Abstract
Autotaxin is an extracellular, two zinc-centered enzyme that hydrolyzes lysophosphatidyl choline to lysophosphatidic acid, involved in various cancerous processes, e.g. migration, proliferation and tumor progression. We examined the autotaxin inhibitory properties of extended structure carbamoylphosphonates (CPOs) PhOC6H4SO2NH(CH2)nNHCOPO3H2, with increasing lengths of methylene chains, (CH2)n, n = 4–8. Carbamoylphosphonates having n = 6, 7, 8 inhibited autotaxin in vitro with IC50 ≈ 1.5 µM. Using an imaging probe we demonstrated that compound n = 6 inhibits recombinant autotaxin activity in vitro and in vivo, following oral CPO administration. Additionally, daily oral administration of compound n = 7 inhibited over 90% of lung metastases in a murine melanoma metastasis model. Both the carbamoylphosphonates and the enzymes reside and interact in the extracellular space expecting minimal toxic side effects, and presenting a novel approach for inhibiting tumor proliferation and metastasis dissemination.
Introduction
For cancer to thrive, it must first develop a suitable supporting environment such as the tumor microenvironment (TME) which enables tumor cells to express the enzymes that can support tumor proliferation or metastasis dissemination. In this current research we focused on three families of enzymes, which share in common having a zinc-atom and their ability to aid the progression of cancerous lesions and metastasis.
Studying the tumor microenvironment's extracellular zinc enzymes, autotaxin (ATX or ENPP2) is the core of this present research. This enzyme was first isolated as an autocrine motility factor from melanoma cells in 1992Citation1. ATX is a secreted hydrolase, the activity of which originates from a threonine residue and from two zinc ions in the active siteCitation2. ATX belongs to the ectonucleotide pyrophosphatase and phosphodiesterase (ENPP) family, which consists of seven membersCitation3. ATX is the only member having lysophospholipase D (lysoPLD) activity, which hydrolyzes lysophosphatidyl choline (LPC) to the bioactive lysophosphatidic acid (LPA)Citation4. The latter is involved in diverse malignant processes such as migration, proliferation and differentiationCitation5. ATX is at the focus of considerable effort to achieve clinically useful inhibitors, however so far without successCitation6. Our research focuses on the inhibiting properties of carbamoylphosphonates (CPO) towards ATX.
In this paper, we show that N-6-[4-phenoxybenzenesulfonamido]hexyl-carbamoylphosphonate (CPO n = 6) inhibits recombinant autotaxin (ATX) in vitro. Furthermore, orally administered CPO n = 6 inhibited ATX in vivo, as established by ATX-Red-2 in an in vivo imaging probe visualizing enzymatic activity in SCID mice, bearing a triple-negative cell lineMDA231-induced breast tumor.
In addition to the CPOs’ activity on ATX, we investigated the enzymatic inhibiting properties of CPO n = 7, 8 on carbonic anhydrase (CA) IX and XII. These enzymes belong to the family of zinc metalloenzymes which facilitate the formation of bicarbonate from carbon dioxide and waterCitation7. The relevance of CAs to cancer was discovered in 1992, with the identification of CAIX and CAXIICitation8. These isoforms are barely expressed in normal tissues, but are predominantly associated with and overexpressed in tumors and are involved in critical processes related to cancer progressionCitation9–11. CAIX and XII are membrane-type isoforms with extracellularly exposed active sites, induced by the transcription factor Hypoxia Induced Factor 1α (HIF-1α). Their upregulation results in a decrease of extracellular pH, and is strongly associated with cancer cell survival and malignant progressionCitation12,Citation13. The activity of CAIX is also important for metastasis, as recently demonstrated by Lou et al.Citation14, who showed that CAIX is an essential factor in the survival of tumor cells in hypoxic areas of breast tumor, and its activity contributes to metastasis generation in breast cancer. Furthermore, both inhibition of CAIX activity and depletion of CAIX expression have been shown to result in regression or growth inhibition of mouse and human breast tumors, as well as inhibition of cancer metastasisCitation15.
In the current research we report the inhibiting effects of CPOs n = 7, 8 on CA, primarily the cancer supporting isoforms CAIX and CAXII. This is an extension of recently reported research, which showed that CPOs n = 5, 6 – previously recognized in vivo as active anti-metastatic matrix metallo-proteinase (MMP) inhibitorsCitation16–18 – also act as CA inhibitorsCitation15.
