Abstract
A new series of anti-bacterial and anti-fungal mono- and di-substituted triazoles (L1)–(L6) have been synthesized and characterized on the basis of their physical, spectral and analytical data. The ligands (L1)–(L6) on reaction with vanadyl(IV) sulphate led to the formation of vanadyl(IV) metal complexes (1)–(4). The structure of the complexes has been established on the basis of their physical, spectral and elemental analyses data. The synthesized ligands and their vanadyl(IV) complexes have been screened in vitro for anti-bacterial activity against six bacterial species such as, Escherichia coli (ATCC 25922), Shigella flexneri (ATCC 12022), Pseudomonas aeruginosa (ATCC 27853), Salmonella typhi (ATCC 14028), Staphylococcus aureus (ATCC 25923) and Bacillus subtilis (ATCC 6051) and for in vitro anti-fungal activity against six fungal strains, Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata. The screening results showed the vanadyl complexes to be more bactericidal/fungicidal against one or more bacterial/fungal species. The synthesized compounds were also subjected to brine shrimp bioassay for scrutinizing their cytotoxicity.
Introduction
Due to emergence of antibiotic resistance to clinically used drugs, it is required that novel, efficient and selective strategies may be designed which could work more aggressively to target the lipoid layer of the microorganisms responsible for drug resistance. Metal complexes have been the best choice so far in designing these candidates with remarkable biological potentialsCitation1–3. Variety of triazole derivatives represent an interesting class of compounds showing diversified clinical and pharmacological applications such as anti-bacterialCitation4, anti-fungalCitation5, anti-tumorCitation6, anti-cancerCitation7, anti-inflammatoryCitation8, anti-convulsantCitation9, anti-proliferativeCitation10 and analgesic propertiesCitation11.
Advances in medicinal properties of vanadium metal complexes have led to designing and use of its bioactive potential in metal-based drugsCitation12. The most important aspect is; its ability to enhance the activity of insulin mimeticCitation13,Citation14 in diabetes mellitus of human pathophysiological condition. Vanadyl(IV) complexes with bi-dentate ligands bound to the vanadium metal through oxygen and nitrogen atoms, have been broadly focused in recent years due to their significant efficiency as insulin mimetic compoundsCitation15,Citation16. Orally active medication of these compounds as drug represents an important advancement in the curative approach of human diabetes mellitus. Other potential applications of vanadyl complexes have also been studied in promoting the biological activities such as anti-bacterialCitation17, anti-fungalCitation18, spermicidalCitation19, anti-tumorCitation20, anti-leukemicCitation21 and anti-amoebicCitation22 activity.
Due to increased interest in bioactive potentials of triazoles and vanadium metal, it was thought valuable to combine both the chemistry of triazoles and vanadium metal to form a novel class of vanadium metal based triazoles that could serve as probable bactericidal and fungicidal against resistant bacterial/fungal strains. For this purpose, a new series of vanadyl complexes (1)–(4) with mono-substituted (L1)–(L3) and di-substituted (L4)–(L6) (Schemes 1 and 2) triazole-derived compounds have been prepared and screened for their bactericidal/fungicidal activity against a number of bacterial/fungal strains.
Experimental
Materials and methods
All the chemicals used were of analytical grades. Triazole was purchased from Sigma Aldrich. Fisher Johns melting point apparatus was used for recording melting points. Infrared spectra were recorded on SHIMADZU FT-IR spectrophotometer. Elemental analysis was carried out on Perkin Elmer (USA model). 1H and 13C NMR spectra were recorded on a Bruker Spectrospin Avance DPX-400 spectrometer using TMS as internal standard and d6-DMSO as a solvent. Electron impact mass spectra (EIMS) were recorded on JEOL MSRoute instrument. In vitro bactericidal, fungicidal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Centre for Chemical Sciences, University of Karachi, Pakistan.
Synthesis
Procedure for the synthesis of mono-substituted Schiff bases (L1)–(L3)
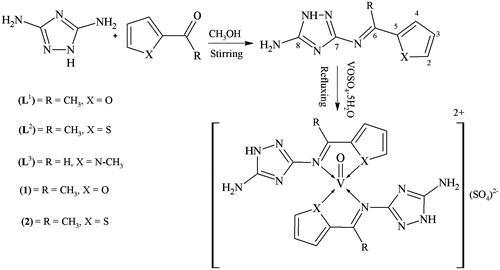
An equimolar solution of 3,5-diamino-1,2,4-triazole (0.99 g, 0.01 moles) and 2-acetyl furan (1 mL, 0.01 moles) in 50-mL dry methanol was stirred for 5 h at room temperature, a precipitated product was formed during stirring. It was filtered, washed with methanol (3 × 5 mL), then with diethyl ether (2 × 5 mL) and dried. Recrystallization in a mixture of methanol:dioxane (1:4) afforded TLC pure product (L1) The same method was applied for the preparation of other ligands (L2)–(L3).
