Abstract
Some of the environmental toxicants acting as endocrine disruptors have been associated with health hazards in human and wildlife by modulating hormonal actions. Atrazine, a strong endocrine disruptor, induces detrimental effects on gonads in male and female, and causes impairment of fertility and developmental problems as well as sex alterations. Atrazine decreases the activities of antioxidant enzymes and thus responsible for oxidative stress. Natural antioxidants have shown ability to reduce/slow down the apoptotic effect of atrazine on testicular tissue. In the present study, some N-phenyl-4-aryl-polyhydroquinolines bearing phenolic or/and alkoxy group(s) (6a–6g) were synthesized and evaluated for antioxidant activity in four different assays. Three best compounds (6e–6g) were studied for their ameliorative effect on testicular tissue supplemented with atrazine in vitro.
Introduction
There is a growing concern among the scientific community, policy makers as well as general public about the adverse impact on health, in general, and reproductive potential, in particular, of a wide range of chemicals released in the environment as herbicides, fungicides, insecticides, etc.Citation1,Citation2 Some of these environmental toxicants strongly act as endocrine disruptors with the potential to alter hormonal action within the body. To cite a few, insecticides (dichlorodiphenyltrichloroethane) DDT and endosulfan, herbicide atrazine, fungicide vinclozolin, various polychlorinated biphenyls used as dielectric and coolant fluids (in transformers, capacitors, electric motors), bisphenol A used in plastics and epoxy resins, polybrominated diphenyl ethers used as a flame retardant, a variety of phthalates as a plasticizers, etc. are some of the commonly accessible endocrine disruptors. Most of these toxicants have been banned in developed countries for agricultural and household purposes due to continuous revelation of their side effects. Unfortunately, they are still being used in developing countries because of their low cost, easy availability as well as the absence of safer and cheaper alternatives. These chemicals are entering into animal and human body through food and drinking water, and their chronic exposure is associated with serious detrimental effects on the body system such as increased incidence of various cancer types, deformations of various body parts besides fertility problems, sexual developmental problems such as feminizing of males or masculine effects on femalesCitation3–6. Low concentration of ROS (reactive oxygen species) generated by sperm is believed to mediate the processes of capacitation, hyperactivation and acrosome reaction which are crucial for effective fertilization of an ovum by a spermCitation7. It has been found that endocrine disrupting chemicals disturb the prooxidant/antioxidant system of the cells leading to the generation of free radicals and thus reactive oxygen species. Mammalian spermatozoa, being rich in membrane bound polyunsaturated fatty acids (PUFA), are more susceptible to ROS attack making them more vulnerable targets of ROS. Mathur and coworkers have noticed that methoxychlorCitation8,Citation9 and lindaneCitation10 force oxidative stress on testes including spermatozoa of mammals, by affecting the normal activities of oxidative enzymes. Atrazine, which is popularly known as “the 21st century DDT,” has shown the capability of turning male frogs to females at concentrations as low as 2.5 parts per billionCitation11,Citation12. Atrazine is believed to decrease the activities of antioxidant enzymes superoxide dismutase, catalase (CAT), glutathuione peroxidase (GSH-Px), glutathione-s-transferase, etc. Continuous atrazine exposure is also known to cause cancer in mammals including humansCitation13, and developmental problems in fishes and amphibiansCitation14. Detrimental effects of atrazine on gonads (testes and ovaries) have been studied in fishesCitation15, amphibiansCitation16 and laboratory mammalsCitation17. While it had been widely acknowledged that exposure to atrazine leads to endocrine disruption, it remained a matter of unsettled debate for long time. Studies reported that atrazine disrupts the hypothalamic control of pituitary–ovarian function as well as of testes, demonstrating the direct role of central nervous system (CNS)Citation18,Citation19 while others believed that there are certain enzymes which are responsible for conversion of an endocrine product into an active hormonal form in the gonads which, when transferred to hypothalamaus/pituitary gland, further controls endocrine system. Unfortunately, these enzymes are inhibited by high doses of atrazine in the gonadsCitation11. Now it is believed that there is involvement of hypothalamo–pituitary–gonadal axis for controlling the whole mechanism and they are interconnected directly and/or indirectly and influence each otherCitation20. However, it is certain that high doses of atrazine slow down the maturation of gonadotrophic system of male as well as female offspring and counteract the gonads’ endocrinal functioning in adults besides causing oxidative stress.
Antioxidants are believed to counteract the harmful effects of ROS and regulate the physiological defense systems and therefore could be useful for prevention or treatment of oxidative-stress related diseasesCitation21–23. Phenolic derivatives such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), butylated hydroxyquinone (TBHQ), rosemarydiphenol, vanillin, syringaldehyde, flavanoids, tannins, phenolic acids, etc. are ubiquitous antioxidants possessing good antioxidant activity. Besides antioxidant potential, alkyl esters of gallic acid are also found to be potent antitumour agents acting by the induction of apoptosisCitation24,Citation25. Lipophilic antioxidants based on galloyl–cinnmaic hybrids have shown synergistic effect, acting as chain breaking antioxidants in biomembranes and other type of lipid systemsCitation26. Vitamin therapy is also playing nowadays a decisive role in attenuating the oxidative stress caused by various endocrine disruptors e.g. vitamin E to ameliorate atrazine-induced oxidative stress in liverCitation27and testicular tissueCitation28. Quercetin, a natural flavanoid found to exhibit significant protection of sertoli germ cells against atrazine-induced oxidative damageCitation29. The very fact that the treatment of oxidative stress produced by atrazine or various other endocrine disruptors on various body parts especially gonads is mainly limited to natural products prompted us to design some new molecules in search of an alternative. QuinolineCitation30,Citation31 skeleton bearing secondary amine group has been tested in recent past specifically for antioxidant activity and has shown promising resultsCitation32,Citation33, e.g. 1,2-dihydro-2,2,4-trimethylquinoline is used in plastic industry and ethoxiquine in fish meal, animal feeds, spices, etc. Polyhydroquinolines bearing phenolic group alone or in combination with alkoxy groups could prove to be a useful scaffold, led us to synthesize some novel N-phenyl-4-aryl-polyhydroquinolines for testing their antioxidant activity. Compounds showing better antioxidant potential were further evaluated for their ameliorative effect on atrazine-induced oxidative stress in testicular tissue of goat (Capra hircus). To the best of our knowledge, this is in itself the first study of laboratory synthesized molecules towards attenuating the oxidative stress produced by atrazine on testicular tissue, particularly on a domestic animal.