Matrix metalloproteinases (MMPs) are a family of about 26 zinc endopeptidases, which collectively have the capacity to degrade all the major components of the extracellular matrix. MMPs expression is increased in tumors due to transcriptional changes: the secreted MMP 2 and 9 are synthesized by stromal cells, and their expression is upregulated by cancer cellsCitation19. In spite of extensive research, no clinically useful inhibitor has yet been identified for MMPs. Their efficient inhibition is, therefore, an important therapeutic targetCitation20.
Experimental section
Chemical synthesis
CPOs used in this work have been synthesized previouslyCitation18.
Inhibitor kinetics
MMPs’ and CAs’ inhibitory constants have been determined as previously described, respectivelyCitation15,Citation18.
In vitro studies of ATX activity
ATX activity was assessed according to manufacturer instructions, by a colorimetric assay (Cayman Chemical, Ann Arbor MI, Kit # 700580), where recombinant ATX cleaves bis-(p-nitrophenyl) phosphate to liberate p-nitrophenol measured at 405–415 nm, in the presence of various putative inhibitors.
In vivo studies
ATX activity in vivo
ATX activity in vivo was estimated using the ATX-Red-2 in vivo imaging probe for the visualization of enzymatic activity (Echelon Bioscience, Salt Lake City, UT, CAT # L-2010)Citation21. ATX-Red-2 is an analog of LPC and contains a near-infrared fluor and a quencher. The compound was injected to SCID mice bearing an MDA-231 cell-induced breast tumor, at a final dose of 0.5 mg/kg. CPO n = 6, an ATX inhibitor was administered per os at a single dose of 50 mg/kg. Fluorescence imaging was monitored at 6, 24 and 48 h following probe and drug administration by an IVIS in vivo imaging system.
Murine melanoma model
C57Bl mice aged 4–6 weeks weighing 20–25 g were used in the melanoma experiments. Experimental metastasis was studied in the murine melanoma model. In this model, B16F10 tumor cells (∼50 000) were injected into the tail vein of C57Bl female mice. Eight mice were used for each CPO examined. The inhibitors were administered either PO or IP at 12.5 and 25 mg/kg doses. Mice were monitored for toxic symptoms. After 21 days, the animals were sacrificed, and the metastases formed on their lungs were counted after appropriate fixation.
Animal care
The joint ethics committee (IACUC) of the Hebrew University and Hadassah Medical Center in Jerusalem, Israel, approved the study protocol for animal welfare (number MD-09-11910-5). The Hebrew University is an AAALAC International accredited institute.
Absorption and resorption studies of CPO on hydroxyapatite
A solution of the carbamoylphosphonic acid (CPO) (50 µM; in 100 mM Tris buffer; pH 7.5) was used. Three aliquots (3 × 4 mL) from the resulting solution were incubated with HAP (1.6 mg) at 37 °C at 170 rpm for 1 h. The remaining CPO solution was used as reference as well as for 2 h and 18 h absorption studies. After 1 h, the three aliquots were centrifuged (10 min, 2600g), and the UV absorbances of the clear solutions were recorded and subtracted from the initial value to obtain the amount of the absorbed CPOs. The same procedure was used to determine the absorbances after the time intervals of 2 h and 18 h, also in triplicates. The concentrations of CPO were calculated from the observed optical densities of the solutions.
To examine the reversibility of the absorption of CPOs to HAP, the samples from the 2 h absorption experiments were taken and centrifuged (5 min, 2600g). The HAP samples were isolated by decantation, then double-distilled water was added (2 × 2 ml) followed by centrifugation. The aqueous layer was decanted and the white precipitate was stirred with 4 mL Tris buffer (50 mM; pH 7.5,) at 37 °C at 170 rpm for 1 h, 2 h and 18 h. After centrifugation (10 min, 2600g), the supernatant solutions were removed, and their UV absorbance was recorded. The data are listed in the bottom part of Table 3. The absorbance was measured at λ = 242 nm, where the absorbance is maximum for CPOs.
Results
Inhibitor kinetics
CA inhibiting effect of CPO n = 5–8 are shown in . All the compounds exhibit similar pharmacological inhibiting profiles, displaying IC50 < 10uM for both CA IX and XII.
