Abstract
In this study, we have synthesized 2-[3- or 4-(2-aryl-2-oxoethoxy)arylidene]benzofuran-3-one derivatives (D1–D38) and evaluated their anti-cancer activities. The final compounds were obtained in multistep synthesis reactions using benzofuranon-3-one derivatives (A1–A4, B) as starting materials which were gained in various synthetic ways. Aurone derivatives (C1–C10) were acquired with the condensation reaction of these starting materials and 3-/4-hydroxybenzaldehyde which were then reacted with α-bromoacetophenones to get final compounds. The anti-cancer activity of the selected compounds was performed by National Cancer Institute (NCI), USA against 60 human tumor cell lines derived from nine neoplastic diseases. Compounds exhibited anti-cancer activity in varying ratios.
Introduction
Flavonoids are secondary metabolites of plants which exhibit various biological properties that are beneficial for human health via interacting with some cellular targets in the body. They can be classified into several subclasses and some of these subclasses are flavonols, flavones, isoflavones, flavanones, flavans, aurones and chalconesCitation1,Citation2. All classes of flavonoids exhibit variety of biological and pharmacological activities, but among them, the chalcones () and their ring analogue flavones () have been considerably explored. Various natural, semi-synthetic and synthetic derivatives of these structures have been investigated and reported with a wide range of biological applicationsCitation3,Citation4.
Aurones, (Z)-2-benzylidenebenzofuran-3-(2H)-ones (), constitute a less studied subclass of flavonoids although they are isosteres of flavonesCitation5,Citation6. However, they have attracted attention with promising biological potential such as anti-microbialCitation7, anti-cancerCitation8, anti-leishmanialCitation9–11, anti-histaminicCitation12, anti-inflammatoryCitation13, antioxidantCitation14, insect anti-feedantCitation15, herbicidalCitation16, anti-HIVCitation17,Citation18, anti-HCV (hepatis C virus)Citation19,Citation20, anti-malarialCitation21,Citation22, ChE inhibitoryCitation23,Citation24, MAO inhibitoryCitation25 activities in the specified studies. The observed biological potential is thought to be due to the similarity of adenine of ATP and the flavonoids, consequently inhibition of the activity of ATP-dependent enzymes and proteins, which is essential for the function of enzymes and receptors. In particular, the aurones are estimated to mimic better the adenine because of the benzofuranone structure than the benzopyranone part of the corresponding flavonesCitation26. Some naturally occurring aurones maritimein, sulfuretin and aureusidin are also studied and among them sulfuretin () has been known to have anti-inflammatory activityCitation27,Citation28. In recent years, flavonoids have been developed as anti-cancer agents and have interested medicinal chemists. In fact, some flavonoids have entered clinical trials such as flavopiridol which was identified as the first cyclindependent kinase inhibitor and entered phase II clinical trialsCitation29,Citation30. Among them, aurones have been first reported in 2003 with high anti-cancer activity and they were determined to exhibite higher cytotoxic property than corresponding chalcone and flavon derivatives at same concentrationsCitation31. In subsequent years, many studies were performed about the anti-cancer activity of the auronesCitation32–39. Along with the identified molecular mechanisms of flavonoids as carcinogen inactivation, anti-proliferation, cell cycle arrest, induction of apoptosis and differentiation, inhibition of angiogenesis, antioxidation and reversal of multidrug resistance; α,β-unsaturated carbonyl structure has been evaluated inducing anti-cancer activity with acting as an alkylating agentCitation40–42.
Considering literature findings and as a continuation of our on-going studiesCitation43, we synthesized new aurone derivatives and evaluated their anti-cancer activity.
Experimental section
Chemistry
All reagents and solvents were obtained from Sigma-Aldrich Chemical Co (Sigma-Aldrich Corp., St. Louis, MO) and Merck KGaA (Darmstadt, Germany). Melting points were determined using a Electrothermal 9100 digital melting point apparatus (Essex, UK) and are uncorrected. All reactions were monitored by thin layer chromatography (TLC) using Silica Gel 60 F254 TLC plates (Merck KGaA, Darmstadt, Germany). Petroleum ether and ethyl acetate were used as mobile phase prepared in various ratios for TLC. Spectroscopic data were recorded as follows: IR spectra were IR Shimadzu 8400S FT-IR spectrometer (Tokyo, Japan), 1H-NMR spectra on a Bruker 500 MHz spectrometer (Billerica, MA) in DMSO-d6 with TMS as internal standard and mass spectra using a Agilent 110 MSD spectrometer (Santa Clara, CA) instruments.