Materials and methods
All reactions were carried out under atmospheric pressure. Melting points were determined in open capillaries in an electrical melting point apparatus and were uncorrected. IR spectra were recorded on ABB MB 3000 DTGS IR instrument using the KBr pellet technique. The 1H NMR and 13C NMR spectra were recorded in DMSO-d6 on Bruker NMR spectrometer at 300 and 75.5 MHz, respectively. The δ values are given in ppm relative to tetramethylsilane (TMS) as internal standard (for 1H and 13C NMR). Mass spectra were recorded on JEOL-AccuTOF JMS-T100LC mass spectrometer having a DART (Direct Analysis in Real Time) source in ES+ mode. The purity of the compounds was checked by 1H NMR and thin layer chromatography (TLC) on silica gel plates using a mixture of chloroform and methanol as eluent. Iodine or UV lamp was used as a visualizing agent. All solvents were dried and/or purified according to the standard procedures prior to use. All reagents employed in the present work were purchased from commercial suppliers and used without further purification. Abbreviations “s” for singlet, “d” for doublet, “m” for multiplet, “ex” for exchangeable proton (detected by disappearance of signal upon D2O addition) are used for NMR assignments and “s” for strong, “m” for medium for IR assignments. “d” stands for decomposition in melting point data.
DPPH (2,2′-diphenyl-1-picryl-hydrazyl), ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)], linoleic acid (LA), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), TPTZ (2,4,6-tripyridyl-s-triazine), ferric chloride (FeCl3·6H2O), sodium acetate, potassium persulfate, atrazine, Dulbecco’s modified Eagle’s Medium (DMEM), antibiotics (penicillin, streptomycin) were purchased from Sigma-Aldrich or other standard suppliers.
Synthesis
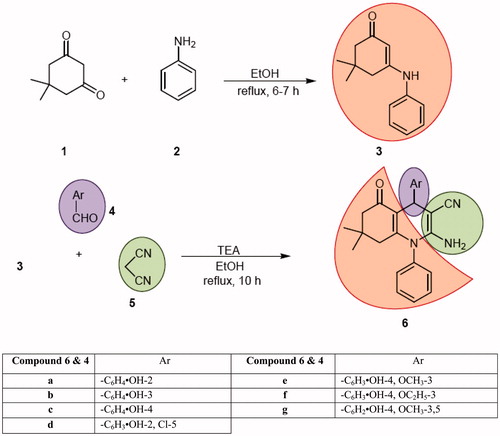
General procedure for synthesis of 3-phenylamino-5,5-dimethylcyclohex-2-enone 3
An ethanolic solution (50 mL) of dimedone (1, 42.8 mmol) and aniline (2, 42.8 mmol) was refluxed for 6–7 h. On reaction completion, it was reduced to half of its volume and cooled to room temperature. Yellow crystalline solid separated out which was filtered, washed with cold ethanol (5 mL) and crystallized from ethanol to afford 3-phenylamino-5,5-dimethylcyclohex-2-enone 3. Yield: 80%; m.p. 180–182 °C; Lit.Citation34 m.p. 184–185 °C; IR (KBr) cm−1: 3232 (m, N–H stretch), 1595 (m, C=O stretch), 1566 (s, C=C stretch), 1520 (s, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 8.79 (s, ex, 1H, NH), 7.34–7.39 (m, 2H, Ar), 7.10–7.19 (m, 3H, Ar), 5.31 (s, 1H, =CH), 2.38 (s, 2H, CH2), 2.05 (s, 2H, CH2), 1.02 (s, 6H, 2 × CH3).
Representative protocol for synthesis of N-phenyl-4-aryl-polyhydroquinolines, 6a–6g
An ethanolic solution (25 mL) of 3-phenylamino-5,5-dimethylcyclohex-2-enone (3, 2.79 mmol), appropriate arylaldehyde (4, 2.79 mmol) and malononitrile (5, 3.35 mmol) containing 4–5 drops of triethylamine was refluxed for 10 h. Solution slowly turned reddish-orange which indicated reaction progression. On reaction completion, solution was reduced to half and cooled to room temperature. Solid started separating out or otherwise added small amount (5–10 mL) of ethyl acetate to the concentrated reaction mixture for solid separation which was filtered, washed with ethyl acetate (10 mL) followed by water (30 mL), dried and crystallized from EtOH:EtOAc (1:1) to afford N-phenyl-4-aryl-polyhydroquinolines (6) as yellow- or orange-coloured solid in moderate yield.
