Abstract
Pyrogallol is found naturally in crops and fruits of many plants. It is also an active ingredient of many pharmaceuticals. For this reason, we employed different in vitro antioxidant assays such as cupric ion Cu2+ reducing power, Fe3+ reducing power, total antioxidant activity by ferric thiocyanate method, ABTS radical scavenging, DMPD radical scavenging, DPPH • scavenging, Fe2+ chelating, scavenging and H2O2 scavenging activities of pyrogallol. Pyrogallol inhibited 77.95% lipid peroxidation of linoleic acid emulsion at 30 μg/mL concentration. BHA, BHT, α-tocopherol and trolox exhibited inhibitions of 89.88, 89.97, 83.82 and 91.85% against peroxidation of linoleic acid emulsion at the same concentration, respectively. In addition, pyrogallol was an effective of all the scavenging and reducing power results. In this study, pyrogallol was also evaluated as potential inhibitor for acethycholinesterse enzyme. The results showed that pyrogallol exhibited potent acetylcholinesteras inhibitory activity with IC50 and Ki values 10.2 and 8.6 μM, respectively.
Introduction
For maintaining the structural integrity of cells and tissues, and in fulfilling the normal functions, it is very remarkable that an important balance is always needed between oxidant and antioxidants. By scavenging free radicals, antioxidants reduce agents and limit oxidative damage in biological structuresCitation1. Being related to the formation of lipid peroxidation, antioxidants play an important role in preventing many diseases and agingCitation2.
Polyphenols are naturally occurring plant metabolites and also called phenolic compounds. They are widely available in vegetables, fruits, seeds, nuts and flowers. They can easily react with reactive oxygen species (ROS), resulting in powerful antioxidant activity. So it could be considered that – the more the intake of polyphenols, less the risk for cancer and various diseases. Polyphenols are a structural class of natural, but also synthetic chemicals characterized by the presence of large multiples of phenol structural unitsCitation3,Citation4. They, integral part of both human and animal diets, constitute one of the most ubiquitous groups of plant metabolites. Because of owing to their antioxidant capacity and their possible beneficial implications in human health, such as in the treatment and prevention of cancer and cardiovascular disease, recent interest in food phenolics has increased greatlyCitation5. Recently, the importance of phenolic compounds is described extensively in the Gulcin’s reviewCitation1.
Pyrogallol (1,2,3-trihydroxybenzene) is a polyphenol found various of fruits and vegetables such as avocado and apricot. It is used as an active reducer for gold, silver and mercury salts; a chemical reagent for antimony and bismuth; and a developer in photography and holography. Additionally, pyrogallol is used in the manufacture of pharmaceuticals and pesticides and has been used for medicinal purposes as a topical antipsoriatic. Owing to its antioxidant properties, pyrogallol is used as a corrosion inhibitor (i.e. oxygen scavenger) in boilers.
On the other hand, Alzheimer’s disease (AD) is characterized by dementia, cognitive impairment and memory loss, and is one of the most common diseases in elderly peopleCitation6,Citation7. Recently, treatment of AD focuses on AChE inhibitors (AChEI), such as tacrine, donepezil, rivastigmine and galantamine. However, the potential effectiveness of such inhibitors in clinical use is often complicated by their associated side effects. It was reported that clinical studies have shown that the AChEI tacrine causes hepatotoxicityCitation8. Since AD is a multi-pathogenic illness, a current drug-discovery strategy is needed to develop novel anti-Alzheimer’s agents with multiple potencies such as inhibition of AChE. In the future, the development of specific and new AChEI may lead to improved clinical outcomes.
In this study, the ferric ion (Fe3+) reducing power, cupric ion (Cu2+) reducing power, total antioxidant activity by ferric thiocyanate method, ABTS radical scavenging, DPPH radical scavenging, DMPD radical scavenging, ferrous ion (Fe2+) chelating, superoxide () scavenging and hydrogen peroxide (H2O2) scavenging activity of pyrogallol were investigated.
