Abstract
Keeping in view the recent success of molecular hybridization technique in drug design, 2,4-diarylpyrano[3,2-c]chromen-5(4H)-ones as conjugates of coumarins and chalcones have been designed and synthesized in the present study. The catalytic efficiency of various Lewis acids for the synthesis of designed conjugates under neat conditions was investigated, and SiO2 (200–400 mesh)-ZnCl2 was optimized as the best catalyst among the tested ones. The conjugates were evaluated for in-vitro xanthine oxidase activity. The results of the in-vitro assay were quite promising as some conjugates were endowed with remarkable inhibitory potential against the enzyme. HV-8, 11 and 12 were found to be high-potent inhibitors with HV-11 (the most potent inhibitor) possessing an IC50 value of 2.21 µM. The most active conjugate HV-11 was evaluated for the type of inhibition and was found to be a mixed type inhibitor. The compliance of some selected conjugates to the Lipinski rule was also calculated.
Introduction
Oxidative hydroxylation of hypoxanthine and xanthine catalyzed by xanthine oxidase to produce uric acid and reactive oxygen species leads to many diseases like gout and at least symptoms of diseases like oxidative damage to the tissueCitation1–3. Therefore, the selective inhibition of XO may result in broad spectrum chemotherapeutic for gout, cancer, inflammation and oxidative damageCitation2–4. AllopurinolCitation3,Citation4, 2-alkyl hypoxanthinesCitation5,Citation6, pterin and 6-formylpterinCitation7 represents the class of purine-based xanthine oxidase inhibitors. All these inhibitors have been successfully utilized and have proved their inhibitory potential towards the enzyme. However, these purine-based inhibitors have been reported to be associated with Steven Johnson syndrome and worsening of renal function induced in some of the patientsCitation2–4. Keeping in view these side effects, we have recently designed some non-purine xanthine oxidase inhibitors such as azaflavonesCitation8, n-acetyl pyrazolinesCitation9, β-acetamido compoundsCitation10, naphthopyransCitation11 and 4,6-diaryl/heteroarylpyrimidin-2(1H)-onesCitation12.
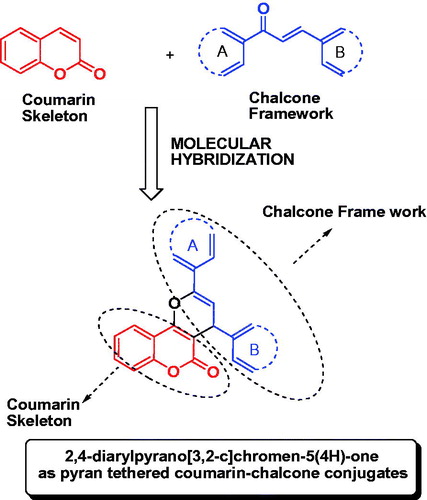
Keeping in view the promising xanthine oxidase inhibitory potential of these non-purine chemical architectures, the present study explores xanthine oxidase inhibitory evaluation of the designed 2,4-diarylpyrano[3,2-c]chromen-5(4H)-one as coumarin–chalcone conjugates ().
Coumarins form an important class of compounds, which occupy a special role in natureCitation13,Citation14. The presence of coumarin architecture in naturally occurring phytoconstituents with anticancerCitation15,Citation16, anti-HIVCitation17, antituberculosisCitation18, anti-influenzaCitation19, anti-alzheimerCitation20,Citation21, anti-inflammatoryCitation22, antiviralCitation23, antimicrobialCitation13 and xanthine oxidase inhibitory activitiesCitation24–26 makes it a privileged structure. Among the diverse array of biological activities possessed by coumarins, we are particularly interested towards their xanthine oxidase inhibitory potential which has also been extensively exploredCitation24–26. Esculetin represents the most potent xanthine oxidase inhibitor of this classCitation24.
Chalcones (1,3-diaryl-2-propen-1-ones) with an enone system between two aromatic rings constitute an important class of natural products which serve as precursors for the preparation of various flavonoids and exhibit interesting pharmacological activitiesCitation27,Citation28. Chalcones are also known as open chain flavonoid derivatives which are extensively reported as xanthine oxidase inhibitorsCitation29–31. Recently 3,5,2′,4′-tetrahydroxychalcone was found to be potent competitive inhibitor of the enzyme as indicated by the kinetic study. In vivo, intragastric administration of the chalcone was able to significantly reduce serum uric acid levels and inhibited hepatic xanthine oxidase activities of hyperuricemic mice in a dose-dependent mannerCitation32.