Table 1. Inhibition constants of CPOs on MMP 2, CA IX and XII and ATX enzymes.*†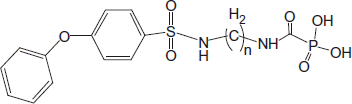 .
.
MMP 2 inhibiting profiles of CPO n = 5–8 are also depicted in . While CPOs n = 5, 6 exhibit significant inhibiting effects of IC50 < 5uM, CPO n = 7, 8 did not show any inhibiting effects (IC50 > 100 µM). This is since the MMP’s S1' pocket has limited depth and it is unable to accommodate the longer linking chain.
In vitro studies
The CPOs examined in this article have similar structures to some published in vitro active ATX inhibitorsCitation6. also depicts the inhibitory constants of the CPOs toward ATX. While CPO n = 5 is too short to inhibit ATX (IC50 = 100), the three longest structures of CPOs n = 6, 7, 8 were found to be active ATX inhibitors at low micromolar IC50.
Despite the apparent similarity between the CPOs’ chemical structures, they differ in ATX-inhibiting profiles. This demonstrates the importance of specific structural requirements and the selectivity it imposes.
In vivo studies
ATX activity in vivo
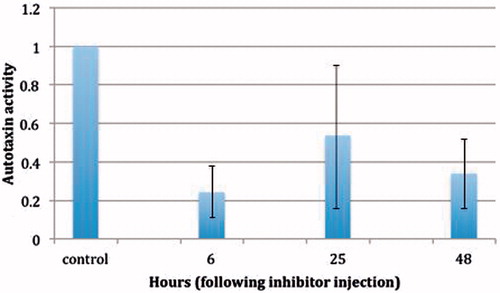
ATX activity in vivo was estimated using ATX-Red-2-in-vivo imaging probe for the visualization of enzymatic activity in SCID mice, bearing a triple-negative cell line MDA231-induced breast tumor (). Focal intensity of the fluorescence of the probe indicates the in vivo activity of ATX enzyme at the site of the tumor. A single oral dose administration of CPO n = 6 resulted in diminished fluorescence intensity at the tumor sites, indicating inhibition of ATX by the compound. When not inhibited (i.e. control group), the enzymatic activity of ATX was measurable even at 48 h after probe injection. At the same time point, the inhibitory effect of CPO n = 6 was significantly evident ( and ).
Figure 1. The fluorescent image of the experimental animals at the different time points. ATX activity in vivo was estimated by the using ATX-Red-2, an in-vivo imaging probe, at a final dose of 0.5 mg/kg, for the visualization of enzymatic activity. The data were collected at three time points as indicated. Circle shows the tumor location.
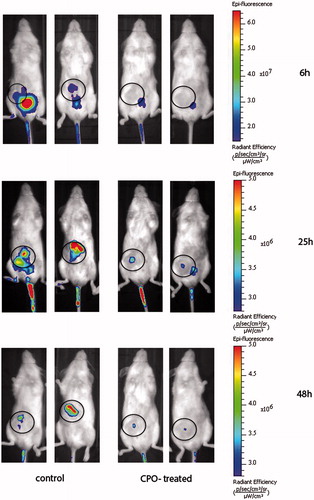
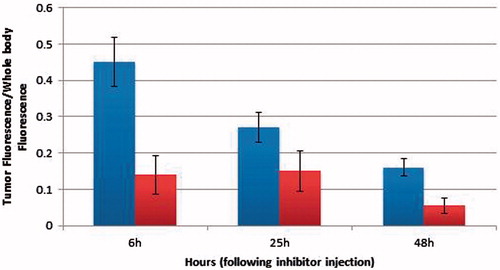
Figure 2. The fluorescence ratio of the tumor versus the whole body fluorescence, as a function of time. There is a gradual decrease in the amount of fluorescence due to dye clearance from the mice, as time progresses. Dark grey bars represent the control mice while the light grey bars represent the treated ones.
Antimetastatic studies: murine melanoma model
Mice were injected intravenously with B16F10 cells and treated daily for three weeks by CPOs n = 6, 7 as indicated in a murine melanoma model. CPO n = 7 was examined when given orally, and compared with the previously published effect of either oral or intraperitoneal administration of CPO n = 6 ().