2-Amino-4-(2-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6a
Yield: 85%; m.p. 286–288 °C (d); IR (KBr) cm−1: 3441 (m, O–H stretch), 3348 (m, N–H stretch), 2206 (s, C≡N stretch), 1643 (s, C=O stretch), 1612 (s, C=N stretch), 1551 (s, C=C stretch), 1497 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 9.39 (s, ex, 1H, OH), 7.51–7.60 (m, 3H, Ar), 7.40 (d, 2H, J = 6.9 Hz, Ar), 7.09 (d, 1H, J = 7.8 Hz, Ar), 6.99 (t, 1H, J = 7.2 Hz, Ar), 6.76 (d, 2H, J = 7.8 Hz, Ar), 5.09 (s, ex, 2H, NH2), 4.71 (s, 1H, CH), 2.16–2.23 (m, 2H, CH2), 1.94–2.00 (m, 1H, CH2), 1.66–1.71 (m, 1H, CH2), 0.88 (s, 3H, CH3), 0.76 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): δ 195.3 (CO), 155.1, 151.8, 151.6, 137.0, 132.6, 130.5, 130.4, 130.1, 129.2, 127.6, 122.1, 119.4, 116.2, 111.2, 60.5, 49.8, 41.5, 32.2, 31.8, 29.6, 26.6; DART-MS: m/z 386.23 (M+H)+, C24H23N3O2H+ calcd. 386.17.
2-Amino-4-(3-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6b
Yield: 75%; m.p. 254–256 °C; IR (KBr) cm−1: 3464 (m, O–H stretch), 3325 (m, N–H stretch), 2183 (s, C≡N stretch), 1651 (s, C=O stretch), 1597 (s, C=C stretch), 1489 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 9.32 (s, ex, 1H, OH), 7.58–7.62 (m, 3H, Ar), 7.38 (d, 2H, J = 6.6 Hz, Ar), 7.10 (t, 1H, J = 8.1 Hz, Ar), 6.70 (d, 2H, J = 8.4 Hz, Ar), 6.57 (d, 2H, J = 7.2 Hz, Ar), 5.29 (s, ex, 2H, NH2), 4.36 (s, 1H, CH), 2.16–2.23 (m, 2H, CH2), 1.98–2.03 (m, 1H, CH2), 1.66–1.71 (m, 1H, CH2), 0.88 (s, 3H, CH3), 0.75 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): δ 195.3 (CO), 157.8, 151.5, 150.5, 148.3, 136.7, 130.6, 130.3, 130.2, 129.7, 122.0, 117.8, 114.1, 113.7, 112.2, 61.0, 56.5, 49.8, 41.4, 36.6, 32.3, 29.5, 26.8; DART-MS: m/z 386.23 (M+H)+, C24H23N3O2H+ calcd. 386.17.
2-Amino-4-(4-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6c
Yield: 70%; m.p. 266–268 °C (d); Lit.Citation35 m.p. 267–268 °C; IR (KBr) cm−1: 3472 (m, O–H stretch), 3325 & 3271 (m, N–H stretch), 2176 (s, C≡N stretch), 1612 (br s, C=O and C=C stretch), 1489 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 9.19 (s, ex, 1H, OH), 7.53–7.61 (m, 6H, Ar), 7.37 (d, 2H, J = 6.9 Hz, Ar), 7.06 (d, 2H, J = 8.4 Hz, Ar), 6.70 (d, 2H, J = 8.4 Hz, Ar), 5.21 (s, ex, 2H, NH2), 4.36 (s, 1H, CH), 2.15–2.20 (m, 2H, CH2), 1.96–2.02 (m, 1H, CH2), 1.65–1.71 (m, 1H, CH2), 0.87 (s, 3H, CH3), 0.73 (s, 3H, CH3).
2-Amino-4-(5-chloro-2-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6d
Yield: 88%; m.p. 302–304 °C (d); IR (KBr) cm−1: 3464 (m, O–H stretch), 3325 (m, N–H stretch), 2183 (s, C≡N stretch), 1643 (s, C=O stretch), 1612 (s, C=C stretch), 1551 (s, C=C stretch), 1497 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 9.75 (s, ex, 1H, OH), 7.56–7.64 (m, 4H, Ar), 7.37 (d, 2H, J = 6.6 Hz, Ar), 7.03–7.05 (m, 2H, Ar), 6.78 (d, 1H, J = 6.3 Hz, Ar), 5.16 (s, ex, 2H, NH2), 4.65 (s, 1H, CH), 2.16–2.22 (m, 2H, CH2), 1.95–2.00 (m, 1H, CH2), 1.66–1.71 (m, 1H, CH2), 0.88 (s, 3H, CH3), 0.77 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): δ 195.4 (CO), 154.5, 152.1, 151.8, 136.9, 134.4, 130.6, 130.4, 130.1, 129.3, 128.9, 127.3, 122.5, 121.9, 117.7, 110.3, 59.6, 49.7, 41.4, 32.8, 32.3, 29.6, 26.5; DART-MS: m/z 420.19 (M+H)+, C24H22ClN3O2H+ calcd. 420.14.
2-Amino-4-(4-hydroxy-3-methoxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6e
Yield: 77%; m.p. 254–256 °C; IR (KBr) cm−1: 3456 (m, O–H stretch), 3340 (m, N–H stretch), 2176 (s, C≡N stretch), 1651 (s, C=O stretch), 1605 (s, C=C stretch), 1512 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 8.78 (s, ex, 1H, OH), 7.57–7.59 (m, 3H, Ar), 7.34 (d, 2H, J = 6.0 Hz, Ar), 6.65–6.77 (m, 3H, Ar), 5.26 (s, ex, 2H, NH2), 4.38 (s, 1H, CH), 3.75 (s, 3H, OCH3), 2.17–2.24 (m, 2H, CH2), 1.99–2.04 (m, 1H, CH2), 1.65–1.71 (m, 1H, CH2), 0.88 (s, 3H, CH3), 0.76 (s, 3H, CH3); Citation13C NMR (75.5 MHz, DMSO-d6): δ 195.3 (CO), 151.4, 150.2, 147.8, 145.5, 138.1, 136.8, 130.6, 130.3, 130.1, 122.0, 119.3, 115.8, 112.6, 111.3, 61.4, 55.9, 49.8, 41.4, 36.0, 32.3, 29.6, 26.6; DART-MS: m/z 416.23 (M+H)+, C25H25N3O3H+ calcd. 416.18.