N3-[(1E)-1-(furan-2-yl)ethylidene]-1H-1,2,4-triazole-3,5-diamine (L1)
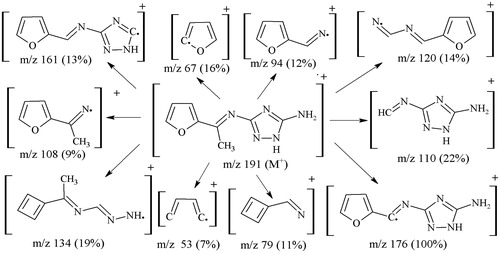
Yield (1.4g, 73%); m.p. 244 °C; IR (KBr, cm−1): 3350 (NH2), 3203 (NH, triazole), 1636 (C = N, azomethine), 1605 (C = N, triazole), 1128 (C–O), 1021 (N-N); 1H NMR (DMSO-d6, δ, ppm): δ 2.34 (s, CH3), 6.02 (s, NH2), 6.95 (dd, J = 4.9, 3.8 Hz, C3-H), 7.38 (d, J = 4.9 Hz, C4-H), 7.69 (d, J = 3.8 Hz, C2-H), 11.88 (s, NH); 13C NMR (DMSO-d6, δ, ppm): δ 15.10 (CH3), 114.32 (C4), 122.43 (C3), 141.81 (C2), 146.68 (C5), 152.71 (C6), 158.48 (C8), 163.59 (C7); EIMS (70eV) m/z (%): 191 ([M]+, 18), 176 (100), 161 (13), 134 (19), 120 (14), 110 (22), 108 (9), 94 (12), 79 (11), 67 (16), 53 (7); anal. calcd. for C8H9N5O (191.19): C, 50.26; H, 4.74; N, 36.63; Found: C, 50.20; H, 4.71; N, 36.58%.
N3-[(1E)-1-(thiophen-2-yl)ethylidene]-1H-1,2,4-triazole-3,5-diamine (L2)
Yield (1.45 g, 70%); m.p. 252 °C; IR (KBr, cm−1): 3350 (NH2), 3204 (NH, triazole), 1635 (C = N, azomethine), 1608 (C = N, triazole), 1021 (N-N), 883 (C-S); 1H NMR (DMSO-d6, δ, ppm): δ 2.38 (s, CH3), 5.98 (s, NH2), 6.90 (d, J = 3.8 Hz, C3-H), 7.50 (d, J = 3.6 Hz, C4-H), 7.65 (d, C2-H), 11.86 (s, NH); 13C NMR (DMSO-d6, δ, ppm): δ 14.90 (CH3), 125.95 (C2), 132.30 (C3), 136.76 (C5), 138.44 (C4), 158.17 (C6), 158.86 (C8), 163.56 (C7); EIMS (70eV) m/z (%): 207 ([M]+, 17), 192 (100), 151 (14), 136 (9), 123 (13), 110 (10), 109 (19), 84 (15); anal. calcd. for C8H9N5S (207.26): C, 46.36; H, 4.38; N, 33.79; S: 15.47; Found: C, 46.30; H, 4.35; N, 33.74; S: 15.43%.
N3-[(E)-(1-methyl-1H-pyrrol-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L3)
Yield (1.3 g, 68%); m.p. 231 °C; IR (KBr, cm−1): 3350 (NH2), 3204 (NH, triazole) 1634 (HC = N, azomethine), 1606 (C = N, triazole), 1020 (N-N); 1H NMR (DMSO-d6, δ, ppm): δ 3.45 (s, CH3), 5.91 (dd, J = 3.8 Hz, C3-H), 6.0 (s, NH2), 6.68 (d, J = 3.7 Hz, C4-H), 6.97 (d, C2-H), 8.69 (s, HC = N), 11.88 (s, NH); 13C NMR (DMSO-d6, δ, ppm): δ 32.20 (N-CH3), 113.65 (C3), 116.96 (C4), 122.44 (C2), 130.13 (C5), 152.73 (C6), 157.61 (C8), 162.85 (C7); EIMS (70eV) m/z (%): 190 ([M]+, 19), 175 (100), 159 (13), 147 (11), 133 (14), 110 (11), 107 (16), 83 (9), 80 (11), 66 (8); anal. calcd. for C8H10N6 (190.21): C, 50.52; H, 5.30; N, 44.18; Found: C, 50.47; H, 5.27; N, 44.12%.
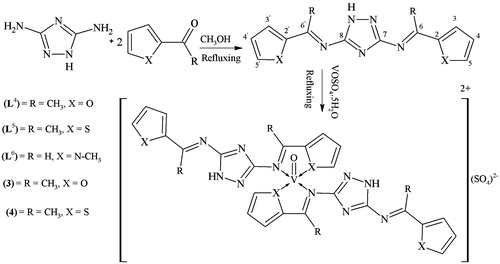
Procedure for the synthesis of di-substituted schiff bases (L4)–(L6)
The ligands (L4)–(L6) have been synthesized by the condensation reaction of 3,5-diamino-1,2,4-triazole with 2-acetyl furan, 2-acetyl thiophene and 1-methylpyrrole-2-carboxaldehyde in (1:2) molar ratio on refluxing. Rest of the procedure was same as for synthesis of (L1).
