 °C. The assay was initiated by the addition of the enzyme. One unit of enzyme activity was defined as the amount of enzyme that converts 1 μmol of xanthine to uric acid per min under defined conditionsCitation29.
Kinetic studies of XO
For the inhibition studies of some cerium vanadate derivatives, different concentrations were added to the enzyme activity. XO enzyme activity with cerium vanadate derivatives was assayed by following the oxidation of xanthine. Activity % values of XO for seven different concentrations of cerium vanadate derivatives were determined by regression analysis using Microsoft Office 2007 Excel (Istanbul Microsoft Office, Turkey). XO enzyme activity without a cerium vanadate was accepted as 100% active. The graphs determined that the inhibitor concentration caused up to 50% inhibition (IC50 values) on the enzymeCitation29.
Results and discussion
In , powder XRD patterns of CeVO4, 0.1 wt. % Ca-CeVO4, 0.5 wt. % Ca-CeVO4, 1 wt. % Ca-CeVO4, 0.1 wt. % Mg-CeVO4, 0.5 wt. % Mg-CeVO4 and 1 wt. % Mg-CeVO4 are shown. When the pattern of CeVO4 is compared to The International Centre for Diffraction Data (ICDD) cards, it gets along with CeVO4 (ICDD: 12–757), crystallized in tetragonal system with unit cell parameters a = 7.399 and c = 6.496 Å and space group I41/and 141. In the XRD pattern of Ca or Mg-doped CeVO4, CaO and MgO peaks were not detected.
Figure 1. The powder X-ray diffraction pattern of undoped cerium vanadate and Ca/Mg-doped cerium vanadate.
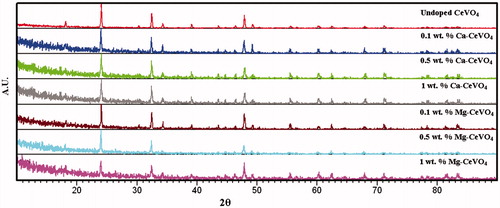
XOR is a molybdenum-containing enzyme, which catalyzes the hydroxylation reaction on sp2-hybridized carbon centers of a variety of substrates including purines, aldehydes and other heterocyclic compounds. There are some studies that present in the literature about XO inhibition by various compounds such as flavonoidsCitation32. In this study, cerium vanadate compounds inhibition of XO resulted in a decreased production of uric acid, which was measured spectrophotometricallyCitation31. The XO inhibitory effect of the cerium vanadate compounds was found to be 0.14–0.2 mM range as listed in . As you can see from the results, the new cerium vanadate compounds also showed a powerful inhibition when we compare other results in the literatureCitation33. Furthermore, the standard inhibitor of XO (allopurinol) had an IC50 value of 7.4 (±0.07) μM was given in the literatureCitation34. In the literature, there are also some studies about naphthopyrans catalyzed by silica supported fluoroboric acid as a new class of non purine XO inhibitorsCitation35. Another study is described an approach for the design and synthesis of 1-acetyl-3,5-diaryl-4,5 dihydro (1H)pyrazoles as a new class of potential non purine XO inhibitorsCitation36. Furthermore, aza-flavones were designed as potential XO inhibitors by Dhiman et al.Citation37. Another approach is N-(1,3-diaryl-3-oxopropyl)amides as a new template for XO inhibitors designed by Nepali group as wellCitation38.
Table 1. Inhibition results of doped and undoped CeVO4.
Conclusion
A more important area in which XO inhibitors clearly need improvement is the reduction of their side effects. As seen from the literature, allopurinol does have a number of serious side effects, and the cellular and molecular mechanisms of these side effects are incompletely understood. Some recent data indicate that the renal toxicity of allopurinol is related to impairment of pyrimidine metabolismCitation35. But the encouraging findings, such as in this study for having novel series of XO inhibitors, may act an important role for various therapeutic indications for XO inhibition.
The goal of this article is to give a critical attempt of the effects of new type of XO inhibitors and also to review the novel emerging therapeutic strategies offered by this promising approach.
Declaration of interest
The authors have no declaration of interest for this study.