2-Amino-4-(3-ethoxy-4-hydroxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6f
Yield: 68%; m.p. 268–270 °C; IR (KBr) cm−1: 3450 (m, O–H stretch), 3333 & 3248 (m, N–H stretch), 2176 (s, C≡N stretch), 1636 (s, C=O stretch), 1558 (s, C=C stretch), 1520 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 8.71 (s, ex, 1H, OH), 7.53–7.61 (m, 3H, Ar), 7.33 (d, 2H, J = 7.2 Hz, Ar), 6.64–6.76 (m, 3H, Ar), 5.27 (s, ex, 2H, NH2), 4.36 (s, 1H, CH), 3.94–4.04 (m, 2H, OCH2), 2.17–2.23 (m, 2H, CH2), 1.98–2.03 (m, 1H, CH2), 1.64–1.70 (m, 1H, CH2), 1.33 (t, 3H, J = 8.7 Hz, CH3), 0.87 (s, 3H, CH3), 0.74 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): δ 195.4 (CO), 151.5, 150.2, 146.8, 145.8, 138.0, 136.8, 136.8, 130.6, 130.2, 130.1, 122.0, 119.4, 115.9, 112.9, 112.6, 64.2, 61.3, 49.8, 41.4, 35.9, 32.2, 29.6, 26.5, 15.3; DART-MS: m/z 430.26 (M+H)+, C26H27N3O3H+ calcd. 430.20.
2-Amino-4-(4-hydroxy-3,5-dimethoxyphenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydro-3-quinolinecarbonitrile 6g
Yield: 65%; m.p. 268–270 °C; IR (KBr) cm−1: 3444 (m, O–H stretch), 3325 & 3294 (m, N–H stretch), 2183 (s, C≡N stretch), 1651 (s, C=O stretch), 1612 (s, C=C stretch), 1558 (s, C=C stretch), 1504 (m, N–H bend); 1H NMR (300 MHz, DMSO-d6): δ 8.19 (s, ex, 1H, OH), 7.57–7.60 (m, 3H, Ar), 7.32 (d, 2H, J = 6.3 Hz, Ar), 6.47 (s, 2H, Ar), 5.29 (s, ex, 2H, NH2), 4.39 (s, 1H, CH), 3.74 (s, 6H, 2× OCH3), 2.19–2.27 (m, 2H, CH2), 2.00–2.06 (m, 1H, CH2), 1.66–1.72 (m, 1H, CH2), 0.89 (s, 3H, CH3), 0.76 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): δ 195.4 (CO), 151.5, 150.4, 148.3, 137.1, 136.8, 134.4, 130.7, 130.2, 130.1, 122.0, 112.4, 112.6, 61.3, 56.5, 56.3, 49.8, 41.5, 36.3, 32.2, 29.7, 26.4, 19.0; DART-MS: m/z 446.26 (M+H)+, C26H27N3O4H+ calcd. 446.20.
In vitro antioxidant assays
DPPH radical scavenging activity
Scavenging activity of compounds against DPPH radical was assessed according to the method of Blois with some modificationsCitation36. Briefly, 2 mL of each compound in EtOAc (1 mM) was mixed with 2 mL of DPPH methanol solution (0.1 mM). The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured at 517 nm. Ascorbic acid (1 mM) and BHT (1 mM) were used as references. The ability to scavenge DPPH radical was calculated by the following equation:
where Abscontrol is the absorbance of DPPH radical in EtOAc (ethyl acetate) and Abssample is the absorbance of DPPH radical solution mixed with sample. All determinations were performed in triplicate (n = 3).
ABTS•+ radical scavenging activity
For ABTS•+ assay, the procedure followed the method of Re et al.Citation37 with some modifications. The stock solutions included 7 mM ABTS•+ solution and 2.4 mM potassium persulfate solution. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 14 h at room temperature in the dark. The solution was then diluted by mixing 2 mL ABTS•+ solution with 30 mL methanol to obtain an absorbance of 0.706 ± 0.01 units at 734 nm using a spectrophotometer. A fresh ABTS•+ solution was prepared for each assay. 0.1 mL of compound in EtOAc (1 mM) was allowed to react with 2 mL of the ABTS•+ solution, and the absorbance was taken at 734 nm after 2 min. The ABTS•+ scavenging capacity of the compound was compared with that of BHT and ascorbic acid and percentage inhibition calculated as:
where Abscontrol is the absorbance of ABTS•+ radical in methanol and Abssample is the absorbance of an ABTS•+ radical solution mixed with sample. All determinations were performed in triplicate (n = 3).
Ferric reducing/antioxidant power (FRAP)
The ferric reducing ability of plasma (FRAP) assay was done according to the method described by Benzie and StrainCitation38 with some modifications. The stock solutions included 300 mM acetate buffer (3.1 g C2H3NaO2 × 3H2O and 16 ml C2H4O2), pH 3.6, 10 mM TPTZ solution in 40 mM HCl and 20 mM FeCl3 × 6H2O solution. The fresh working solution was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ solution and 2.5 mL FeCl3 × 6H2O solution and then warmed at 37 °C before using 0.15 mL of compound in EtOAc (1 mM) was allowed to react with 2.8 mL of the FRAP solution for 30 min in the dark condition. Readings of the colored product (ferrous tripyridyltriazine complex) were then taken at 593 nm. Results are expressed in mM trolox equivalent (TE). Ascorbic acid and BHT were used as references. All determinations were performed in triplicate (n = 3).