Thus, the proved potential of coumarin and chalcones as xanthine oxidase inhibitors and recent success of molecular hybridization technique in drug designCitation33 tempted us to design such conjugates. Pyran was selected as a linker for tethering coumarin with chalcones. The presence of pyran as an structural motif in various non-purine xanthine oxidase inhibitors supports the suitability of its selection as a linkerCitation10,Citation28–31.
Results and discussion
Chemistry
The usual strategy for the synthesis of 2,4-diaryl pyrano[3,2-c]coumarins is to react 4-hydroxy coumarin with α,β-unsaturated compounds. There are some methodologiesCitation34–36 utilizing various catalysts reported for the synthesis of 2,4-diaryl pyrano[3,2-c]coumarins, but each one suffers from some drawbacks such as environmentally hazardous (POCl3), expensive catalysts (AuCl3/3AgOTf) and some unavailable bronsted acids. Recently, Bagdi et al. reported an efficient regioselective methodology for the synthesis of 2,4-diaryl pyrano[3,2-c]coumarins via copper (II) triflateCitation37. However, in view of limited efficient and environmental friendly synthetic schemes and in continuation of our efforts to develop cost effective, highly yielding and environmental friendly methodologies for non-purine xanthine oxidase inhibitors, we investigated the effects of some inexpensive and easily available Lewis acids under neat conditions for the synthesis of desired compounds.
In an attempt to investigate the catalytic efficiency of various Lewis acids (), a model reaction was performed with different catalyst for the synthesis of target compound (Scheme 1). All reactions were carried out using 1 mmol of hydroxy coumarin and chalcone.
Table 1. Percentage yield of the model reaction with various catalysts.
presents the yield of the model reaction at 100 °C and a reaction time of 4 h with various Lewis acids. Further increase in temperature and time resulted in slight decrease in the yields with all the catalyst. Among the various Lewis acids tested, ZnCl2 was found to be the best catalyzing model reaction in 50% yield. Thus catalytic efficiency of ZnCl2 was further investigated by adsorbing it over different grades of silica. The yields of the model reaction were higher with ZnCl2 adsorbed on SiO2 (200–400 mesh) than the other grades of silica. The results clearly demonstrates the effect of increased effective surface area on the catalytic efficiency of silica-supported zinc chloride as the yield of the model reaction with SiO2 (200–400 mesh)-ZnCl2 was around 1.5-folds higher than silica (60–120 mesh). Thus the optimum reaction conditions involve the use of SiO2 (200–400 mesh)-ZnCl2 at 100 °C for 4 h. In order to examine the substrate scope of this reaction, variety of chalcone derivatives was used. indicates that all the reactions with electronically and sterically diverse chalcone derivatives proceeded smoothly to afford the target compounds in moderate to good yields. It was observed that the reaction of 4-hydroxy coumarin with chalcones possessing nitro and halo-substituted phenyl rings resulted in higher yields as compared to chalcones (methyl and methoxy substitutions). This could be attributed to the stronger −I effect of the halogens and nitro group as comparison to methoxy and methyl groups with stronger +R effect. The reaction of hydroxy coumarin and chalcone (synthesized from heteroaryl aldehyde) resulted in a clean reaction and afforded to yield the pyrano[3,2-c]coumarins in moderate yield; however, the reaction with chalcones synthesized from heteroaryl ketones did not produce the desired compound, rather an unidentified product was obtained. The characterization of the unidentified product is being dealt separately in our laboratory. The yields of the target compound obtained by our investigations were quite similar to one of the earlier reported methodology by Bagdi et al.Citation37 Thus, this protocol presents an alternative methodology employing the use of silica supported Lewis acid with an increased surface area under solvent-free condition as the advantages over the other existing methodologies.
Table 2. Isolated yields, IC50 values and type of inhibition.