Materials and methods
Chemicals
Pyrogallol, butylated hydroxyanisole (BHA), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), butylated hydroxytoluene (BHT), α-tocopherol, N,N-dimethyl-p-phenylenediamine dihydrochloride (DMPD), 2,9-dimethyl-1,10-phenanthroline (neocuproine), 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), trichloroacetic acid (TCA), acetylcholinesterase enzyme and other chemicals were purchased from Sigma-Aldrich GmbH (Sternheim, Germany).
Fe3+ reducing power
For measuring power of pyrogallol, Oyaizu’s the ferric reducing antioxidant powder method, with slight modification, was used. According to this method, Fe3+(CN−)6 is reduced to Fe2+(CN−)6 and absorbance is measured by using spectrophotometerCitation9. The absorbance was measured at 700 nm in a spectrophotometerCitation10.
Cu2+ reducing method
We measured reducing power of pyrogallol with a slight modification of Cuprac methodCitation11. Zero-point 25 mL CuCl2 (0.01 M), 0.25 mL ethanol neocuproine (7.5 mM), 0.25 mL CH3COONH4 buffer solution (1.0 M) and standard antioxidants (10, 20 and 30 μg/mL) were added to each test tube. Total volume was adjusted to 2 mL and kept at room temperature during 30 min. Absorbance was measured at 450 nm in spectrophotometerCitation12.
Ferric thiocyanate method (total antioxidant activity)
The ferric thiocyanate method was used to evaluate the effects of pyrogallol. With this method, peroxide formation occurred during the oxidation of linoleic acid oxidationCitation13,Citation14. These compounds oxidized Fe2+–Fe3+ and ions form a complex with thiocyanate. Reaction mixture has a maximum absorbance at 500 nm. The percent inhibition of lipid peroxidation was calculated by the following equation:
AS is the absorbance of the sample in the presence of pyrogallol or other test compounds. AC is the absorbance of the control reaction contains only sodium phosphate buffer and linoleic acid emulsionCitation15,Citation16.
ABTS•+ scavenging activity
ABTS•+ scavenging activity assay was improved by Re et al.Citation17 with minor modification. After an antioxidant is added to a pre-formed ABTS•+ radical solution, the remaining ABTS•+ is determined at 734 nm in a spectrophotometer. The ABTS radical solution was prepared by using 2 mM ABTS in H2O with potassium persulfate (2.45 mM). Then, ABTS•+ solution stored in the dark at room temperature until used. Finally, 1 mL of ABTS•+ solution was added to 3 mL of pyrogallol solution in ethanol at different concentrations (10–30 μg/mL). The mixture was incubated in the dark for 30 min and measured at 734 nmCitation18.
DPPH• scavenging activity
This activity was measured by the method of Blois described previously by GulcinCitation19. DPPH solution has deep purple color and if the sample contains an antioxidant this color disappearsCitation16,Citation20. One microliter of ethanolic DPPH solution (0.1 mM) was added to the different concentrations (10–30 μg/mL) of pyrogallol. These samples were vortexed and incubated in the dark at 30 °C for 30 min. The absorbance was measured at 517 nm against blank samples.
DMPD•+ scavenging activity
DMPD•+ scavenging activity was determined with minor modifications by GülcinCitation15 according to the method of Fogliano et al. Firstly, 105 mg DMPD was dissolved in 5 L of distilled water and 1 mL of this solution was added to 100 ml of acetate buffer (0.1 M, pH 5.3). DMPD•+ was obtained by adding 0.3 ml ferric chloride (0.05 M) to this solution. After the addition of different concentrations (10–30 μg/mL) of pyrogallol standard antioxidants and 1 μL of DMPD•+ solution was added to the reaction mixture, they incubated in dark for 15 min. Absorbance of mixture was measured at 505 nm.
Fe2+ ions chelating activity
Pyrogallol’s chelating activity was measured using the method of Dinis et al.Citation21 and the absorbance was measured at 562 nm spectrophotometrically. This method is based on the absorbance measurement of Fe2+–ferrozine complex. In the experiment, 0.1 mL of FeCl2 (0.6 mM) solution was added to pyrogallol (10–30 μg/mL) in 0.4 mL methanol. The reaction was initiated by adding 5 mM ferrozine. Then, it was incubated for 10 min and the mixture was measured spectrophotometrically at 562 nm.
 scavenging activity
scavenging activity
scavenging activity was measured according to the method of Zhishen et al. with slight modificationCitation22. In this method, the total volume of the reaction mixture was 3 mL. The reaction mixture comprising riboflavin, methionine and NBT were illuminated at 25 °C for 40 min. The absorbance was measured at 560 nm. Different concentrations (10–30 μg/mL) of pyrogallol were added to the reaction mixture, in which
was scavenged, thereby inhibiting the NBT reductionCitation23.