Antioxidant activity in linoleic acid system by the ferric thiocyanate (FTC) method
The antioxidant activity of compounds against lipid peroxidation was measured through ammonium thiocyanate assay, as described by Mistuda et al.Citation39, with some modifications. The reaction solution, containing 0.2 mL of 1 mM compound in EtOAc, 0.2 mL of linoleic acid emulsion (25 mg/mL in 99% ethanol) and 0.4 mL of 50 mM phosphate buffer (pH 7.4), was incubated in the dark at 40 °C. A 0.1-mL aliquot of the reaction solution was then added to 3 mL of 70% (v/v) ethanol and 0.05 mL of 30% (w/v) ammonium thiocyanate. Precisely 3 min after the addition of 0.05 mL of 20 mM ferrous chloride in 3.5% (v/v) hydrochloric acid to the reaction mixture, the absorbance of the resulting red color was measured at 500 nm. Aliquots were assayed every 24 h until the day after the absorbance of the control solution (without compound) reached maximum value. BHT and ascorbic acid were used as references. All determinations were performed in triplicate (n = 3).
Cytotoxic assays
Collection of materials
Goat (Capra hircus) testes were procured from the slaughter houses around Kuruskshetra (26°6′N, 76°5′E), India, and brought to laboratory in normal saline at 4 °C. Then the testes were decapsulated, cut into smaller pieces (1 mm3) and further processed for testicular tissue cultureCitation40.
Reagents/Chemicals
Atrazine, DMSO, phosphate buffered saline (PBS), DMEM and antibiotics (penicillin, streptomycin) were used.
Testicular tissue culture in vitro
The testicular tissues (1 mm3) were washed three times with PBS and then cultured in DMEM medium supplemented with antibiotics (200-unit having concentration of penicillin 100 IU/mL and streptomycin 100 IU/mL) in CO2 incubator (5% CO2, 95% humidity, 38 °C). The testicular tissues were incubated with 10 μm of each tested compound in DMSO along with 100 nmol/mL concentration of atrazine in DMSO. Harvesting was done after 6 h of culture duration. Then the harvested testicular tissue cultures were washed with PBS and fixed in alcoholic Bouin’s fixative for 24 h. Following dehydration in series of ethanol grades, the testicular tissues were cleared in xylene and embedded in paraffin wax. The paraffin blocks were prepared, and sections were cut at 5 μm thickness. These sections were then stained with haematoxylin and eosin, and were observed under Olympus microscope (Japan).
Results and discussion
Chemistry
The synthesis of N-phenyl-4-aryl-polyhydroquinolines 6 was achieved in two steps starting from aniline (2) involving one pot multi-component reaction strategy according to Scheme 1. 3-Phenylamino-5,5-dimethylcyclohex-2-enone (3) was obtained by condensation of dimedone (1) with aniline (2) in refluxing ethanol. Treating 3 with phenolic aldehydes (4) and malononitrile (5) in a molar ratio of (1:1:1.2) in refluxing ethanol for 10 h containing catalytic amount of triethylamine yielded the corresponding N-phenyl-4-aryl-polyhydroquinolines 6 as target compounds in moderate yield. 3-Phenylamino-5,5-dimethylcyclohex-2-enone 3 was characterized by comparing its spectral data as well as melting pointCitation34. The structure of 6a–6g was assigned on the basis of its FT-IR, 1H NMR and Citation13C NMR spectral data and was confirmed on the basis of mass spectra (DART-MS). A characteristic absorption band in the region 2176–2206 cm−1 was assigned to C≡N stretching in IR spectra of all the compounds besides displaying other important absorption bands at 3441–3472 cm−1 corresponding to O–H stretching, 3325–3348 cm−1 due to N–H stretching and 1636–1651 cm−1 due to C=O stretching. Phenolic OH was further confirmed by the presence of an exchangeable singlet in the region δ 8.19–9.75 in 1H NMR spectra. NH2 protons and methine (CH) proton of the dihydropyridine skeleton were assigned in the region δ 5.09–5.33 and δ 4.36–4.89, respectively, whereas two methyl groups were confirmed in the aliphatic region at δ 0.87–0.89 and δ 0.72–0.83. Methoxy groups in 6e and 6g were clearly assigned in the region δ 3.74–3.75. A multiplet–triplet pattern due to two and three protons at δ 3.94–4.04 and δ 1.33, respectively, present in compound 6f confirmed the presence of –OCH2CH3 group. Other appropriate signals were easily assigned in aromatic and aliphatic regions.
In vitro antioxidant activity
It has been reported that sometimes compounds show antioxidant activity in vitro in extracellular environment but unfortunately, their efficacy in the intracellular environment is often decreased, probably due to their poor solubility as well as poor permeability across the cell membraneCitation41,Citation42. Therefore, to make an effective antioxidant compound in a biological context, solubility in different media as well as transport across the cell membrane are extremely important besides other governing factors. That is why various methods are employed to check the antioxidant potential of a compound which vary in environment as well as generation of free radicalsCitation41,Citation42. In our study, we used three different simple redox-based assays, each having its own uniqueness as well as importance but all involving one redox reaction with the oxidant, for measuring the reducing capacity of the test compounds. Among these assays, the DPPH assay is influenced by the kinetic behaviour of the antioxidant compound, while the FRAP and ABTS•+ assays are carried out at acidic and neutral conditions, respectivelyCitation43. Importance of the DPPH method lies in the fact that this method is independent of compound’s polarity, while the ABTS•+ method is applicable for both lipophilic and hydrophilic compoundsCitation42. The FRAP assay is inexpensive, reagents are simple to prepare, the results are highly reproducible, and the procedure is straightforward and speedyCitation38. Other assay employed in this study has been selected to evaluate the antioxidant activity of the polyhydroquinolines under investigation in linoleic acid system by FTC method. The radical scavenging and total antioxidant activity of compounds (1 mM) were compared with those of ascorbic acid and BHT at the same concentration and expressed as % inhibition against DPPH, ABTS and mM TE, respectively ().