In-vitro xanthine oxidase assay
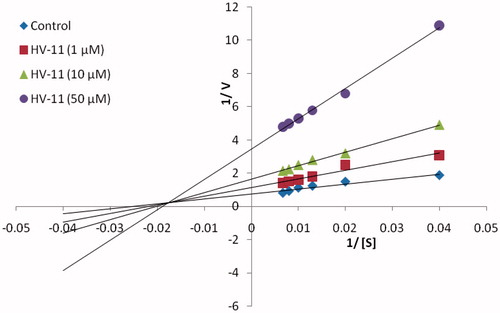
In vitro screening of the conjugates using bovine milk xanthine oxidase (grade 1, ammonium sulfate suspension) enzymatic assay was performed as described in the literatureCitation38. Allopurinol was employed as reference inhibitorCitation39. The results of the in-vitro assay () indicated that HV-8, HV-11 and HV-12 were endowed with significant inhibitory potential against the enzyme. HV-11 with an unsubstituted phenyl ring (Ring A) and thiophenyl ring (Ring B) was the most potent among the series with an IC50 value of 2.21 μM followed by HV-12 (unsubstituted phenyl ring – Ring A and 1-naphthyl – Ring B, IC50 value 4.31 μM) and HV-8 (unsubstituted phenyl – Ring A and 2-naphthyl – Ring B, IC50 value 8.21 μM). Conjugates HV-11 and HV-12 were found to be more active than allopurinol (IC50 = 8.79 μM), whereas HV-8 displayed a similar inhibitory profile to allopurinol. Enzyme kinetics study was performed for HV-11 (most active inhibitor). The Lineweaver–Burk plot () revealed that compound HV-11 was a mixed-type XO inhibitor. The pattern of graph shows that it is a form of mixed inhibition scenario. The Km, Vmax and slope are all affected by the inhibitor. The inhibitor has increased the Km and slope (Km/Vmax) while decreasing the Vmax. Moreover, carefully observing , it was found that intersecting lines on the graph converge to the left of the y-axis and above the x-axis which indicates that the value of α (a constant which defines the degree to which inhibitor binding affects the affinity of the enzyme for substrate) is greater than 1. This confirms that the inhibitor preferentially binds to the free enzyme and not the enzyme substrate complex. also revealed some interesting generalizations about the structure–activity relationship (SAR). SAR study revealed (i) placement of any substituent on Ring A irrespective of its electronic effects resulted in decreased activity profile (compare HV-1 with HV-13, 20 and HV-10 with HV-11. (ii) Conjugates with phenyl rings possessing electron donating substituents (Ring B) were more active than the conjugates with unsubstituted phenyl rings and phenyl rings possessing electron withdrawing substituents (Ring B) (compare HV-6, HV-7, HV-14 and HV-17 with HV-2, 3, 4, 5, 9, 16, 19. Over all the preference order of substituents for Ring B is as follows: OCH3 > CH3 > H > halo and nitro. (iii) Placement of a heteroaryl ring as Ring B significantly enhanced the inhibitory profile (compare HV-1 with HV-11). (iv) Placement of a bicyclic ring as Ring B also led to enhancement of activity (HV-1 with HV-8 and HV-12). (v) 1-Naphthyl was favored over 2-naphthyl as Ring B (Compare HV-8 with HV-12).
We also calculated the compliance of compounds to the Lipinski’s rule of fiveCitation40. Briefly, this simple rule is based upon the observation that most biological active drugs have a molecular weight (MW) of 500 or less, a log P not higher than 5, five or fewer hydrogen bond donor sites and ten or fewer hydrogen bond acceptor sites (N or O atoms). Conjugates HV-6 (moderately active) HV-8, 11, 12 (potent), HV-13, 16 (least active) were selected for the calculation of molecular properties on the basis of the degree of variation of the activity profile. The three most potent conjugates, i.e. HV-8, 11, 12, possessed an exactly same total polar surface area, number of rotatable bonds, and number of OH and NH bonds. Among HV-8, 11 and 12, the most potent inhibitor, i.e. HV-11 possessed a lower ClogP as well as molecular volume. Thus the increase in ClogP value and molecular volume could be responsible for the variation in XO inhibitory activity among the three most potent inhibitors. The increase in TPSA, nON, nrotb, volume and the ClogP by introducing the nitro group either on Ring A and B (HV-13, -16) in comparison to the value displayed by HV-11 (the most potent inhibitor) might be responsible for their diminished activity profile. HV-6, a conjugate with moderate inhibitory profile possessed TPSA value in between HV-11 (the most potent) and HV-16 (the least potent), significantly increased molecular volume, nON as well as nrotb as compared to HV-11. Thus, indicates a clear correlation between the inhibitory potential of the conjugates with the molecular properties. Among the six conjugates, only HV-6 and -11 are completely consistent with Lipinski’s rule.