N,N′-bis[(1E)-1-(furan-2-yl)ethylidene]-1H-1,2,4-triazole-3,5-diamine (L4)
Yield (2.1g, 74%); m.p. 229–231 °C; IR (KBr, cm−1): 3205 (NH, triazole), 1637 (C = N, azomethine), 1607 (C = N, triazole), 1130 (C–O), 1020 (N-N); 1H NMR (DMSO-d6, δ, ppm): δ 2.34 (s, 6H, CH3), 6.95 (dd, J = 4.9, 3.8 Hz, C3-H, -H), 7.38 (d, 2H, J = 4.9 Hz, C4-H,
-H), 7.69 (d, 2H, J = 3.8 Hz, C2-H,
-H), 11.88 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 15.10 (2×CH3), 114.34 (C4,
), 122.45 (C3,
), 141.80 (C2,
), 146.69 (C5,
), 152.72 (C6,
), 164.44 (C7, C8); EIMS (70eV) m/z (%): 283 ([M]+, 24), 268 (35), 254 (11), 190 (100), 176 (23), 120 (20), 108 (11), 94 (18), 78 (13); anal. calcd. for C14H13N5O2 (283.28): C, 59.36; H, 4.63; N, 24.72; Found: C, 59.48; H, 4.59; N, 24.68%.
N,N′-bis[(1E)-1-(thiophen-2-yl)ethylidene]-1H-1,2,4-triazole-3,5-diamine (L5)
Yield (2.35g, 75%); m.p. 226–227 °C; IR (KBr, cm−1): 3206 (NH, triazole), 1635 (C = N, azomethine), 1609 (C = N, triazole), 1021 (N-N), 885 (C-S); 1H NMR (DMSO-d6, δ, ppm): δ 2.38 (s, 6H, CH3), 6.90 (d, 2H, J = 3.8 Hz, C3-H, -H), 7.50 (d, 2H, J = 3.6 Hz, C4-H,
-H), 7.65 (d, 2H, C2-H,
-H), 11.86 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 14.90 (2×CH3), 125.95 (C2,
), 132.30 (C3,
), 136.76 (C5,
), 138.44 (C4,
), 158.17 (C6,
), 163.36 (C7, C8); EIMS (70eV) m/z (%): 315 ([M]+, 23), 300 (100), 286 (17), 206 (21), 192 (14), 136 (26), 124 (9), 110 (8), 83 (9), 78 (7); anal. calcd. for C14H13N5S2 (315.42): C, 53.31; H, 4.15; N, 22.20; S: 20.33; Found: C, 53.27; H, 4.19; N, 22.17; S: 20.35%.
N,N′-bis[(1E)-(1-methyl-1H-pyrrol-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L6)
Yield (2.2g, 78%); m.p. 218–219 °C; IR (KBr, cm−1): 3204 (NH, triazole) 1634 (HC = N, azomethine), 1606 (C = N, triazole), 1020 (N-N); 1H NMR (DMSO-d6, δ, ppm): δ 3.45 (s, 6H, CH3), 5.91 (dd, 2H, J = 3.8 Hz, C3-H, -H), 6.68 (d, 2H, J = 3.7 Hz, C4-H,
-H), 6.97 (d, 2H, C2-H,
-H), 8.69 (s, 2H, HC = N), 11.88 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 32.20 (N-CH3), 113.65 (C3,
), 116.96 (C4,
), 122.44 (C2,
), 130.13 (C5,
), 152.73 (C6,
), 160.15 (C7, C8); EIMS (70eV) m/z (%): 315 ([M]+, 12), 300 (4), 207 (93), 192 (100), 174 (4), 151 (20), 136 (11), 124 (11), 110 (16), 97 (8), 84 (8); anal. calcd. for C14H15N7 (281.32): C, 59.77; H, 5.37; N, 34.85; Found: C, 59.72; H, 5.34; N, 34.81%.
Synthesis of vanadyl(IV) complexes (1)
To a hot magnetically stirred dioxane (20 mL) solution of N3-[(1E)-1-(furan-2-yl)ethylidene]-1H-1,2,4-triazole-3,5-diamine (0.382 g, 0.002 moles), a methanol solution (20 mL) of VOSO4.5H2O (0.163g, 0.001 moles) was added. The mixture was refluxed for 3 h during which a precipitated product was formed. It was then cooled to room temperature. The precipitates thus formed were filtered, washed with methanol, dioxane and then with diethyl ether and dried. It was recrystallized in a mixture of water:dioxane (1:3) to obtain TLC pure complex (1) (Schemes 1 and 2). All other complexes (2)–(4) were prepared following the same method.
Pharmacology
In vitro bactericidal, fungicidal, minimum inhibitory concentration (MIC) and cytotoxicity were studied according to following procedures.
Bactericidal activity (in vitro)
All the newly synthesized triazole Schiff bases (L1)–(L6) and their oxovanadium(IV) complexes (1)–(4) were screened in vitro for their bactericidal activity against four Gram-negative (Escherichia coli (ATCC 25922), Shigella flexneri (ATCC 12022), Pseudomonas aeruginosa (ATCC 27853), Salmonella typhi (ATCC 14028), and two Gram-positive Staphylococcus aureus (ATCC 25923) and Bacillus subtilis (ATCC 6051) bacterial strains by the agar-well diffusion methodCitation23. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centers at least 24-mm apart. Two to eight hours old bacterial inocula containing approximately 104–106 colony-forming units (CFU/mL) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration of the test sample (1mg/mL in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference anti-bacterial drug, imipenum, served as negative and positive controls, respectively. The plates were incubated at 37 °C for 24 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). In order to confirm the effect of DMSO in the biological screening, alternate studies on DMSO solution showed no activity against any bacterial strains.