Table 1. In vitro antioxidant activities of compounds 6 through DPPH, ABTS and FRAP methods.
DPPH and ABTS assays are based on the ability of the test compounds to scavenge synthetic-free radicals, using a variety of radical-generating systems and methods for detection of the oxidation end-point. ABTS or DPPH radical scavenging methods are common spectrophotometer procedures for determining the antioxidant capacities of components. In DPPH method, the DPPH radical is used as a substrate to evaluate free radical scavenging activities of compounds 6a–6g. It involves the reaction of specific antioxidant with a stable-free radical DPPH. As a result, there is reduction of DPPH concentration by the antioxidant that leads to a decrease in the optical absorbance of DPPH that is detected by spectrophotometer at 517 nm. All the seven compounds (6a–6g) tested showed antioxidant activity less than ascorbic acid but three compounds 6e–6g showed activity comparable to BHT.
The ABTS activity of all the compounds 6a–6g was found to be higher than BHT (15.3 ± 0.7%), and the four compounds 6a, 6e–6g were found to exhibit excellent activity (>90%) while the other two compounds 6d and 6c showed moderate activity when compared with ascorbic acid (>100%).
In FRAP assay, reduction of ferric tripyridyl triazine (Fe3+- TPTZ) complex to ferrous form (which has an intense blue color) at low pH can be monitored by measuring the change in absorption at 593 nm. The change in absorbance is therefore directly related to the combined or “total” reducing power of the electron donating antioxidants present in the reaction mixture. Only three compounds (6e–6g) could manifest FRAP activity, showing capability to inhibit free radical chain reaction by donating a hydrogen atom. Compound 6g showed much higher FRAP activity than the remaining two compounds and BHT which was comparable to ascorbic acid.
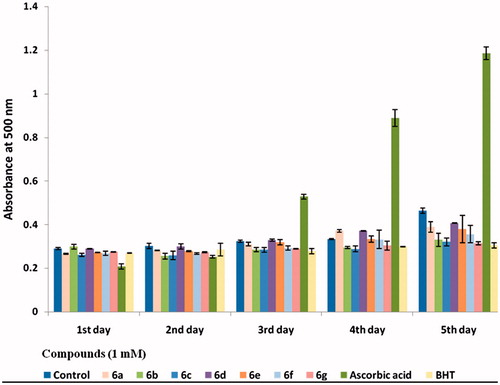
During linoleic acid peroxidation, peroxides are formed and these compounds oxidize Fe2+ to Fe3+. The Fe3+ ion forms a complex with SCN–, which has a maximum absorbance at 500 nm. Thus, a high-absorbance value was an indication of high-peroxide formation. This method measures the amount of peroxide produced during the initial stages of oxidation, which is the primary product of oxidation. All tested polyhydroquinolines hindered the oxidation of linoleic acid for all 5 days, and the antioxidant activity of compound 6g was comparable to that of BHT. Ascorbic acid lost its peroxide quenching ability very soon (after 2 days) and started demonstrating pro-oxidative effect at this concentration from the 3rd day (). Antioxidant activity data can be seen in supplementary information (Table 2).
A common structure–activity relationship (SAR) was clearly noticed amongst tested polyhydroquinolines in the sense that antioxidant potential decreases as the position of phenolic hydroxyl group varies from ortho to para i.e. away from (hydro)quinolinic system. It means that the synthesized system could show better antioxidant potential when phenolic OH is present at ortho-position. Addition of (5)-Cl group in 6a, i.e. compound 6d, showed decrease in antioxidant activity. Combination of (para) phenolic hydroxyl group with alkoxy group was also studied and was found that (4-)phenolic hydroxyl flanked by two (3- & 5-) methoxy groups, i.e. 6g, showed enhanced antioxidant activity as compared to phenolic group associated with single methoxy group as in 6e. Changing the mehoxy group (6e) to ethoxy (6f) showed a slight decrease in antioxidant potential.
In vitro ameliorative study on atrazine-induced oxidative stress on testicular tissue
In the present investigation, three compounds 6e–6g showing promising antioxidant activity in all the four employed methods were further evaluated for their ability to ameliorate the histopathological changes induced by atrazine in testicular tissue of goat in vitro. The concentration of synthesized compounds and atrazine was kept at 10 μmol/mL and 100 nmol/mL (DMSO), respectively, in the media for carrying out the experiments. Testicular tissue sections (5 μm) stained with haematoxylin and eosin revealed normal histoarchitecture of the seminiferous tubules in control group under microscope (×1000) (). Seminiferous tubules were packed with loose connective tissue having blood vessels. Normal Leydig cells were present outside the seminiferous tubules. Sertoli cells and all types of the germ cells were present in the seminiferous tubules well arranged in the specific manner. Spermatogonia were present at the basal portion and next to these, spermatocytes occupied the middle portion. Spermatids formed the next layer and then mature elongated spermatozoa were present toward the lumen of the seminiferous epithelium. Sertoli cells were having broad basal portion with round or oval nuclei resting on the basal lamina and its cytoplasmic part with irregular extensions scattered in between the germ cells up to the lumen of the seminiferous tubules ().