H2O2 scavenging activity
The H2O2 scavenging was described following the procedure of Ruch et al.Citation24. According to this method, a solution of 40 mM H2O2 was prepared in 0.1 M phosphate buffer (pH 7.4). Pyrogallol (10 μg/mL) in 3.4 mL phosphate buffer was added to 0.6 mL of H2O2 solution (40 mM) and of the reaction mixture’s absorbance was measured at 230 nm.
Acetylcholinesterase activity method
Acetylcholinesterase enzyme catalyzes the breakdown of acetylcholine to acetate and thiokols. Pyrogallol was assayed for cholinesterase by the method of Ellman et al.Citation25 using 3 mM acetylthiocholine iodide as substrate. According to the Ellman method thiocholine, produced by AChE, reacts with 5,5′-dithiobis(2-nitrobenzoic acid) to form a colorimetric product. The absorbance at 412 nm was measured immediately. In this method, 100 μL Tris–HCl (pH 8), 50 μL DTNB and 40 μL enzyme were added into the test tubes and total volume was adjusted with distilled water to 950 μL. Then, 50 μL of acetylthiocholine iodide was added to this mixture and absorbance was measured at 412 nm. Distilled water was used instead of enzyme for blank sample.
Results
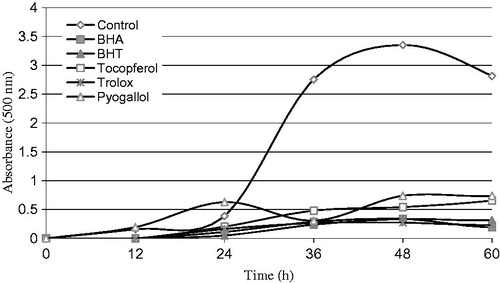
In the linoleic acid emulsion system, pyrogallol showed effective antioxidant activity. The effect of 30 μg/mL concentration of pyrogallol on the lipid peroxidation of linoleic acid emulsion was found to be 77.95% (). On the other hand, the same concentration of standard antioxidants (BHA, BHT, α-tocopherol and trolox) exhibited 89.88, 89.97, 83.82 and 91.85% peroxidation of linoleic acid emulsion, respectively. The autoxidation of linoleic acid emulsion without pyrogallol or standard compounds was accompanied by a rapid increase in peroxides. According to results of this experiment, pyragallol was found to have potent activity. On account of the deleterious role of free radicals in biological systems and foods, radical scavenging activity is very significant. Chemical assays relate to the ability of scavenge synthetic free radicals, using a variety of radical generating methods and systems for detection of the oxidation endpoint. Antioxidant capacities of components are determined with commom spectrophotometric procedures such as DMPD•+, DPPH• and ABTS•+ radical scavenging methodsCitation26. All of the tested compounds showed remarkable radical scavenging activity. As shown in Supplementary Figure 2A, there was a significant decrease in the concentration of ABTS•+ owing to the scavenging capacity at all pyrogallol concentrations (10–30 μg/mL). EC50 values for standard antioxidants (BHA, BHT, α-tocopherol and trolox) were found to be 9.26, 8.02, 12.98 and 11.49 μg/mL, respectively. Pyrogallol and standards’ EC50 values on the ABTS•+ scavenging decreased in the following order: pyrogallol (7.08 μg/mL) > BHT (8.02 μg/mL) > BHA (9.26 μg/mL) > troloks (11.49 μg/mL) > α-tokoferol (12.98 μg/mL). Lower EC50 values point out high ABTS•+ scavenging ability.
Figure 1. Total antioxidant activities of pyrogallol and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox at the same concentration (30 μg/mL) assayed by ferric thiocyanate methodCitation13,Citation14.