References
- Urso ML, Clarkson MP. Oxidative stres, exercise, and antiooxidant supplemetation. Toxicology 2003;189:41–54
- Yun-Zhong F, Sheng Y, Guoyao Wu. Free radicals, antioxidants, and nutrition. Nutrition 2002;18:872–9
- Enroth C, Eger BT, Okamoto K, et al. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA 2000;97:10723–8
- Kooij A. A re-evaluation of the tissue distribution and physiology of xanthine oxidoreductase. Histochem J 1994;26:889–915
- Della Corte E, Stirpe F. The regulation of rat liver xanthine oxidase. Biochem J 1972;126:739–45
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 2004;555:589–606
- Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev 2004;36:363–75
- Boumerfeg S, Baghiani A, Djarmouni M, et al. Inhibitory activity on xathine oxidase and antioxidant properties of Teucrium polium. Chin Med 2012;3:30–41
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhbitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114
- Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 2002;105:2619–24
- Barceloux DG. Vanadium. Clin Toxicol 1999;37:265–78
- Mukherjee B, Patre B, Mahapatra S, et al. Vanadium: an element of atypical biological significance. Toxicol Lett 2004;150:135–43
- Nriagu JP. Vanadium in the environment, Part 2. Health effects. New York: John Wiley and Sons, Inc.; 1998
- Stern A, Yin X, Tsang SS, et al. Vanadium as a modulator of cellular regulatory cascades and oncogene expression. Biochem Cell Biol 1993;71:103–12
- Rashwan NM, Al-Firdous FA. Insulin-mimetic activity of vanadium and zinc in diabetic experimental rats. J Am Sci 2011;7:407–16
- Bishayee A, Waghraya A, Patel MA, Chatterjee M. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence (mini-review). Cancer Lett 2010;294:1–12
- Desoize B. Metals and metal compounds in cancer treatment. Anticancer Res 2004;24:1529–44
- Pol A, Barends TRM, Dietl A, et al. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 2014;16:255–64
- Emsley J. Nature’s building blocks: an A-Z guide to the elements (New ed.). New York, NY: Oxford University Press; 2011:375–83, 412–15, 475–81, 511–20, 529–33, 582. ISBN 978-0-19-960563-7
- Liochev S, Fridovich I. The vanadate-stimulated oxidation of NAD(P)H by biomembranes is a superoxideinitiated free radical chain reaction. Arch Biochem Biophys 1986;250:139–45
- Rau M, Patole MS, Vijaya S, et al. Vanadate-stimulated NADH oxidation in microsomes. Mol Cell Biochem 1987;75:151–9
- Liochev SI, Fridovich I. Further studies of the mechanism of enhancement of NADH oxidation by vanadate. J Free Radic Biol Med 1985;1:287–92
- Darr D, Fridovich I. Vanadate and molybdate stimulate the oxidation of NADH by superoxide radical. Arch Biochem Biophys 1984;232:562–5
- Liochev S, Fridovich I. A study on the mechanism of the vanadate-dependent NADH oxidation. Free Radic Biol Med 1988;5:349–54
- Ullrich V, Bachschmid M. Superoxide as a messenger of endothelial function. Biochem Biophys Res Commun 2000;278:1–8
- Huang C, Zhang Z, Ding M, et al. Vanadate induces p53 transactivation through hydrogen peroxide and causes apoptosis. J Biol Chem 2000;275:32516–22
- Özdemir Z, Ozbayoglu G, Aysen Y. Investigation of thermoluminescence properties of metal oxide doped lithium triborate. J Mater Sci 2007;42:8501–8
- Özer N, Müftüoglu M, Ataman D, et al. Simple, highyield purification of xanthine oxidase from bovine milk. J Biochem Biophys Methods 1999;39:153–9
- Beyaztas S, Arslan O. Effects of some antibiotics on xanthine oxidase enzyme activities in vitro. Hacettepe J Biol Chem 2011;39:195–205
- McManaman JL, Shellman V, Wright RM, Repine JE. Purification of rat liver xanthine oxidase and xanthine dehydrogenase by affinity chromatography on benzamidine-sepharose. Arch Biochem Biophys 1996;332:135–41
- Massey V, Brumby PE, Komai H. Studies of milk xanthine oxidase: some spectral and kinetic properties. J Biol Chem 1969;244:1682–91
- Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem 1999;63:1787–90
- Bytyqi-Damoni A, Genç H, Zengin M, et al. In vitro effect of novel β-lactam compounds on xanthine oxidase enzyme activity. Artif Cells Blood Substit Immobil Biotechnol 2012;40:369–77
- Ahmad I, Ijaz F, Fatima I, et al. Xanthine oxidase/tyrosinase inhibiting, antioxidant, and antifungal oxindole alkaloids from Isatis costata. Pharm Biol 2010;48:716–21
- Sharma S, Sharma K, Ojha R, et al. Microwave assisted synthesis of naphthopyrans catalysed by silica supported fluoroboric acid as a new class of non purine xanthine oxidase inhibitors. Bioorg Med Chem Lett 2014;24:495–500
- Nepali K, Singh G, Turan A, et al. A rational approach for the design and synthesis of 1-acetyl-3,5-diaryl-4,5 dihydro (1H)pyrazoles as a new class of potential non purine xanthine oxidase inhibitors. Bioorg Med Chem 2011;19:1950–8
- Dhiman R, Sharma S, Singh G, et al. Design and synthesis of aza-flavones as a new class of xanthine oxidase inhibitors. Archie der Pharmazie (Chemistry in Life Sciences) 2013;346:7–167
- Nepali K, Agarwal A, Sapra S, et al. N-(1,3-diaryl-3-oxopropyl)amides as a new template for xanthine oxidase inhibitors. Bioorg Med Chem 2011;19:5569–76