Table 3. Molecular properties of some selected hybrids.
Conclusion
The present study reports the xanthine oxidase inhibitory potential of 2,4-diarylpyrano[3,2-c]chromen-5(4H)-ones designed and synthesised as coumarin–chalcone conjugates for the first time. Some of the conjugates were endowed with potent inhibitory potential. The most potent inhibitor was found to be a mixed type inhibitor. Overall HV-11 and -12 seems to be good hits among the series. Further detailed investigation on HV-11 and -12 is under progress.
Experimental
The reagents were purchased from Sigma Aldrich, Merck, CDH, Loba chem., Spectro chem., India, and used without further purification. All yields refer to isolated products after purification. Biotage Microwave Synthesizer (Model: Initiator) operating at 150 °C with the microwave power maximum level of 400 W was used for the fries rearrangement. Products were characterized by spectral data. 1H NMR and 13C NMR spectra were recorded on Bruker Advance II 400 NMR Spectrometer and JEOL AL 300 NMR Spectrometer. The spectra were measured in CDCl3 relative to TMS (0.00 ppm). Melting points were determined in open capillaries and were uncorrected.
Procedure for the synthesis of 2,4-diaryl pyrano[3,2-c]coumarins
The mixture of 4-hydroxy coumarin (1 mmol), differently substituted chalcones (1 mmol) and SiO2 (200–400 mesh)-ZnCl2 (10 mol%), was heated on an oil bath for 4 h. The reaction mixture was extracted with water and ethyl acetate. The ethyl acetate fraction was concentrated and subjected to column chromatography. The product was eluted with increasing % age of ethyl acetate in hexane. The remaining reactions were carried out following these general procedures. In each occasion, the spectral data (1H NMR, 13C NMR and MASS) of known compounds are 2,4-diphenylpyrano[3,2-c]chromen-5(4H)-oneCitation37, 4-(3-nitrophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-oneCitation37, 2-phenyl-4-p-tolylpyrano[3,2-c]chromen-5(4H)-oneCitation37, 2-(4-chlorocyclohexa-2,4-dienyl)-4-(4-methoxyphenyl)pyrano[3,2-c]chromen-5(4H)-oneCitation37, 4-(4-chlorophenyl)-2-(4-methylcyclohexa-2,4-dienyl)pyrano[3,2-c]chromen-5(4H)-oneCitation37, 2-(4-methylcyclohexa-2,4-dienyl)-4-phenylpyrano[3,2-c]chromen-5(4H)-oneCitation37.
The physical data of the 14 new compounds are provided below.
Characterization data
The characterization data for the 14 newly synthesized compounds are given below.
4-(4-Chlorophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-2): m.p.: 87–88 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.01 (1H, d, J = 7.8 Hz), 7.73 (2H, d, J = 7.8 Hz), 7.60 (1H, m), 7.25–7.48 (9H, m), 5.79 (1H, d, J = 4.8 Hz), 4.68 (1H, d, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 31.172, 102.923, 103.480, 114.560, 116.862, 122.761, 124.237, 124.905, 125.532, 127.028, 128.736, 129.465, 132.148, 147.646, 161.482. Anal. Calc. for C24H15ClO3: C, 74.52; H, 3.91; Found: C, 74.68; H, 4.07.
4 -(4-Bromophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-3): m.p.: 168–169 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.02 (1H, d, J = 6.6 Hz), 7.73 (2H, d, J = 6.3 Hz), 7.57 (1H, m), 7.39–7.45 (5H, m), 7.26–7.36 (4H, m), 5.80 (1H, d, J = 4.8 Hz), 4.68 (1H, d, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 36.207, 103.114, 103.220, 114.428, 116.905, 121.207, 122.718, 124.274, 124.723, 128.744, 129.443, 130.242, 131.720, 132.204, 132.449, 142.564, 147.255, 152.798. Anal. Calc. for C24H15BrO3: C, 66.84; H, 3.51; Found: C, 67.02; H, 3.76.