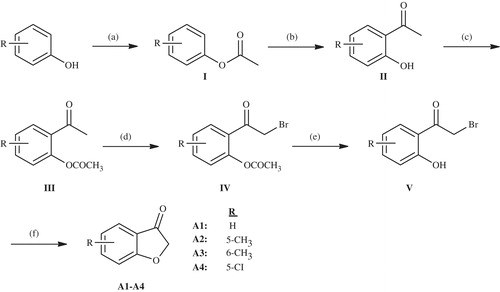
Benzofuran-3-one derivatives (A1–A4)
Phenole derivative (0.5 mol) (R: H, 5-methyl, 5-chloro, 6-methyl, 6-chloro) was first refluxed with acetic anhydride (0.6 mol) in pyridine for 30 min and then the reaction mixture was treated with water after cooling, the precipitated material was filtered or not solidified material was extracted with appropriate solvents. The obtained phenyl acetate derivative (I) (0.4 mol) was reacted with AlCl3 (0.48 mol) in a flask placed in an oil bath kept under control at 160–170 °C. After exiting oil bath, viscous mixture was poured into ice water and dried up to get 2′-hydroxyacetophenone derivatives (II). Compound II (0.3 mol) was stirred with acetic anhydride (0.45 mol) at 50–100 °C for 3–4 h which was acetylated in order to prevent reaction ability of hydroxyl group. The obtained 2′-acetyloxyacetophenone derivatives (III) (0.25 mol) compound was brominated with Br2 (0.3 mol) in ether or acetic acid to get 2′-acetyloxy-2-bromoacetophenone derivatives (IV) which was then hydrolized with 10% HCl acid (300 ml) to afford 2′-hydroxy-2-bromoacetophenone (V). Finally, compounds A1, A2, A3 and A4 (0.2 mol) were achieved from compound V and sodium acetate (0.6 mol) at reflux conditions in ethanol by a cyclization reaction. The intermediate products were gained by filtration from reaction medium which were then crystallized from petroleum ether. The obtained compounds and melting points indicated in the literature is given below.
Benzofuran-3-one (A1): 101–103 °CCitation44. 5-Methlbenzofuran-3-one (A2): 83–85 °CCitation45.
5-Chlorobenzofuran-3-one (A3): 87–89 °CCitation45. 6-Metilbenzofuran-3-one (A4): 83–85 °CCitation45.
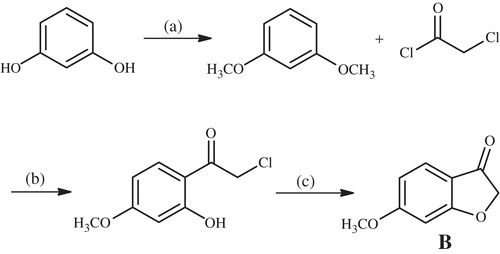
6-Methoxybenzofuran-3-one (B)
Resorcinol was refluxed with dimethylsulfate and sodium hydroxide in acetone to give 1,3-dimethoxybenzene using standard procedures. The obtained compound was then reacted with chloroacetyl chloride and anhydrous aluminium chloride in carbon disulfide by Friedel-Crafts acetylation. The obtained 2′-hydroxy-4-methoxy-2-chloroacetophenone was reacted with sodium acetate in ethanol to give 6-methoxybenzofuran-3-one (B) m.p.120–122 °CCitation46.
2-(3- or 4-Hydroxybenzylidene)benzofuran-3-one derivatives (C1–C10)
Benzofuranone derivative (20 mmol) (A1–A4, B) was refluxed with 3-/4-hydroxybenzaldehyde derivatives (22 mmol) in 70 mL n-butanol or isobutanol for 1 h using hydrochloric acid as a catalyst (1 ml). The precipate formed upon cooling was filtered and crytallized. The chemical properties of the obtained ten derivatives (C1–C10) were given in .
Table 1. Some characteristics of the compounds (C1–C10).
General procedure of the synthesis 2-[3- or 4-(2-aryl-2-oxoethoxy)arylidene]benzofuran-3-one derivatives (D1–D38)
2-(3- or 4-Hydroxybenzylidene)benzofuran-3-one derivatives (C1–C10) (3 mmol) were refluxed with brominated acetophenones (3 mmol) for 6 h in acetone with the presence of potassium carbonate (0.03 mmol). After TLC, the reaction solvent was evaporated and the obtained residue was washed with water and crystallized from ethanol to achieve 2-[3- or 4 -(2-Aryl-2-oxoethoxy)arylidene]benzofuran-3-one compounds (D1–D38). The chemical properties of the obtained 10 derivatives (D1–D38) were given in .