Figure 2. Photomicrograph of testicular tissue (a) showing normal arrangement of different types of germ cells [spermatogonia (Sg), spermatocytes (Sp), spermatids (Sd) & sperms (Spm)] in control group; (b) treated with atrazine (100 nmol/ml) showing a number of pycnotic nuclei (P), crescent-shaped nuclei (arrow), chromolysis (ch) and vacuolization (V) within the seminiferous tubule; (c) treated with atrazine (100 nmol/ml) supplemented with compound 6e showing reduction in atretic changes induced by atrazine after 6 h of exposure; (d) treated with atrazine (100 nmol/ml) supplemented with compound 6g showing decline in number of chromolysis, condensation and fragmentation; (e) treated with atrazine (100 nmol/ml) supplemented with compound 6f showing protection against atrazine-induced cytotoxicity. H&E (×1000).
![Figure 2. Photomicrograph of testicular tissue (a) showing normal arrangement of different types of germ cells [spermatogonia (Sg), spermatocytes (Sp), spermatids (Sd) & sperms (Spm)] in control group; (b) treated with atrazine (100 nmol/ml) showing a number of pycnotic nuclei (P), crescent-shaped nuclei (arrow), chromolysis (ch) and vacuolization (V) within the seminiferous tubule; (c) treated with atrazine (100 nmol/ml) supplemented with compound 6e showing reduction in atretic changes induced by atrazine after 6 h of exposure; (d) treated with atrazine (100 nmol/ml) supplemented with compound 6g showing decline in number of chromolysis, condensation and fragmentation; (e) treated with atrazine (100 nmol/ml) supplemented with compound 6f showing protection against atrazine-induced cytotoxicity. H&E (×1000).](/cms/asset/14cbfb13-1541-4a6d-ae28-d26e5d2b8907/ienz_a_960864_f0002_c.jpg)
In the experimental group, treated with atrazine having concentration 100 nmol/mL in the culture medium, histomorphological alterations were revealed in testicular tissues after 6 h of atrazine exposure. Many atretic changes were noticed in different types of germ cells such as hyalinization, margination of chromatin, pinching off of the nuclear material, pycnotic nuclei, crescent shaped nuclei, chromolysis, vacuolization, fragmentation of nuclei, decondensation of nuclear materials, appearance of meganucleated cells, disruption in the normal arrangement of cells, etc. (). Degenerating Leydig cells were also observed in the testicular sections. Basal lamina was also detached from the underlying cells in few portions of seminiferous tubules (). Supplementation of compounds 6e–6g (10 μmol/mL) separately in next three experiments along with the exposure of atrazine promisingly showed antioxidant-type attenuation/prevention of degenerating changes induced by atrazine in testicular tissue. Reduction/delaying in atretic changes were clearly observed in each case such as decline in chromolysis, condensation, fragmentation and vacuolization ().
The results of the present investigation clearly demonstrated that atrazine-induced atretic changes like vacuolization, chromolysis, pycnosis, fragmentation, hyalinization, condensation were dramatically reduced when the atrazine-cultured testicular tissue was simultaneously supplemented with compounds 6e–6g separately, thereby indicating the protecting effect of these compounds against atrazine-induced cytotoxicity. Although all the three compounds (6e–6g) showed ameliorative effect, protecting ability (delaying in degenerative changes) of 6e was found to be much higher followed by 6g and then 6f.
Conclusions
The objective of the present work was to investigate some new organic molecules which could have potential ability to act like natural antioxidants (Vitamin C & E) and attenuate the apoptotic effect of atrazine on testicular tissue in vitro. We could find N-phenyl-4-aryl-polyhydroquinolines as a potential scaffold which could be synthesized by following one pot multi-component reaction strategy and might possess antioxidant activity comparable to natural antioxidants. Seven N-phenyl-4-aryl-polyhydroqunilines (6a–6g) were synthesized by treating 3-phenylamino-5,5-dimethylcyclohex-2-enone with various phenolic aldehydes and malononitrile in refluxing ethanol containing catalytic amount of triethylamine. All seven target compounds were tested in vitro for antioxidant activity by three different assays (DPPH, ABTS+ and FRAP) and by ferric thiocyanate method. Three compounds (6e–6g) exhibited better antioxidant activity in all the assays which were further tested for their ameliorative effect on degenerative changes in testicular tissue induced by atrazine in vitro and showed promising results. Data suggested that introduction of alkoxy group significantly enhanced the antioxidant activity of polyhydroquinolines and thus ameliorative effect on atrazine-induced histopathological changes in testicular tissue.
Supplementary material available online
Supplemental Material.pdf
Download PDF (12.6 KB)Acknowledgements
The authors are thankful to Sophisticated Analytical Instrument Facility, Central Drug Research Institute, Lucknow, India, for Mass spectra.
Declaration of interest
Navneet Chandak is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the award of senior research fellowship. The authors report no conflict of interest.