4-(4-Fluorophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-4): m.p.: 142–143 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.03 (1H, d, J = 7.5 Hz), 7.75 (2H, d, J = 7.8 Hz), 7.59 (1H, m), 7.34–7.49 (7H, m), 7.00 (2H, m), 5.83 (1H, d, J = 4.8 Hz), 4.72 (1H, d, J = 4.8 Hz). Anal. Calc. for C24H15FO3: C, 77.83; H, 4.08; Found: C, 78.11; H, 3.95.
4-(4-Nitrophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-5): m.p.:190–191 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.18 (2H, d, J = 9.00 Hz), 8.04 (1H, d, J =7.5 Hz), 7.73–7.81 (2H, m), 7.58–7.61 (3H, m), 7.35–7.48 (5H, m), 5.78 (1H, d, J = 4.8 Hz), 4.85 (1H, d, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 36.721, 102.141, 102.425, 114.200, 116.986, 117.586, 122.807, 123.013, 123.251, 123.489, 123.929, 124.070, 124.220, 124.466, 124.795, 125.041, 128.026, 128.308, 128.556, 128.826, 128.939, 129.427, 129.740, 130.283, 130.536, 131.566, 132.119, 132.560, 133.077, 147.101, 147.834, 150.613, 152.867, 156.315, 161.289. Anal. Calc. for C24H15NO5: C, 72.54; H, 3.80; N, 3.52; Found: C, 72.48; H, 4.02; N, 3.76.
4-(3,4-Dimethoxyphenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-6): m.p.: 85–86 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.02 (1H, d, J = 7.8 Hz), 7.74 (2H, d, J = 6.3 Hz), 7.57 (1H, m), 7.36–7.46 (5H, m), 6.99 (1H, s), 6.93 (1H, d, J = 8.4 Hz), 6.80 (1H, d, J = 8.1 Hz), 5.85 (1H, d, J = 5.1 Hz), 4.66 (1H, d, J = 4.8 Hz), 3.86 (6H, s), 5.85 (1H, d, J = 5.1 Hz), 4.66 (1H, d, J = 4.8 Hz). Anal. Calc. for C26H21O5: C, 75.72; H, 4.89; Found: C, 75.84; H, 5.05.
4-(4-Methoxyphenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-7): m.p.: 132–133 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.00 (1H, d, J = 7.5 Hz), 7.72 (2H, d, J = 7.8 Hz), 7.42 (1H, m), 7.41 (2H, d, J = 7.5 Hz), 7.15–7.35 (5H, m), 6.84 (2H, d, J = 8.4 Hz), 5.81 (1H, d, J = 5.1 Hz), 4.66 (1H, d, J = 5.1 Hz), 3.84 (3H, s). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 35.745, 55.299, 103.873, 114.005, 114.613, 116.816, 122.685, 124.182, 124.650, 126.130, 128.475, 128.697, 129.230, 129.587, 131.964, 132.685, 135.832, 146.770, 152.715, 155.510, 158.754, 161.568. Anal. Calc. for C25H18O4: C, 78.52; H, 4.74; Found: C, 78.69; H, 4.99.
4-(Naphthalen-2-yl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-8): m.p.: 178–179 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 7.74–8.14 (4H, m), 7.43–7.58 (5H, m), 7.28–7.36 (7H, m), 5.90 (1H, d, J = 3.9 Hz), 4.89 (1H, d, J = 3.9 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 18.405, 36.823, 58.455, 103.706, 116.882, 122.743, 124.220, 124.735, 125.810, 126.103, 126.511, 127.199, 127.616, 127.940, 128.428, 128.706, 129.304, 132.068, 132.710. Anal. Calc. for C28H18O3: C, 83.57; H, 4.51; Found: C, 83.33; H, 4.77.
4-(3-Chlorophenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-9): m.p.: 132–133 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.02 (1H, d, J = 8.1 Hz), 7.72 (2H, d, J = 7.8 Hz), 7.59 (1H, m), 7.19–7.31 (5H, m), 7.31–7.46 (4H, m), 5.80 (1H, d, J = 3.0 Hz), 4.69 (1H, d, J = 3.00 Hz): 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 36.422, 102.858, 102.995, 114.317, 116.800, 122.745, 124.321, 124.676, 126.716, 127.397, 128.451, 128.705, 129.434, 129.841, 132.272, 134.431, 145.574, 147.152, 152.726, 156.025, 161.308. Anal. Calc. for C24H15ClO3: C, 74.52; H, 3.91; Found: C, 74.68; H, 3.82.