Table 2. In vivo effect of selected CPO inhibitors on metastasis formation in mice.* 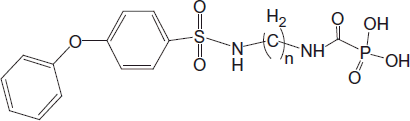
It is evident that daily treatment of the examined CPOs is effective in reducing metastasis when administered orally or intraperitoneally. This correlates well with the enzyme-inhibiting properties displayed above and the long pharmacokinetic half-life previously reported.
Absorption and resorption studies of CPO on hydroxyapatite
The pharmacokinetic examination of CPOs n = 5, 6 reported in our previous publicationCitation18 shows volumes of distribution which considerably exceeded the volumes of the extracellular fluid (EF) in rat, indicating that in addition to its presence in the EF, a significant proportion of the drug may be accumulated in certain organs. In this work we examined the extent of CPO n = 6, 7 absorption using a recently published method for studying bisphosphonate absorption to hydroxyapatite (HAP) as a model for boneCitation22, and compared it with CPO n = 5 which was re-examined. As shown in , the absorption of CPOs to HAP increases as the lengths of the aliphatic chains increase. In contrast, the rate of resorption of CPOs from HAP decreases in the homologous series from CPO n = 5 to n = 7, as observed for alkyl bisphosphonates in bone-resorptionCitation23.
Table 3. Carbamoylphosphonates absorption to (A), and resorption from (B) hydroxyapatite (HAP).
Discussion
In the course of their growth, tumors avoid the inhibitory signals from the adjacent cells and subjugate the surrounding cells to their own needs. This results in unlimited proliferation, invasion into neighboring tissues and ultimate establishment of distant metastases. One characteristic feature of such microenvironment is a more acidic extracellular pH than that of normal tissuesCitation24. This higher lactate production by tumors in the presence of oxygen provides a growth advantage for tumor cells in vivo. One of the tumor cells’ responses to acidosis and hypoxia is the induction of certain genes. Of note is the HIF-1α gene, which in turn induces the expression of numerous additional genes, such as ATX, which hydrolyzes LPC to the bioactive lipid LPACitation4.
High expression of ATX is found in various tumor cell types, including neuroblastoma, hepatocellular carcinoma, breast cancer, renal cell carcinoma, glioblastoma, non-small cell lung cancer, B cell lymphomas, and thyroid carcinoma. Therefore, the inhibition of this membrane-bound enzyme is essential for abolishing the growing tumor. To our best knowledge, no ATX inhibitor reached clinical trial so far.
Similar to ATX, CA IX and XII are also induced by HIF-1αCitation13. In the present context we focus on the inhibition properties of CPOs on these two CAs, CA IX and XII, which are membrane-type isoforms with extracellularly exposed active sites. Isozymes CA IX and CA XII are transmembranal enzymes and are critical for cell proliferation; their up-regulation is also strongly associated with cancer cell survival and malignant progressionCitation12.
Using various breast cancer cell linesCitation14, it has been recently demonstrated that CAIX is essential to the survival of tumor cells under hypoxic conditions of breast tumor, and its activity contributes to metastasis generation. It has been proposed that CAIX could serve as a specific biomarker for this kind of tumor. The necessity of CAIX for tumor and metastasis formation has also been recently demonstrated by using the knock-down approachCitation25. Further, inhibition of CAIX has been shown to result in regression or growth inhibition of mouse and human breast tumors as well as inhibition of cancer metastasis formationCitation25.
MMPs aid tumor progression by increasing cancer cell growth, migration and invasion, angiogenesis and metastasisCitation26,Citation27. In recent years, we have reported in a series of papers that show CPOs are able to act both in vitro and in vivo as inhibitors of the extracellular MMP 2Citation16–18 and possess zinc-binding abilityCitation28.
The three enzyme families (ATX, CA IX and XII and MMP 2) have significant differences in their substrates and their products, but share similarities in the fact that they all have zinc ions at their catalytic sites and furthermore, they function in the extracellular medium. In light of this, we trust that our proposed CPOs, which exhibit a dual or triple inhibiting effect on these enzymes, as shown in , display anticancer and antimetastatic activities.
The duration of the ATX inhibition for more than 48 h after a single administration of CPO n = 6 coincides with the biological half-life of this compound, measured in rats to be over 14 hCitation18. Such lengthy activity in rodents may indicate an even longer duration in humans due to the differences in metabolic rates.