In Supplementary Figure 2B, pyrogallol was an efficient DMPD•+ radical scavenger in a concentration-dependent manner (10–30 μg/mL). Pyrogallol and standards’ EC50 values on the DMPD•+ decreased in the following order: pyrogallol (10.44 μg/mL) > BHA (44.75 μg/mL) > troloks (59.38 μg/mL). Owing to the scavenging capacity at all the phenolic compounds concentrations, there was a notable decrease in the concentration of DMPD•+. Reportedly, when hydrophobic antioxidants such as BHT or α-tocopherol were used, the main drawback of the DMPD•+ method is that its sensitivity and reproducibility dramatically reducedCitation2.
In Supplementary Figure 2C, owing to the scavenging ability of pyrogallol and the reference compounds, there was a significant reduction in the concentration of DPPH radical. Standard antioxidants (BHA, BHT, α-tokoferol and trolox) were used as references for radical scavenger activity. Pyrogallol and standard antioxidants’ (BHA, BHT, α-tokoferol and trolox) EC50 values on the DPPH radical were found to be 10.71, 15.07, 31.45, 11.48 and 8.63 μg/mL and decreased in the order of troloks > pyrogallol > α-tokoferol > BHA > BHT. A lower EC50 values point higher DPPH radical scavenging activity.
Due to the fact that antioxidant compounds are able to donate electrons to reactive radicals, reducing them into more stable and unreactive species, the reduction capacity of a compound may serve as a significant indicator of its potential antioxidant activityCitation27. In this article, Fe3+ reducing power and CUPRAC assays were used for reducing power of pyrogallol. When pyrogallol was compared to the standards, both methods were found to be effective. As seen in Supplementary Figure 3A, pyrogallol demonstrated powerful Fe3+ reducing activity. So these differences were statistically found to be major. The reducing power of pyrogallol and standards (BHA, BHT, α-tocopherol and trolox) increased with increasing concentration of samples. The Fe3+–Fe2+ reducing powers of the phenolic and standard compounds at the same concentration (20 μg/mL) were as follows: pyrogallol > BHA > BHT > troloks ≥ α-tokoferol.
Cu2+ reducing capability of pyrogallol was measured by CUPRAC method was found to be concentration dependent (10–30 μg/mL). As shown in Supplementary Figure 3B, Cu2+ ions reducing power of pyrogallol and standard compounds at the same concentration (20 μg/mL) were as follows: pyrogallol > BHA > BHT > α-tokoferol > troloks. Similar results were observed for pyrogallol between Cu2+ ions reducing and Fe3+ ions reducing ability methods.
Among the various species of metal ions, Fe2+ ion is the most strong pro-oxidant. Ferrozine and Fe2+ can quantitatively form complexes. The complex formation is disrupted, resulting in a decrease in the red color of the complex in the presence of chelating agents. For this reason, measurement of color reduction provides estimating the metal chelating activity of the coexisting chelator. If absorbance is lower this means that metal chelating activity is higher. Fe2+ chelating activities of pyrogallol and standard antioxidants (BHA, BHT, α-tocopherol and trolox) are shown in Supplementary Figure 4. EC50 values for pyrogallol, standard antioxidants (BHA, BHT, α-tokoferol and trolox) on the Fe2+ chelating activity were found to be 44.08, 19.08, 17.65, 15.76 and 17.27 μg/mL, respectively, and decreased in the order of α-tokoferol > troloks > BHT > BHA > pyrogallol. A lower EC50 value exhibits a higher Fe2+ chelating activity.
Oxidative stress can generate included in free radical species. This radical is a factor that can destruct cell membranes and induce aging. In Supplementary Figure 5A, EC50 values for pyrogallol and standards on the
scavenging were as follows: BHA (17.92 μg/mL) > pyrogallol (18.93 μg/mL) > BHT (28.53 μg/mL). EC50 value was not determined for α-tocopherol and its water-soluble analog, trolox.