2-(4-Methoxyphenyl)-4-(thiophen-2-yl)pyrano[3,2-c]chromen-5(4H)-one (HV-10): m.p.: 134–135 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.04 (1H, d, J = 7.8 Hz), 7.92 (2H, d, J = 8.7 Hz), 7.67 (1H, d, J = 9.00 Hz), 7.54 (1H, m), 7.32–7.38 (3H, m), 7.11–7.18 (2H, m), 6.91–6.99 (2H, m), 6.93 (2H, d, J = 9.00 Hz), 5.81 (1H, d, J = 5.1 Hz), 5.02 (1H, d, J = 5.1 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 31.118, 55.435, 101.146, 102.864, 103.523, 113.695, 114.109, 114.607, 116.724, 116.853, 122.751, 124.031, 124.215, 124.839, 125.081, 125.442, 126.360, 127.026, 130.329, 130.622, 132.107, 132.606, 147.445, 147.981, 152.326, 152.685, 155.551, 160.573, 161.595, 164.396. Anal. Calc. for C23H16SO4: C, 71.12; H, 4.15; S, 8.25; Found: C, 70.92; H, 3.96; S, 7.99.
2-Phenyl-4-(thiophen-2-yl)pyrano[3,2-c]chromen-5(4H)-one (HV-11): m.p.: 168–169 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.00 (1H, d, J = 7.5 Hz), 7.76 (2H, d, J = 6.3 Hz), 7.58 (1H, m), 7.34–7.48 (5H, m), 7.19 (1H, d, J =4.5 Hz), 7.12 (1H, d, J = 3.6 Hz), 6.95 (1H, dd, J = 3.6 Hz and 5.1 Hz), 5.95 (1H, d, J = 5.1 Hz), 5.06 (1H, d, J = 5.1 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 31.161, 102.907, 103.452, 114.539, 116.846, 122.762, 124.254, 124.894, 125.522, 127.028, 128.736, 129.468, 132.162, 132.498, 147.575, 147.643, 152.700, 155.539, 161.491. Anal. Calc. for C22H14SO3: C, 73.72; H, 3.94; S, 8.95; Found: C, 73.93; H, 4.12; S, 9.11.
4-(Naphthalen-1-yl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-12): m.p.: 192–193 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.39 (1H, d, J = 8.7 Hz), 8.08 (1H, d, J =7.8 Hz), 7.89 (1H, d, J = 7.8 Hz), 7.74 (1H, d, J = 7.2 Hz), 7.50–7.68 (5H, m), 7.32–7.45 (7H, m), 5.96 (1H, d, J = 4.5 Hz), 5.57 (1H, d, J = 4.5 Hz). 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 32.100, 103.029, 103.775, 114.538, 116.964, 122.702, 122.939, 124.270, 124.640, 125.799, 125.931, 126.584, 127.736, 128.613, 129.022, 129.181, 130.839, 132.153, 132.665, 134.084, 140.024, 146.361, 152.892, 156.972, 161.420. Anal. Calc. for C28H18O3: C, 83.57; H, 4.51; Found: C, 83.49; H, 4.66.
2-(4-Nitrophenyl)-4-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-13): m.p.: 120–124 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.31 (1H, d, J = 9.00 Hz), 7.92 (1H, d, J = 8.7 Hz), 7.25–7.61 (11H, m), 6.06 (1H, d, J = 5.4 Hz), 4.76 (1H, d, J = 4.5 Hz). Anal. Calc. for C24H15NO5: C, 72.54; H, 3.80; N, 3.52; Found: C, 72.44; H, 4.04; N, 3.78.
4-(2-Methoxyphenyl)-2-phenylpyrano[3,2-c]chromen-5(4H)-one (HV-14): m.p.: 168–169 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 8.03 (1H, d, J = 7.5 Hz), 7.70 (2H, d, J = 7.5 Hz), 7.36–7.49 (5H, m), 7.17–7.25 (3H, m), 6.87–6.98 (2H, m), 5.86 (1H, d, J = 5.4 Hz), 5.12 (1H, d, J = 4.5 Hz), 3.89 (3H, s) 13C NMR (CDCl3, 75 MHz, δ, TMS = 0): 27.225, 30.624, 32.969, 55.785, 102.480, 103.468, 111.091, 114.646, 116.371, 116.717, 116.860, 121.004, 122.134, 122.611, 122.793, 124.118, 124.545, 125.225, 125.686, 128.177, 128.543, 128.638, 128.929, 129.430, 131.658, 131.909, 132.012, 132.968, 146.401, 152.872, 156.802, 157.128, 161.414, 192.114. Anal. Calc. for C25H18O4: C, 78.52; H, 4.74; Found: C, 78.69; H, 4.56.