Table 2. Some characteristics of the compounds (D1-D38).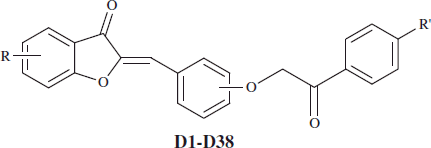 .
.
2-[3-(2-Phenyl-2-oxoethoxy)benzylidene]benzofuran-3-one (D1).
IR (KBr) νmax (cm−1): 3098, 3046 (Aromatic C–H), 2975, 2945, 2844 (Aliphatic C–H), 1704, 1669 (C = O), 1602–1477 (C = C), 1288, 1217 (C–O). 1H-NMR δ (ppm): 5.69 (2H, s, OCH2), 6.93 (1H, s, = CH−), 7.11 (1H, dd, J: 1.48 Hz, J: 8.25 Hz, Ar-H), 7.29–7.33 (2H, m, Ar-H), 7.42–7.45 (2H, m, Ar-H), 7.58–7.66 (4H, m, Ar-H), 7.72–7.83 (2H, m, Ar-H), 8.08 (2H, d, J: 8.10 Hz, Ar-H). MS [M + 1]+: m/z 357.1.
2-[3-[2-(4-Methylphenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D2)
IR (KBr) νmax (cm−1): 3074, 3035 (Aromatic C–H), 2975, 2834 (Aliphatic C–H), 1699, 1669 (C = O), 1598–1475 (C = C), 1275, 1234 (C–O). 1H-NMR δ (ppm): 2.45 (3H, s, CH3), 5.66 (2H, s, OCH2), 6.95 (1H, s, = CH−), 7.17 (1H, dd, J: 1.34 Hz, J: 8.22 Hz, Ar-H), 7.29–7.36 (2H, m, Ar-H), 7.43 (2H, d, J: 8.35 Hz, Ar-H), 7.47 (1H, s, Ar-H), 7.60–7.68 (2H, m, Ar-H), 7.78–7.83 (2H, m, Ar-H), 8.00 (2H, d, J: 8.12 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[3-[2-(4-Methoxyphenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D3)
IR (KBr) νmax (cm−1): 3077, 3039 (Aromatic C–H), 2960, 2862 (Aliphatic C–H), 1705, 1681 (C = O), 1647–1464 (C = C), 1271, 1224 (C–O). 1H-NMR δ (ppm): 3.90 (3H, s, OCH3), 5.63 (2H, s, OCH2), 6.95 (1H, s, = CH−), 7.10 (1H, dd, J: 1.32 Hz, J: 8.41 Hz, Ar-H), 7.12 (2H, d, J: 7.05 Hz, Ar-H), 7.34 (2H, d, J: 7.30 Hz, Ar-H), 7. 55 (1H, t, J: 8.20 Hz, Ar-H), 7.61–7.63 (2H, m, Ar-H), 7.77–7.83 (2H, m, Ar-H), 8.07 (2H, d, J: 7.0 Hz, Ar-H). MS [M + 1]+: m/z 387.
2-[3-[2-(4-Chlorophenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D4)
IR (KBr) νmax (cm−1): 3088, 3036 (Ar-H ve = CH−), 2965, 2844 (Alifatik–H), 1706, 1665 (C = O), 1602–1477 (C = C), 1288,1217 (C–O). 1H-NMR δ (ppm): 5.79 (2H, s, OCH2), 6.94 (1H, s, = CH−), 7.14 (1H, dd, J: 1.34 Hz, J: 8.22 Hz, Ar-H), 7.31–7.36 (2H, m, Ar-H), 7.58–7.63 (1H, m, Ar-H), 7.77–7.83 (2H, m, Ar-H), 7.72 (2H, d, J: 8.59 Hz, Ar-H), 7.83 (2H, d, J: 7.46 Hz, Ar-H), 8.10 (2H, d, J: 8.59 Hz, Ar-H). MS [M + 1]+: m/z 390.5.
2-[3-[2-(4-Nitrophenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D5)
IR (KBr) νmax (cm−1): 3078, 3056 (Aromatic C–H), 2970, 2921, 2854 (Aliphatic C–H), 1697, 1649 (C = O), 1601–1478 (C = C, N = O), 1288,1217 (C–O). 1H-NMR δ (ppm): 5.75 (2H, s, OCH2), 6.94 (1H, s, = CH), 7.14 (1H, dd, J: 1.43 Hz, J: 8.26 Hz, Ar-H), 7. 34 (1H, t, J: 7.50 Hz, Ar-H), 7.44–7.48 (2H, m, Ar-H), 7.63–7.67 (2H, m, Ar-H), 7.81 (2H, d, J: 7.52 Hz, Ar-H), 8.29 (2H, d, J: 8.73 Hz, Ar-H), 8.42 (2H, d, J: 8.68 Hz, Ar-H). MS [M + 1]+: m/z 402.