As suggested earlierCitation18, the biological activity of the CPOs is the result of a certain threshold inhibitory concentration required for the inhibition of the extracellular zinc enzymes. This explains the evident activity seen following oral treatment, despite the relatively poor oral bioavailability (0.4%)Citation18. It is also in accordance with the relatively similar activity seen for CPO n = 6, 7 following PO and IP administrations. The prolonged activity of the CPOs seen at this preclinical stage indicates the suitability of a once a day treatment (either as anti-metastatic or antitumoral drugs) that also seem to exhibit flexibility in case of poor complianceCitation29.
Acknowledgements
We highly appreciate Yael Ben-Nun's assistance with imaging analysis.
Declaration of interest
The authors declare no competing financial interest. This work was supported in part by the Grass Center for Drug Design and Synthesis of Novel Therapeutics to E.B. and R.R. A.H., R.R., and E.B. are affiliated with the David R. Bloom Center of Pharmacy and the Brettler Center for Pharmacology, in the School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem.
References
- Stracke ML, Krutzsch HC, Unsworth EJ, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 1992;267:2524–9
- Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett 2003;538:60–4
- Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal 2006;2:361–70
- Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 2002;277:39436–42
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol 2009;9:15–23
- Albers HM, Ovaa H. Chemical evolution of autotaxin inhibitors. Chem Rev 2012;112:2593–603
- Potter CPS, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer 2003;89:2–7
- Pastorekova S, Zavadova Z, Kostal M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992;187:620–6
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT, Scozzafava A, Conway J. Carbonic anhydrase: its inhibitors and activators. 1st ed. Boca Raton, NY, London: CRC Press; 2004
- Cecchi A, Supuran CT. Fluorescence- and spin-labeled carbonic anhydrase inhibitors. Curr Pharm Des 2008;14:699–707
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45
- Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76
- Reich R, Hoffman A, Veerendhar A, et al. Carbamoylphosphonates control tumor cell proliferation and dissemination by simultaneously inhibiting carbonic anhydrase IX and matrix metalloproteinase-2. Toward nontoxic chemotherapy targeting tumor microenvironment. J Med Chem 2012;55:7875–82
- Breuer E, Salomon CJ, Katz Y, et al. Carbamoylphosphonates, a new class of in vivo active matrix metalloproteinase inhibitors. 1. Alkyl- and cycloalkylcarbamoylphosphonic acids. J Med Chem 2004;47:2826–32
- Reich R, Katz Y, Hadar R, Breuer E. Carbamoylphosphonate matrix metalloproteinase inhibitors 3: in vivo evaluation of cyclopentylcarbamoylphosphonic acid in experimental metastasis and angiogenesis. Clin Cancer Res 2005;11:3925–9
- Frant J, Veerendhar A, Chernilovsky T, et al. Orally active, antimetastatic, nontoxic diphenyl ether-derived carbamoylphosphonate matrix metalloproteinase inhibitors. ChemMedChem 2011;6:1471–7
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74
- Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol 2009;21:645–53
- Madan D, Ferguson CG, Lee WY, et al. Non-invasive imaging of tumors by monitoring autotaxin activity using an enzyme-activated near-infrared fluorogenic substrate. PLoS One 2013;8:e79065
- Jahnke W, Henry C. An in vitro assay to measure targeted drug delivery to bone mineral. ChemMedChem 2010;5:770–6
- Shinoda H, Adamek G, Felix R, et al. Structure–activity relationships of various bisphosphonates. Calcif Tissue Int 1983;35:87–99
- Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 2005;5:786–95
- McIntyre A, Patiar S, Wigfield S, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res 2012;18:3100–11
- Stellas D, Patsavoudi E. Inhibiting matrix metalloproteinases, an old story with new potentials for cancer treatment. Anticancer Agents Med Chem 2012;12:707–17
- Lia NG, Shib ZH, Tang YP, Duan JA. Selective matrix metalloproteinase inhibitors for cancer. Curr Med Chem 2009;16:3805–27
- Farkas E, Katz Y, Bhusare S, et al. Carbamoylphosphonate-based matrix metalloproteinase inhibitor metal complexes: solution studies and stability constants. Towards a zinc-selective binding group. J Biol Inorg Chem 2004;9:307–15
- Hoffman A, Stepensky D. Pharmacodynamic aspects of modes of drug administration for optimization of drug therapy. Crit Rev Ther Drug Carrier Syst 1999;16:571–639