Scavenge H2O2 ability of pyrogallol is shown in Supplementary Figure 5B and compared with standard antioxidants (BHA, BHT, α-tocopherol and trolox). BHA, BHT, α-tocopherol and trolox exhibited 32.9, 57.5, 53.2 and 56.6% H2O2 scavenging activity, respectively, at 10 μg/mL. On the other side, H2O2 scavenging activity of pyrogallol was found to be 20.9% at the same concentration. Then, EC50 values were calculated. EC50 values for pyrogallol, BHA, BHT, α-tokoferol and trolox were found to be 23.92, 15.19, 8.69, 9.30 and 8.80 μg/mL. These results indicate that pyrogallol has a powerful O2 and H2O2 anion radical scavenging activity.
The mechanism of action of AChE might lead to the design of the mechanism-based inhibitors, which could be of future therapeutic use. Different types of AChEIs have been studied for the treatment of AD. Some of the AChEI differ in their mechanism of action, metabolism and brain selectivity. AChE was very effectively inhibited by pyrogallol, with Ki value of 8.6 μM (IC50: 10.22, r2: 0.9952). On the other hand, comparing to inhibitor effect, tacrine was used. Tacrine, which is used for the treatment of mild, inhibited AChE with Ki value of 0.19 μM (IC50: 0.48, r2: 0.9933). IC50 and Ki values for pyrogallol and tacrine are shown in .
Discussion
Polyphenols, or phenolic compounds, is a common term that refers to a large number of compounds (>8000). They have at least one aromatic ring attached with one or more hydroxyl group and they are produced in plants as secondary metabolites via the Shikimic acid pathwayCitation4. As a result of studies, many new plant component parts, among these are the polyphenols, on antioxidant activity have been identified. Antioxidant activity of phenolic compounds is mainly owing to their redox properties, which permit them to act as reducing agents, singlet oxygen quenchers or hydrogen donors. In addition to their roles as antioxidants, they exhibit a wide spectrum of medicinal properties, such as anti-inflammatory, cardioprotective, antimicrobial, antithrombotic and anti-allergic effectsCitation28.
In this study, we have used different in vitro bioanalytical methodologies ( and ) and studied the antioxidant and radical scavenging activity of pyrogallol. Its antioxidant and radical scavenging activities were compared to standard antioxidants (BHA, BHT, α-tocopherol and its water-soluble analog, trolox). These collations were made using a series of in vitro tests. For this purpose, ABTS•+ scavenging, DPPH• free radical scavenging, DMPD•+ scavenging, scavenging, Fe3+–Fe2+ transformation and CUPRAC methods were used.
Table 1. Reducing ability of pyrogallol by Fe3+–Fe2+ transformation and CUPRAC method and standards such as BHA, BHT, α-tocopherol and trolox at the same concentration (20 μg/mL).
Table 2. Concentration required for 50% scavenging (IC50) of DPPH• scavenging activity, ABTS•+ scavenging activity, DMPD•+ scavenging activity,  scavenging activity, Fe2+ chelating activity and H2O2 scavenging activity of pyrogallol and standard compounds such as BHA, BHT, α-tocopherol and trolox.
scavenging activity, Fe2+ chelating activity and H2O2 scavenging activity of pyrogallol and standard compounds such as BHA, BHT, α-tocopherol and trolox.
Table 3. IC50 and Ki values, and inhibition types of tacrine and pyrogallol.
Varied methods such as DPPH•, ABTS•+ and DMPD•+ are currently used to assess the antioxidant activity of plant phenolic compounds and these methods are rapid; no sophisticated instrumentation is required, a sample analysis takes 15 min in total, or no expensive reagentsCitation17,Citation26.
DPPH• and ABTS•+ are the most common methods used to show significant differences in their response to antioxidants. The ABTS•+ must be generated by enzymes or chemical reactions, conversely the DPPH• does not require any special preparation. Furthermore, ABTS•+ can be dissolved in aqueous and organic media due to the hydrophilic and lipophilic nature of the compounds. However, DPPH can only be dissolved in organic media, especially in ethanol. When interpreting the roles of hydrophilic antioxidants, this is a very important limitationCitation29.
ABTS free radicals occur by oxidation of ABTS and potassium persulfate. This assay is based on the inhibition of the absorbance of ABTS•+, which has a characteristic long-wave length absorption spectrum showing absorption at 734 nm. In this method, reaction with ABTS•+ is both faster and is completed in a short time (0.25–0.5 min)Citation30.