2-[4-(2-Phenyl-2-oxoethoxy)benzylidene]benzofuran-3-one (D6)
IR (KBr) νmax (cm−1): 3049, 3035 (Aromatic C–H), 2922, 2895 (Aliphatic C–H), 1709, 1693 (C = O), 1650–1479 (C = C), 1261,1230 (C–O). 1H-NMR δ (ppm): 5.64 (2H, s, OCH2), 6.99 (1H, s, = CH−), 7.21 (2H, d, J: 8.70 Hz, Ar-H), 7. 34 (1H, t, J: 7.50 Hz, Ar-H), 7.58 (2H, d, J: 8.84 Hz, Ar-H), 7.63 (2H, d, J: 7.65 Hz, Ar-H), 7.62–7.84 (2H, m, Ar-H), 8.0 (2H, d, J: 8.69 Hz, Ar-H), 8.07 (2H, d, J: 7.66 Hz, Ar-H). MS [M + 1]+: m/z 357.2.
2-[4-[2-(4-Methylphenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D7)
IR (KBr) νmax (cm−1): 3084, 3025 (Aromatic C–H), 2972, 2832 (Aliphatic C–H), 1712, 1669 (C = O), 1622–1475 (C = C), 1275,1234 (C–O). 1H-NMR δ (ppm): 2.43 (3H, s, Ar–CH3), 5.68 (2H, s, O–CH2), 6.98 (1H, s, = CH−), 7.14 (2H, d, J: 8.60 Hz, Ar-H), 7. 34 (1H, t, 7.43 Hz, Ar-H), 7.42 (2H, d, J: 7.93 Hz, Ar-H), 7.53 (1H, d, J: 8.61 Hz, Ar-H), 7.81–7.84 (2H, m, Ar-H), 7.97 (2H, d, J: 8.04 Hz, Ar-H), 8.0 (2H, d, J: 8.72 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[4-[2-(4-Methoxyphenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D8)
IR (KBr) νmax (cm−1): 3070, 3032 (Aromatic C–H), 2965, 2844 (Aliphatic C–H), 1694, 1659 (C = O), 1598–1475 (C = C), 1275,1224 (C–O). 1H-NMR δ (ppm): 3.89 (3H, s, OCH3), 5.65 (2H, s, OCH2), 6.98 (1H, s, = CH−), 7.12 (2H, d, J: 7.77 Hz, Ar-H), 7.14 (2H, d, J: 7.78 Hz, Ar-H), 7.14 (1H, t, 7.40 Hz, Ar-H), 7.58 (1H, d, J: 8.58 Hz, Ar-H), 7.80–7.84 (2H, m, Ar-H), 7.77 (2H, d, J: 8.82 Hz, Ar-H), 8.0 (2H, d, J: 8.83 Hz, Ar-H). MS [M + 1]+: m/z 387.
2-[4-[2-(4-Chlorophenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D9)
IR (KBr) νmax (cm−1): 3085, 3020 (Aromatic C–H), 2975, 2832 (Aliphatic C–H), 1710, 1670 (C = O), 1622–1475 (C = C), 1271,1234 (C–O). 1H-NMR δ (ppm): 5.66 (2H, s, OCH2), 6.96 (1H, s, = CH−), 7.12 (2H, d, J: 7.90 Hz, Ar-H), 7. 14 (1H, t, 7.40 Hz, Ar-H), 7.58–7.62 (1H, m, Ar-H), 7.66 (2H, d, J: 8.59 Hz, Ar-H), 7.80–7.84 (2H, m, Ar-H), 7.94 (2H, d, J: 7.98 Hz, Ar-H), 8.10 (2H, d, J: 8.59 Hz, Ar-H). MS [M + 1]+: m/z 390.5.
2-[4-[2-(4-Nitrophenyl)-2-oxoethoxy]benzylidene] benzofuran-3-one (D10)
IR (KBr) νmax (cm−1): 3074, 3035 (Aromatic C–H), 2975, 2834 (Aliphatic C–H), 1699, 1669 (C = O), 1598–1475 (C = C, N = O), 1275,1234 (C–O). 1H-NMR δ (ppm): 5.76 (2H, s, OCH2), 6.94 (1H, s, = CH–), 7.12 (2H, d, J: 7.90 Hz, Ar-H), 7. 32–7.34 (1H, m, Ar-H), 7.44–7.48 (2H, m, Ar-H), 7.63–7.67 (1H, m, Ar-H), 7.80–7.84 (2H, m, Ar-H), 8.31 (2H, d, J: 8.82 Hz, Ar-H), 8.43 (2H, d, J: 8.84 Hz, Ar-H). MS [M + 1]+: m/z 402.