References
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009;30:293–341
- Pocar P, Brevini TA, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction 2003;125:313–25
- Colborn T. Founder: TEDX. Available from: http://endocrinedisruption.org/endocrine-disruption/bisphenol-a/overview [last accessed 15 Jun 2014]
- Hayes TB, Anderson LL, Beasley VR, et al. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J Steroid Biochem Mol Biol 2011;127:64–73
- Murray TJ, Lea RG, Abramovich DR, et al. Endocrine disrupting chemicals: effects on human male reproductive health. Early Pregnancy 2001;5:80–112
- Nicolopoulou-Stamati P, Pitsos MA. The impact of endocrine disrupters on the female reproductive system. Human Reprod Update 2001;7:323–30
- da Silva FM, Marques A, Chaveiro A. Reactive oxygen species: a double-edged sword in reproduction. Open Vet Sci J 2010;4:127–33
- Gangadharan B, Murugan MA, Mathur PP. Effect of methoxychlor on antioxidant system of goat edpididymal sperm in vitro. Asian J Androl 2001;3:285–8
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of methoxyxhlor on the epididymal antioxidant system of adult rats. Reprod Toxicol 2002;16:161–72
- Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl 2001;3:205–8
- Hayes TB, Collins A, Lee M, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA 2002;99:5476–80
- Kriger K. Founder: Save the frogs. Available from: http://www.savethefrogs.com/threats/pesticides/atrazine/index.html [last accessed 25 Jun 2014]
- Fan W, Yanase T, Morinaga H, et al. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect 2007;115:720–7
- Scahill JL. Effects of atrazine on embryonic development of fathead minnows (Pimephales promelas) and Xenopus laevis. BIOS 2008;79:139–49
- Spanò L, Tyler CR, van Aerle R, et al. Effects of atrazine on sex steroid dynamics, plasma vitellogin concentration and gonad development in adult gold fish (Carrassius auratus). Aquat Toxicol 2004;66:369–79
- Hecker M, Park JW, Murphy MB, et al. Effects of atrazine on CYP19 gene expression and aromatase activity in testes and on plasma sex steroid concentrations of male African clawed frogs (Xenopus laevis). Toxicol Sci 2005;86:273–80
- Dehkhargani SF, Malekinejad H, Shahrooz R, Sarkhanloo RA. Detrimental effect of atrazine on testicular tissue and sperm quality: implication for oxidative stress and hormonal alterations. Iran J Toxicol 2011;5:426–35
- Cooper RL, Stocker TE, Tyrey L, et al. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci 2000;53:297–307
- Cooper RL, Laws SC, Das PC, et al. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol 2007;80:98–112
- Foradori CD, Hinds LR, Quihuis AM, et al. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol Reprod 2011;85:684–9
- Cocheme HM, Murphy MP. Can antioxidants be effective therapeutics? Curr Opin Investig Drugs 2010;11:426–31
- Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem 2011;18:3871–88
- Bursal E, Gülçin I. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–9
- Locatelli C, Filippin-Monteiro FB, Creczynski-Pasa TB. Alkyl esters of gallic acid as anticancer agents: a review. Eur J Med Chem 2013;6:233–9
- Gülçin I, Beydemir S, Topal F, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst and kit. J Enzyme Inhib Med Chem 2012;27:587–94
- Teixeira J, Silva T, Benfeito S, et al. Exploring nature profits: development of novel and potent lipophilic antioxidants based on galloyl–cinnamic hybrids. Eur J Med Chem 2013;62:289–96
- Singh M, Sandhir R, Kiran R. Effects on antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp Toxicol Pathol 2011; 63:269–76
- Sharma RK, Fulia A, Chauhan PK. Antioxidant attenuation of atrazine induced histopathological changes in testicular tissue of goat in vitro. Toxicol Int 2012;19:260–6
- Abarikwu SO, Pant AB, Farombi EO. Dietary antioxidant, quercetin protects sertoli-germ cells coculture from atrazine-induced oxidative damage. J Biochem Mol Toxicol 2012;26:477–85
- Liu ZQ, Han K, Lin YZ, Luo XY. Antioxidtive and prooxidative effect of 4-hydroxyquinoline derivative on free-radical-initiated hemolysis of erythrocytes is due to its distributive status. Biochim Biophys Acta 2002;1570:97–103
- Yoon MA, Zeong TS, Park DS, et al. Antioxidative effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull 2006;29:735–9
- Zhang Y, Fang Y, Liang H, et al. Synthesis and antioxidant activities of 2-oxo-quinoline-3-carbaldehde Schiff-base derivatives. Bioorg Med Chem Lett 2013;23:107–11
- Yang XH, Zhang PH, Zhou YH, et al. Synthesis and antioxidant evaluation of novel 4-aryl-hexahydroquinoline from lignin. ARKIVOC 2011;x:335–45
- Yamada K, Konakahara T, Iida H. Photochemical reactions of enamino ketones. Bull Chem Soc Jpn 1973;46:2504–11
- Gao S, Tsai CH, Tseng C, Yao CF. Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron 2008;64:9143–9
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200
- Re R, Pellegrini N, Proteggente A, et al. Antioxidnt activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 1999;26:1231–7
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6
- Mistuda H, Yasumoto K, Iwami I. Antioxidative action of indole compounds during the autooxidation of linoleic acid. Eiyoto Shokuryo 1966;19:210–14
- Chandak N, Bhardwaj JK, Sharma RK, Sharma PK. Inhibitors of apoptosis in testicular germ cells: Synthesis and biological evaluation of some novel IBTs bearing sulfonamide moiety. Eur J Med Chem 2013;59:203–8
- Barzegar A, Pedersen JZ, Incerpi S, et al. The mechanism of antioxidant activity of IRFI005 as a synthetic hydrophilic analogue of vitamin E. Biochimie 2011;93:1880–8
- Hadjipavlou-Litina D, Magoulas GE, Bariamis SE, et al. Synthesis and evaluation of the antioxidative potential of minoxidil-polyamine conjugates. Biochimie 2013;95:1437–49
- Morabito G, Trombetta D, Brajendra KS, et al. Antioxidant properties of 4-methylcoumarins in in vitro cell-free systems. Biochimie 2010;92:1101–7