To evaluate the free radical scavenging effectiveness of different antioxidant substances, DPPH method is used. In this method, DPPH decreases in the presence of a hydrogen-donating antioxidant owing to the formation of the non-radical form DPPH-H in the reaction. DPPH accepts an electron or hydrogen radical and is a stable free radical to become a stable diamagnetic moleculeCitation31.
DMPD radical scavenging method is more suitable for hydrophilic antioxidants, but is less sensitive to hydrophobic bioactive compounds. In spite of the ABTS procedure, the DMPD•+ method guarantees a very stable endpoint. When a large-scale screening is required, this is especially important. According to the studies, the main drawback of the DMPD method when using the hydrophobic antioxidants such as α-tocopherol or BHT, its reproducibility and sensitivity decrease. It was reported that organic acids might cause interference. Therefore, these standard antioxidants were not used in the antiradical assay.
Antioxidant compounds are able to donate electrons to reactive radicals, reducing them into more stable and unreactive speciesCitation27. Fe3+ and CUPRAC methods were used for reducing power of pyrogallol.
Antioxidant compounds decrease Fe3+–ferricyanide complexes to the ferrous (Fe2+) form. The Prussian blue-colored complex is formed by adding FeCl3 to the ferrous (Fe2+) ions. In this method, depending on the reducing power of the antioxidant, the yellow color of the test solution changes to blue or green. The higher the absorbance, the higher the ferric reducing power.
Cu2+ is reduced to Cu+ by antioxidants according to the CUPRAC method. This method is suitable for a variety of antioxidants regardless of chemical type or hydrophilicity; furthermore, it is cost-effective, rapid and stable. Studies also indicate that in vitro Cu2+-reducing measurement results might be more efficiently extended to the possible in vivo reactions of antioxidants. This method can be used for both hydrophilic and lipophilic antioxidants.
Metal chelating capacity is important, on account of the fact that it reduces the concentration of the catalyzing transition metal in lipid peroxidationCitation1. According to this method, free Fe2+ and ferrozine forms a complex, but not with Fe2+ bound to other chelators. Therefore, if there is a presence of antioxidant chelators, this means that there is a decrease in the amount of ferrozine–Fe2+ complex formed after treatment. This complex is measured spectrophotometrically at 562 nm. A significant drawback of this complexation in measuring the presence of antioxidant chelator is – the reaction is affected by both the antioxidant–Fe2+ and ferrozine–Fe2+ complex formation constants. Therefore, a weak antioxidant iron chelator could be underestimated in quantitative determination.
When one electron transfers to molecular oxygen, are formed. In this study, we used riboflavin/methionine/illuminate system for generation of O2 anion radicals. According to the method, producing the blue formazan, O2 anion reduces the yellow dye (NBT2+), which is measured at 560 nm. Antioxidants inhibit the blue NBT formation.
Riboflavin activating photochemically reacts with NBT to generate NBTH, eventually it leads to formazan according to reaction. Radical species are controlled by a quasi-equilibrium in the presence of oxygen. For this reason, when the assay is performed under aerobic conditions, appear indirectlyCitation32.
H2O2 can be produced by biological systems and can be formed in vivo by several oxidizing enzymes such as O2 dismutase. When H2O2 is added to cells in culture, it can cause transition of metal dependent on OH radicals and lead to DNA damageCitation33. For this reason, removing H2O2 as well as , is very significant for protection of food systems and pharmaceuticalsCitation15.
For food and medicinal bioactive components, total antioxidant capacity is used as a parameter. In this method, thiocyanate method, a compound inhibits oxidative degradation like lipid peroxidation and is used to measure the peroxide level along the initial stage of lipid oxidationCitation34. During the linoleic acid oxidation, peroxides are formed and react with Fe2+ to form Fe3+. Then, ions and thiocyanate (SCN−) form a complex, which has a maximum absorbance at 500 nm. In the presence of antioxidants, oxidation of linoleic acid will be slow. Moreover, the color development will be slow as a result of formation of thiocyanateCitation35.