4-(4-Hydroxy-3-methoxyphenyl)-2-(3,4-dimethoxyphenyl)pyrano[3,2-c]chromen-5(4H)-one (HV-15): m.p.: 122–123 °C; 1H NMR (CDCl3, 300 MHz, δ, TMS = 0): 7.98 (1H, bs), 7.36–7.56 (6H, m), 6.75–7.00 (4H, m), 6.06 (1H, d, J = 4.5 Hz), 4.76 (1H, d, J = 4.5 Hz), 3.95 (3H, s), 3.93 (3H, s), 3.87 (3H, s). Anal. Calc. for C27H22O7: C, 70.73; H, 4.84; Found: C, 71.01; H, 5.06.
Xanthine oxidase assay
Bovine milk xanthine oxidase (grade 1, ammonium sulfate suspension, Sigma–Aldrich) activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a Hitachi U-3010 UV–visible spectrophotometer at 25 °C. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.6), 75 µM xanthine and 0.08 units of xanthine oxidase. Inhibition of xanthine oxidase a ctivity by various inhibitors was measured by following the decrease in the uric acid formation at 293 nm at 25 °C. The enzyme was pre-incubated for 5 min, with test compound, dissolved in DMSO (1% v/v), and the reaction was started by the addition of xanthine. Final concentration of DMSO (1% v/v) did not interfere with the enzyme activity. All the experiments were performed in triplicate, and values were expressed as means of three experimentsCitation38.
Supplementary material available online.
Supplemental Material.pdf
Download PDF (2 MB)Declaration of interest
The authors declare no conflict of interest.
References
- Stockert AL, Shinde SS, Anderson RF, Hille R. The reaction mechanism of xanthine oxidase: evidence for two-electron chemistry rather than sequential one-electron steps. J Am Chem Soc 2002;124:14554–5
- Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 2002;9:195–217
- Hille R. Structure and function of xanthine oxidoreductase. Eur J Inorg Chem 2006;10:1913–26
- Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114
- Biagi G, Giorgi I, Pacchini F, et al. 2-Alkyloxyalkylthiohypoxanthines as new potent inhibitors of xanthine oxidase. Farmaco 2001;56:809–13
- Brien DE, Springer RH, Albert TNA, et al. Purine analog inhibitors of xanthine oxidase – structure–activity relationships and proposed binding of the molybdenum cofactor. J Heterocycl Chem 1985;22:601–34
- Oettl K, Reibneggar G. Pteridines as inhibitors of xanthine oxidase: structural requirements. Biochim Biophys Acta 1999;1430:387–95
- Nepali K, Singh G, Turan A, et al. A rational approach for the design and synthesis of 1-acetyl-3,5-diaryl-4,5-dihydro(1H)pyrazoles as a new class of potential non-purine xanthine oxidase inhibitors. Bioorg Med Chem 2011;19:1950–8
- Nepali K, Agarwal A, Sapra S, et al. N-(1,3-Diaryl-3-oxopropyl)amides as a new template for xanthine oxidase inhibitors. Bioorg Med Chem 2011;19:5569–76
- Dhiman R, Sharma S, Singh G, et al. Design and synthesis of aza-flavones as a new class of xanthine oxidase inhibitors. Arch Pharm Chem Life Sci 2012;346:7–16
- Sharma S, Sharma K, Ojha R, et al. Microwave assisted synthesis of naphthopyrans catalysed by silica supported fluoroboric acid as a new class of non purine xanthine oxidase inhibitors. Bioorg Med Chem Lett 2014;24:495–500
- Shukla S, Kumar D, Ojha R, et al. 4,6-Diaryl/heteroarylpyrimidin-2(1H)-ones as a new class of xanthine oxidase inhibitors. Arch Pharm Chem Life Sci 2014;347:1–10
- Shi Y, Zhou C. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 2011;21:956–60
- Hur SY, Kim TE, Park YG, et al. Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp Mol Med 2005;37:436–46
- Devji T, Reddy C, Woo C, et al. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg Med Chem Lett 2011;21:5770–3
- Reddy NS, Mallireddigari MR, Cosenza S, et al. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg Med Chem Lett 2004;14:4093–7