Fungicidal activity (in vitro)
Fungicidal activity of all compounds was studied against six fungal strains (T. longifusus, C. albican, A. flavus, M. canis, F. solani and C. glabrata). Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 (cfu) mL−1 fungal spore suspensions and transferred to petri plates. Discs soaked in 20 mL (200 µg/mL in DMSO) of the compounds were placed at different positions on the agar surface. The plates were incubated at 32 °C for 7 d. The results were recorded as percentage of inhibition and compared with standard drugs miconazole and amphotericin B.
Minimum inhibitory concentration
Compounds containing promising anti-bacterial activity were selected for MIC studiesCitation24. The MIC was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μg mL−1 concentrations of the compounds along with standards at the same concentrations.
Cytotoxicity (in vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the matter compartment was opened to ordinary light. After 2 d a pipette collected nauplii from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMF. From this stock solutions 500, 50 and 5 µg/mL were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After 2 d, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/ dilution) and the volume was adjusted with seawater to 5 mL per vialCitation25. After 24 h the number of survivors was counted. Data were analyzed by Finney, computer program to determine the LD50 valuesCitation26.
Statistical analysis
Standard deviationCitation27 is a generally used measure of variability or diversity in statistics and probability theory. It indicates how much variation or dispersion is there from the average or mean values. A low standard deviation shows that the data points tend to be very close to the average, while high standard deviation indicates that the data are distributed over a wide range of values. Standard deviation was used as a statistical tool to check the variation of triplicate anti-bacterial and anti-fungal results.
Results and discussion
Chemistry
New series of triazole derived Schiff bases (L1)–(L6) have been synthesized by the condensation reaction of 3,5-diamino-1,2,4-triazole with 2-acetyl furan, 2-acetyl thiophene and 1-methylpyrrole-2-carboxaldehyde in (1:1) molar ratio on stirring (Scheme 1) and (1:2) molar ratio on refluxing (Scheme 2). The most stable structures of the synthesized ligands have been established on the basis of their physical, spectral (IR, 1H and 13C NMR and mass spectrometry) and analytical (CHN analysis) data. All the prepared ligands were stable at room temperature, non-hygroscopic and colored amorphous solid. The vanadyl(IV) complexes (1)–(4) of these ligands were prepared in a molar ratio of 1:2 (metal:ligand). The ligands were soluble in dioxane at room temperature, soluble in methanol on heating and the vanadyl(IV) complexes were only soluble in DMF and DMSO. Physical and analytical data of the complexes () showed these complexes were monomers having stoichiometry of the type ML2 (metal:ligand).
Table 1. Physical and analytical data of the vanadyl(IV) complexes (1)–(4).
IR spectra
The most important and distinguishing IR spectral bands are reported in experimental part and . All ligands possessed potentially active donor sites such as, azomethine nitrogen (–HC = N), triazole nitrogens, acetylfuran-O and acetylthiophene-S which have ability to coordinate with the vanadium metal atom. Originally, 3,5-diamino-1,2,4-triazole had two bands at 3310 and 3350 cm−1 due to two amino groups. Ligands of Scheme 1 showed an absence of a band at 3310 cm−1 emerging into a new band due to the azomethine linkage at 1634–1636 cm−1 however, a band at 3350 cm−1 remained unchanged, thus giving evidence for condensation of only one amino group of the triazole moiety to produce mono-Schiff basesCitation28. However, the ligands of Scheme 2 showed the absence of the bands at 1735, 3310 and 3350 cm−1 originally assigned to carbonyl v(C = O) of aldehydes and two v(NH2) groups of triazole moiety. The absence of these bands and instead, appearance of a strong new band of azomethine v(HC = N) linkage at 1634–1637 cm−1 gave a clue of condensation of carbonyl v(C = O) group with both amino groups of triazole moiety, thus giving bi-Schiff basesCitation29. The bands appearing in the ligands at 883–885 and 1128–1130 cm−1 were assigned to (C–S) and (C–O) vibrations. All these ligands displayed bands at 3203–3206, 1605–1608 and 1020–1021 cm−1 respectively due to the presence of N–H, C = N and N–N vibrations of triazole moietyCitation30.
Table 2. Physical, spectral and IR data of the vanadyl(IV) complexes.