Acetylcholine-mediated neurotransmission is fundamental for nervous system function. While the physiological role of the AChE in neural transmission has been well-known, it is still the focus of pharmaceutical research, targeting in treatments of some diseases such as gravis, glaucoma and AD. It has been elucidated that cholinergic deficiency is associated with AD. Therefore, one of the major therapeutic strategies is to inhibit the biological activity of AChE and hence, to increase the acetylcholine level in the brain. Recently, most of the drugs used for the treatment of AD are AChEI such as tacrine, donepezil and rivastigmineCitation6. In our study, the inhibition study made for tacrine and pyrogallol, and IC50 and Ki values were calculated (). It has been observed pyrogallol was effective inhibitor for AChE with non-competitive inhibition.
Conclusion
According to these results, pyrogallol was powerful antioxidant in different in vitro assays, including the ferric thiocyanate method, DPPH• scavenging, ABTS•+ scavenging, DMPD•+ scavenging, Fe2+ chelating, scavenging and H2O2 scavenging activities, Fe3+–Fe2+ and Cu2+–Cu+ reducing power assays when compared to the standard antioxidant compounds such as BHA, BHT, α-tocopherol as a natural antioxidant and trolox. An anticholinergic agent is a substance that blocks the neurotransmitter acetylcholine in the central and the peripheral nervous system. It has been observed pyrogallol was effective inhibitor for AChE. For this reason, pyrogallol can show effect acting as an anticholinergic substance. Also, it is known that drugs which inhibit acetylcholinesterase are used for the treatment of Alzheimer’s disease. In conclusion, pyrogallol can be used anticholinergic compound or nutritional product as a source of natural antioxidant for pharmaceutical industry.
Supplementary material available online
Supplementary Figures 2–5.
Supplemental Material.pdf
Download PDF (451 KB)Declaration of interest
The author reports no conflicts of interest.
References
- Gülcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345–91
- Gülcin I, Topal F, Sarıkaya SB, et al. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–75
- Sarikaya SB, Gulcin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
- Sarikaya SB, Topal F, Senturk M, et al. In vitro inhibition of a-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62
- Köksal E, Gülcin I. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk J Agric For 2008;32:65–78
- Gocer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch Pharm 2013;346:783–92
- Akıncıoğlu A, Topal M, Gülçin I, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76
- Gocer H, Cetinkaya Y, Goksu S, et al. Carbonic anhydrase and acetylcholine esterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2014. doi:10.3109/14756366.2014.928704
- Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37
- Cetinkaya Y, Gocer H, Menzek A, Gulcin I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl) (2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Pharm 2012;345:323–34
- Koksal E, Gulcin I, Sarikaya SB, Bursal E. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395–405
- Talaz O, Gulcin I, Goksu S, Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–9
- Gulcin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-dopa. Amino Acids 2007;32:431–8
- Gulcin I, Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–20
- Gulcin I, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 2006;223:759–67
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 1999;26:1231–7
- Gülçin İ, Beydemir Ş, Şat İG, Küfrevioğlu Öİ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment 2005;34:193–202
- Gulcin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nutr 2005;56:491–9
- Gulcin I, Sat IG, Beydemir S, et al. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 2004;87:393–400
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9
- Gulcin I, Elias R, Gepdiremen A, et al. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25:44–53
- Gulcin I. Antioxidant activity of eugenol: a structure and activity relationship study. J Med Food 2011;14:975–85
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989;10:1003–8
- Ellman GL, Courtney KD, Andres V, Featherstıne RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95
- Gulcin I, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil-a powerful antioxidant source. Arab J Chem 2012;5:489–99
- Gulcin I, Elmastas M, Aboul-Enein HY. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 2007;21:354–61
- Serbetci HT, Gulcin I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71
- Gulcin I, Bursal E, Sehitoglu HM, et al. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–38
- Gulcin I, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212
- Gulcin I, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin. Phytother Res 2006;20:130–4
- Gulcin I, Topal F, Cakmakci R, et al. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:585–93
- Gulcin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43–53
- Roginsky V, Lissi EA. Review of methods to determine chainbreaking antioxidant activity in food. Food Chem 2005;92:235–54
- Gulcin I, Gagua N, Beydemir S, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587–94