- Xue H, Lu X, Zheng P, et al. Highly suppressing wild-type HIV-1 and Y181C mutant HIV-1 strains by 10-chloromethyl-11-demethyl-12-oxo-calanolide a with druggable profile. J Med Chem 2010;53:1397–401
- Manvar A, Bavishi A, Radadiya A, et al. Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37Rv strains. Bioorg Med Chem Lett 2011;21:4728–31
- Yeh J-Y, Coumar MS, Horng J-T, et al. Anti-influenza drug discovery: structure-activity relationship and mechanistic insight into novel angelicin derivatives. J Med Chem 2010;53:1519–33
- Anand P, Singh B, Singh N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem 2012;20:1175–80
- Piazzi L, Cavalli A, Colizzi F, et al. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg Med Chem Lett 2008;18:423–6
- Lin CM, Huang S-T, Lee F-W, et al. 6-Acyl-4-aryl/alkyl-5,7-dihydroxycoumarins as anti-inflammatory agents. Bioorg Med Chem 2006;14:4402–9
- Curini M, Epifano F, Maltese F, et al. Synthesis of collinin, an antiviral coumarin. Aust J Chem 2003;56:59–60
- Chang WS, Chiang HC. Structure-activity relationship of coumarins in xanthine oxidase inhibition. Anticancer Res 1995;15:1969–73
- Lin HC, Tsai SH, Chen CS, et al. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem Pharmacol 2008;75:1416–25
- Umamaheswari M, Madeswaran A, Asokkumar K, et al. Discovery of potential xanthine oxidase inhibitors using in silico docking studies. Der Pharm Chem 2011;3:240–7
- Dhar DN. The chemistry of chalcones and related compounds. New York (NY): John Wiley & Sons; 1981:5–9
- Star AE, Marby TJ. Flavonoid frond exudates from two Jamaican ferns. Pityrogramma tartarea and P. calomelanos. Phytochemistry 1971;10:2817–18
- Cos P, Ying L, Calomme M, et al. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine-oxidase and superoxide scavengers. J Nat Prod 1998;61:71–6
- Da-Silva SL, Da-Silva A, Honorio KM, et al. The influence of electronic, steric and hydrophobic properties of flavonoid compounds in the inhibition of the xanthine oxidase. J Mol Struct Theochem 2004;684:1–8
- Lin CM, Chen CS, Chen CT, et al. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun 2002;294:167–9
- Niu Y, Zhu H, Liu J, et al. 3,5,2′,4′-Tetrahydroxychalcone, a new non-purine xanthine oxidase inhibitor. Chem Biol Interact 2011;189:161–6
- Nepali K, Sharma S, Sharma M, et al. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem 2014;77:422–87
- Moreau J, Hubert C, Batany J, et al. Metal-free bronted acid catalyzed formal [3+3] annulation. Straight synthesis of dihydro-2H-chromenones, pyranones, and tetrahydroquinolinones. J Org Chem 2009;74:8963–73
- Jacquot Y, Refouvelet B, Bermont L, et al. Synthesis and cytotoxic activity of new 2,4-diaryl-4H,5H-pyrano[3,2-c]benzopyran-5-ones on MCF-7 cells. Pharmazie 2002;57:233–7
- Liu Y, Zhu J, Qian J, et al. Gold(III)-catalyzed tandem conjugate addition/annulation of 4-hydroxycoumarins with α,β-unsaturated ketones. J Org Chem 2011;76:9096–101
- Bagdi AK, Majee A, Hajra A. Regioselective synthesis of pyrano[3,2-c]coumarins via Cu(II)-catalyzed tandem reaction. Tetrahedron Lett 2013;54:3892–5
- Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 2002;9:195–217
- Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inihbitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114
- Guru Nanak Dev University. New class of non purine xanthine oxidase inhibitors. Calculated from http://www.molinspiration.com/cgi-bin/properties [last accessed 1 Jun 2014]


![Scheme 1. Synthesis of 2,4–diphenyl pyrano[3,2-c]coumarins.](/cms/asset/5983ddd5-12dd-4131-880c-de373e2a8708/ienz_a_961446_sch0001_b.jpg)