On comparison of IR spectral data of all the vanadyl(IV) complexes with the data of Schiff base ligands, the absorption modes indicated that the triazole Schiff bases were principally coordinated to the vanadium(IV) metal ion bidentately. The coordination modes of bonding are discussed below:
all the ligands showing IR spectral bands at 1634–1637 cm−1 due to azomethine, ν(HC = N) shiftedCitation31 to lower frequency (15–17 cm−1) at 1618–1622 cm−1thus indicating the coordination of the azomethine-N with the vanadium(IV) metal atom.
the bands at 883–885 and 1128–1130 cm−1 assigned to ν(C–S) and ν(C–O) of thiophene and furan moiety shifted to lower frequency side (15 cm−1) at 868 and 1115 cm−1 suggesting the coordination of thiophene-S and furan-O with the vanadium(IV) metal atom.
the appearance of weaker low-frequency new bands at 417–422, 475–476 and 495–497 cm−1 were attributed to and v(vanadium-N), v(vanadium-S) and v(vanadium-O) confirmingCitation32 the coordination of vanadium metal atom with the ligands via azomethine-N, thiophene-S and furan-O.
two new bands which were not observed in the spectra of the ligands but appeared in the spectra of complexes at 1082–1086 and 976–980 cm−1 were assigned to the presence of (SO4) groupCitation33 outside the coordination sphere and (V = O) of vanadylCitation34, respectively.
all the ligands showed bands at 3205–3208, 1606–1609 and 1020–1021 cm−1 respectively due to the presence of N–H, C = N and N–N vibrations of triazole moiety that remained unchanged in all the complexes indicating that these groups did not participate in the coordination phenomenon.
1H NMR spectra
The 1H NMR spectra of mono-substituted Schiff bases demonstratedCitation35 characteristic amino (NH2) protons at 5.98–6.02 ppm as a singlet, which provided evidence for condensation of one amino group of the triazole moiety. The ligands (L1)-(L6) showed methyl (CH3) and NH protons at 2.42–3.45and 11.86–11.88 ppm, respectively as a singlet. However, the C3–H & –H protons of (L1) and (L4) were observed at 6.95 ppm as a double doublet. In addition, the C4–H &
–H and C2–H &
–H protons were found at 7.38 and 7.69 ppm, respectively as a doublet. Similarly, C3–H and
–H protons found in ligand (L2) and (L5) were observed at 6.90 ppm as a double doublet and C4–H &
–H and C2–H &
–H protons at 7.50 and 7.65 ppm as a doublet. But in case of ligand (L3) & (L6), C3–H and
–H protons appeared at 5.91 ppm as a double doublet, C4–H &
–H and C2–H &
–H protons at 6.68 and 6.97 ppm as a doublet and were shielded due to methyl group and shifted upfield. Azomethine protons were observed at 8.69 ppm as a singlet in ligand (L3) and (L6). All the protons due to heteroaromatic groups were found in their expected regionCitation36. However, the number of proton calculated from the 1HNMR integration curves and those obtained from the values of the expected microanalysis (CHN) confirmed the proposed structures of the Schiff bases. The conclusions drawn from this study provided further support to the modes of bonding discussed earlier in IR spectra.
13C NMR spectra
The 13C NMR spectra of the Schiff bases showed triazole carbons (C7) and (C8) in the region at 157.61–164.44 ppm, respectively. The azomethine carbons (C6) and () of the ligands appeared in the region at 152.71–158.17 ppm. The furanyl and thienyl carbons were found in the region at 113.65–149.86 ppm. The methyl groups of the ligands (L1)–(L6) appeared in the region at 14.9–32.20 ppm. All the protons due to heteroaromatic groups were found in their expected region. The present studies are well supported by their IR and 1H NMR spectral data. Furthermore, all these studies indicated that expected values of carbon atoms compromised well with the number of carbon atoms present in the proposed structures of the compounds.
Mass spectra
The mass spectral data and fragmentation pattern of the triazole ligands strongly confirmed the formation of the ligands possessing proposed structures and also, their bonding pattern. The molecular masses of ligands (L1)–(L6), were found at m/z 191 (calcd. 191.19) for fragment [C8H9N5O]+, m/z 207 (calcd. 207.26) for fragment [C8H9N5S]+, m/z 190 (calcd. 190.21) for fragment [C8H10N6]+, m/z 283 (calcd. 283.28) for fragment [C14H13N5O2]+, m/z 315 (calcd. 315.42) for fragment [C14H13N5S2]+ and m/z 281 (calcd. 281.32) for fragment [C14H15N7]+, respectively. The most stable base peak fragment [C7H6N5O]+ of ligand (L1) was observed at m/z 176. Moreover, ligand (L2) displayed the base peak stable fragment [C7H6N5S]+ at m/z 192 and (L3) showed the stable base peak having fragment [C7H7N6]+ at m/z 175. Similarly, the most stable base peak fragment [C8H8N5O]+ of ligand (L4) was observed at m/z 190. Moreover, ligand (L5) displayed the base peak stable fragment [C8H8N5S]+ at m/z 207 and (L6) showed the stable base peak having fragment [C8H10N6]+ at m/z 192, as these were the most expected stable fragments. The fragmentation pattern followed the cleavage of C = N (exocyclic as well as endocyclic), C = C, C–C, C–O and C–S bonds. Fragmentation pattern of one ligand (L1) is shown as .
Molar conductance and magnetic susceptibility
The molar conductance values of the complexes (1)–(4) were taken in DMF which fall in the range 92–98 Ω−1cm2 mol−1 () indicating electrolytic natureCitation22 of the vanadyl(IV) complexes. The magnetic moment (1.71–1.73 BM) values of the complexes were carried out at room temperature () and supported the square-pyramidal geometryCitation37.
UV/visible spectra
The UV/Visible spectra of the vanadyl(IV) complexes in DMF showed three () distinct low to high intensity bands (v1, v2 and v3) which were assigned to B2 (dxy) → Eπ(dxz, dyz), B2 (dxy) → B1 () and B2 (dxy) → A1 (
) transitions, respectively. The first band found at 13 275–13 341 cm−1was assigned to B2→ Eπ d-d transitions. The second band was observed at 18 438–18 528 cm−1 which can be attributed to B2→ B1 and the presence of third band at 26 869–26 892 cm−1 can be assigned to the transitions B2→ A1. The fourth band of high intensity shown at 36 094–36 142 cm−1 was assigned to metal → ligand charge transfer (MLCT). All these findings provided a strong evidence for the complexes to have a square–pyramidal geometryCitation38.
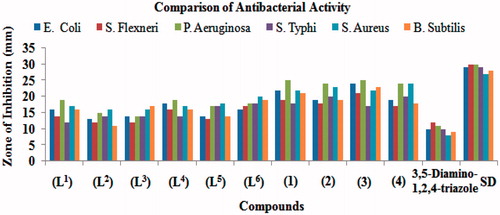
Table 3. Bactericidal activity (concentration used 1 mg/mL of DMSO) of Triazole schiff bases and their vanadyl(IV) complexes [Zone of Inhibition (mm)].
Pharmacology
Bactericidal activity (in vitro)
The results obtained from in vitro bactericidal activity are summarized in () and as (). All the synthesized compounds were tested against four Gram-negative (E. Coli, S. Flexneri, P. Aeruginosa, S. Typhi) and two Gram-positive (S. Aureus, B. Subtilis) bacterial strains according to literature protocol. The results obtained were compared with that of the standard drug imipenum. The percentage activity was compared with the activity of the standard drug considering its activity as 100%. All these ligands and their vanadyl(IV) complexes showed bactericidal activity against all Gram-negative and Gram-positive bacterial strains. The ligand (L1) showed a significant (16–19 mm, 55–63%) activity against all bacterial strains except S. Flexneri and S. Typhi which possessed moderate (12–14 mm, 41–47%) activity. The ligand (L2) exhibited significant (16 mm, 59%) activity against S. Aureus and remaining strains possessed moderate (11–15 mm, 39–50%) activity. The ligand (L3) possessed significant (16–17 mm, 59–61%) activity against S. Aureus and B. Subtilis bacterial strains and rest of strains showed moderate (12–14 mm, 40–48%) activity. The ligand (L4) possessed overall significant (16–19 mm, 57–63%) activity against all bacterial strain except S. Typhi which displayed moderate (14 mm, 48%) activity. Similarly, the ligand (L5) exhibited significant (17–18 mm, 59-67%) activity against P. aeruginosa, S. typhi and S. aureus, and rest of the strains E. coli, S. flexneri and B. Subtilis showed moderate (13–14 mm, 43–50%) activity. However, the ligand (L6) displayed overall significant (16–20 mm, 55–74%) activity against all bacterial strains. Moreover, all the vanadyl(IV) complex (1)–(4) showed overall significant (17–25 mm, 57–89%) activity against all bacterial strains. The results revealedCitation39 that all the ligands and their vanadyl(IV) complexes contributed significantly towards enhancing the bactericidal activity. It is evident from the reported data that coordination makes the ligands to inhibit the growth of bacteria more than the parent ligand.
Fungicidal activity (in vitro)
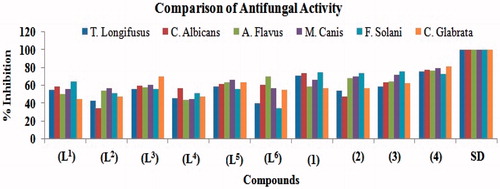
In vitro fungicidal activity of all the compounds were carried out against six fungal strains; T. Longifusus, C. Albican, A. Flavus, M. Canis, F. Solani and C. Glabrata () according to the literature protocol. The results of inhibition obtained were compared with those of the standard drugs, miconazole and amphotericin B (). The activity was measured in percentage inhibition. The ligand (L1) had shown significant (55–65%) activity against all fungal strain except A. Flavus and C. Glabrata which showed moderate (45–51%) activity. The (L2) possessed significant (54–57%) activity against A. Flavus and M. Canis, and rest of strains displayed moderate (35–52%) activity. On the other hand, (L3) displayed overall significant (56–70%) activity against all fungal strains. The ligand (L4) exhibited significant (57%) activity against C. Albican and remaining strains showed moderate (44–52%) activity. The (L5) displayed overall significant (56–67%) activity against all strains. However, (L6) exhibited weaker (35–40%) activity against T. Longifusus and C. Albican, and remaining strains displayed significant (55–70%) activity, respectively. On comparison with the vanadyl(IV) complexes, all the complexes (1)–(6) possessed overall significant (54–82%) activity against all fungal strains except C. Albicans fungal strain of (2) which possessed moderate (48%) activity. It was also concluded that the heterocyclic aldehyde ring system and imine (–CH = N) group play a key role in enhancing the activity. On the other hand, the comparison of average values of the complexes versus ligands, a conclusion can be drawn that the fungicidal activity is overall enhanced upon chelation with the vanadyl(IV) metal atomCitation40.
Table 4. Fungicidal activity (concentration used 200 µg/mL) of triazole schiff bases and their vanadyl(IV) complexes [% inhibition].
Minimum inhibitory concentration (MIC)
The synthesized ligands and their vanadyl(IV) complexes showing promising bactericidal activity (above 80%) were selected for MIC studies and obtained results are reported in . The obtained results indicated that all the vanadyl(IV) complexes (1)–(4) were found to display activity more than 80%, therefore, these complexes were selected for their MIC screening. The MIC values of these compounds (1)–(4) fall in the range 4.54 × 10−8 to 1.64 × 10−7 M. Among these, the compound (1) was found to be the most active possessing maximum inhibition 4.54 × 10−8 M against bacterial strain P. Aeruginosa.
Table 5. Minimum inhibitory concentration (M/mL) of the selected compounds (1)–(4) against selected bacteria and in vitro cytotoxic bioassay of ligands, (L1)–(L6) and vanadyl(IV) complexes (1)–(4).
Cytotoxicity (in vitro)
The Schiff bases and their vanadyl(IV) complexes were screened for their cytotoxicity (brine shrimp bioassay) by using Meyer protocolCitation25. The data recorded in clearly indicated that no compound either ligands or complexes showed potent cytotoxic activity against Artemia salina. This activity relationship may help to serve as a basis for the future research pursuits in designing and development of cytotoxic agents used for clinical purposes.
Conclusion
The synthesized triazole Schiff bases act as bidentate ligands for coordination with the vanadium metal atom. Physical (magnetic and molar conductance), spectral (IR, NMR, electronic) and analytical (C, H, N and V percentage) data revealed that the Schiff base ligands are coordinated with the vanadium metal atom via azomethine-N, furanyl-O and/or thienyl-S showing a square-pyramidal geometry. The obtained results of bactericidal and fungicidal activities indicated that the vanadyl(IV) complexes possessed more biological activity against one or more bacterial and/or fungal strains as compared to their parent uncomplexed ligands. Generally, it is claimed that functional groups found in the compounds like azomethine-N and other heteroatoms (N, O and S) are responsible for the enhancement of bactericidal and fungicidal activities. Our present studies are reported with the conclusion that those compounds which were biologically active, became more active and less biologically active became more upon complexation/coordination with the vanadium metal atom.
Acknowledgements
The authors are thankful to Higher Education Commission (HEC), Government of Pakistan for the award to carry out this research work. The authors are also indebted to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for providing their help in taking NMR, mass spectra and for the help in carrying out bactericidal, fungicidal and brine shrimp bioassays.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the article.
References
- Bagihalli GB, Badami PS, Patil SA. Synthesis, spectral characterization and in vitro biological studies of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. J Enz Inhib Med Chem 2009;24:381–94
- Chohan ZH, Hanif M. Design, synthesis, and biological properties of triazole derived compounds and their transition metal complexes. J Enzyme Inhib Med Chem 2010;25:737–49
- Haasnoot JG. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1, 2, 4-triazole derivatives as ligands. Coord Chem Rev 2000;200–202:131–85
- Chohan ZH, Hanif M. Synthesis and characterization of biologically active new Schiff bases containing 3-functionalized 1,2,4-triazoles and their zinc(II) complexes: crystal structure of 4-bromo-2-[(E)-(1H-1,2,4-triazol-3-ylimino)-methyl]phenol. Appl Organomet Chem 2011;25:753–60
- Chohan ZH, Sumrra SH. Metal based biologically active compounds: Design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem 2010;45:2739–47
- Guo-Qiang H, Li-Li H, Song-Qiang X, Wen-Long H. Design, synthesis and antitumor activity of asymmetric bis(s-triazole Schiff-bases) bearing functionalized side-chain. Chin J Chem 2008;26:1145–9
- Holla BS, Veerendra B, Shivanada MK, Poojary B. Synthesis, characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–67
- Hussein MA, Shaker RM, Ameen MA, Mohammed MF. Synthesis, anti-inflammatory, analgesic, and antibacterial activities of some triazole, triazolothiadiazole, and triazolothiadiazine derivatives. Arch Pharm Res 2011;34:1239–50
- Cui XS, Jing C, Chai KY, Lee JS, Quan ZS. Synthesis and anticonvulsant evaluation of 3-substituted-4-(4-hexyloxyphenyl)-4H-1,2,4-triazoles. Med Chem Res 2009;18:49–58
- Manfredini S, Vicentini CB, Manfrini M, et al. Pyrazolo-triazoles as light activable DNA cleaving agents. Bioorg Med Chem 2000;8:2343–6
- Turan-Zitouni G, Kaplancikli ZA, Erol K, Kilic FS. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Farmaco 1999;54:218–23
- Chohan ZH, Sumrra SH. Some biologically active oxovanadium(IV) complexes of triazole derived Schiff bases: their synthesis, characterization and biological properties. J Enz Inhib Med Chem 2010;25:599–607
- Sakurai H, Kojitane Y, Yoshikawa Y, et al. Antidiabetic vanadium(IV),and zinc(II) complexes. Coord Chem Rev 2002;226:187–9
- Fedorova EV, Buryakina AV, Zakharov AV, et al. Design, synthesis and pharmacological evaluation of novel vanadium-containing complexes as antidiabetic agents. PLoS ONE 2014;9:7:e100386
- Fedorova1 EV, Buryakina AV, Vorobieva NM, Baranova NI. The vanadium compounds: chemistry, synthesis, insulinomimetic properties. Biomed Chem 2013;7:259–70
- Butler A, Carrano CJ. Coordination chemistry of vanadium in biological systems. Coord Chem Rev 1991;109:61–105
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda TB. Design and synthesis of triazole Schiff bases and their oxovanadium(IV) complexes as antimicrobial agents. J Coord Chem 2010;63:3981–98
- Chohan ZH, Sumrra SH. Design, synthesis and biological properties of thiophene derived triazole Schiff bases and their oxovanadium(IV) complexes. J Enz Inhib Med Chem 2012;27:187–93
- D'Cruz OJ, Dong Y, Uckun FM. Spermicidal activity of oxovanadium(IV) complexes of 1, 10-phenanthroline, 2,2'-bipyridyl, 5'-bromo-2'-hydroxyacetophenone and derivatives in humans. Biol Reprod 1999;60:435–44
- Noblia P, Vieites M, Parajon-Costa BS, et al. Vanadium(V) complexes with salicylaldehyde semicarbazone derivatives bearing in vitro anti-tumor activity toward kidney tumor cells (TK-10): Crystal structure of [VVO2(5-bromosalicylaldehyde semicarbazone)]. J Inorg Biochem 2005;99:443–51
- Dong Y, Narla RK, Sudbeck E, Uckun FM. Synthesis, X-ray structure, and anti-leukemic activity of oxovanadium(IV) complexes. J Inorg Biochem 2000;78:321–30
- Maurya MR, Agarwal S, Abid M, et al. Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes. Dalton Trans 2006;7:937–47
- Rahman AU, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. The Netherlands: Harwood Academic Publishers; 2001
- McLaughlin JL, Chang CJ, Smith DL. In: Atta-ur-Rahman, ed. Studies in natural products chemistry, ‘‘Bentch-Top’’ bioassays for the discovery of bioactive natural products: an update, structure and chemistry (part-B), Vol. 9. The Netherlands: Elsevier Science Publishers; 1991:383
- Meyer BN, Ferrigni NR, Putnam JE, et al. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982;45:31–4
- Finney DJ. Probit analysis, 3rd ed. Cambridge University Press, 1971
- Brown GW. Standard deviation, standard error: which ‘standard’ should we use? Am J Dis Child 1982;136:937–41
- Chohan ZH, Sumrra SH. Synthesis, characterization and biological studies of oxovanadium(IV) complexes with triazole-derived Schiff bases. Appl Organomet Chem 2010;24:122–30
- Holla BS, Mahalinga M, Karthikeyan MS, et al. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur J Med Chem 2005;40:1173–8
- Maurya MR, Singh H, Pandey A. (hydroxyalkyl/ aryl]benzimidazolato) Dioxovanadates(V) α Potassium bis(2-[through base assisted aerial oxidation of the corresponding oxovanadium(IV) complexes. Synth React Inorg Met-Org Chem 2002;32:231–42
- Sumrra SH, Chohan ZH. Antibacterial and antifungal oxovanadium(IV) complexes of triazole-derived Schiff bases. Med Chem Res 2013;22:3934–42
- Sumrra SH, Chohan ZH. Metal based new triazoles: their synthesis, characterization and antibacterial/antifungal activities. Spectrochim Acta Part A: Mol Biomol Spectr 2012;98:53–61
- Stoilova D, Georgiev D, Marinova D. Infrared study of the vibrational behavior of guest ions matrix-isolated in metal (II) chromates (Me = Ca, Sr, Ba). Vibrat Spectros 2005;39:46–58
- Xie M, Gao L, Li L, et al. A new orally active antidiabetic vanadyl complex–bis(alpha-furancarboxylato)oxovanadium(IV). J Inorg Biochem 2005;99:546–51
- Nyquist RA. Interpreting infrared, raman, and nuclear magnetic resonance spectra. Orlando: Academic Press; 2001:2–15
- Freeman RA. Handbook of nuclear magnetic resonance, 2nd ed. Longman Publishing, 1997
- Raman N, Raja JD, Sakthivel A. Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition metal complexes derived from a tridentate Schiff base. Russ J Coord Chem 2008;34:400–9
- Raman N, Mitub L, Sakthivel A, Pandi MSS. Studies on DNA cleavage and antimicrobial screening of transition metal complexes of 4-aminoantipyrine derivatives of N2O2 type. J Iran Chem Soc 2009;6:738–48
- Hanif M, Chohan ZH. Design, spectral characterization and biological studies of transition metal(II) complexes with triazole schiff bases. Spectrochim Acta Part A: Mol Biomol Spectr 2013;104:468–76
- Sumrra SH, Chohan ZH. In vitro antibacterial, antifungal and cytotoxic activities of some triazole schiff bases and their vanadyl(IV) complexes. J Enz Inhib Med Chem 2013;28:1291–9