2-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-5-methylbenzofuran-3-one (D11)
IR (KBr) νmax (cm−1): 3088, 3056 (Aromatic C–H), 2975, 2921, 2854 (Aliphatic C–H), 1702, 1664 (C = O), 1601–1477 (C = C), 1288,1217 (C–O). 1H-NMR δ (ppm): 2.35 (3H, s, CH3), 5.68 (2H, s, OCH2), 6.87 (1H, s, = CH−), 7.09 (1H, dd, J: 2.50 Hz, J: 8.23 Hz, Ar-H), 7.14 (1H, d, J: 8.91 Hz, Ar-H), 7.42 (2H, t, J: 8.0 Hz, Ar-H), 7.55–7.63 (5H, m, Ar-H), 7.74 (1H, t, J: 7.4 Hz, Ar-H), 8.08 (2H, d, J: 7.24 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-5-methylbenzofuran-3-one (D12)
IR (KBr) νmax (cm−1): 3053, 3012 (Aromatic C–H), 2918, 2841 (Aliphatic C–H), 1685, 1655 (C = O), 1601–1487 (C = C), 1275,1238 (C–O). 1H-NMR δ (ppm): 2.36 (3H, s, CH3), 3.88 (3H, s, OCH3), 5.58 (2H, s, OCH2), 6.86 (1H, s, = CH−), 7.08 (1H, m, Ar-H), 7.11 (2H, d, J: 8.91 Hz, Ar-H), 7.16 (2H, d, J: 8.0 Hz, Ar-H), 7.41 (1H, t, J: 8.10 Hz, Ar-H), 7.54–7.56 (3H, m, Ar-H), 8.08 (2H, d, J: 7.24 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-5- methylbenzofuran-3-one (D13)
IR (KBr) νmax (cm−1): 3077, 3029 (Aromatic C–H), 2987, 2860 (Aliphatic C–H), 1702, 1681 (C = O), 1621–1476 (C = C), 1274, 1223 (C–O). 1H-NMR δ (ppm): 5.66 (2H, s, OCH2), 6.86 (1H, s, = CH−), 7.10 (1H, dd, J: 2.30 Hz, J: 8.12 Hz, Ar-H), 7.22 (1H, d, J: 8.86 Hz, Ar-H), 7.42 (1H, t, J: 7.40 Hz, Ar-H), 7.54–7.62 (4H, m, Ar-H), 7.68 (2H, d, J: 8.54 Hz, Ar-H), 8.08 (2H, d, J: 8.54 Hz, Ar-H). MS [M + 1]+: m/z 404.5.
2-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-5-methylbenzofuran-3-one (D14)
IR (KBr) νmax (cm−1): 3079, 3057 (Aromatic C–H), 2981, 2932, 2867 (Aliphatic C–H), 1703, 1666 (C = O), 1601–1477 (C = C), 1288, 1217 (C–O). 1H-NMR δ (ppm): 2.34 (3H, s, CH3), 5.70 (2H, s, OCH2), 6.89 (1H, s, = CH−), 7.04 (2H, d, J: 8.15 Hz, Ar-H), 7.44–7.46 (3H, m, Ar-H), 7.48 (1H, s, Ar-H), 7.57–7.65 (2H, m, Ar-H), 7.72 (2H, d, J: 8.20 Hz, Ar-H), 8.05 (2H, d, J: 7.40 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[4-(2 -(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-5- methylbenzofuran-3-one (D15)
IR (KBr) νmax (cm−1): 3058, 3010 (Aromatic C–H), 2915, 2853 (Aliphatic C–H), 1687, 1656 (C = O), 1610–1482 (C = C), 1258,1224 (C–O). 1H-NMR δ (ppm): 2.35 (3H, s, CH3), 3.91 (3H, s, OCH3), 5.59 (2H, s, OCH2), 6.87 (1H, s, = CH−), 7.10–7.16 (3H, m, Ar-H), 7.24 (2H, d, J: 8.50 Hz, Ar-H), 7.48 (2H, d, J: 8.0 Hz, Ar-H), 7.58–7.62 (2H, m, Ar-H), 8.09 (2H, d, J: 7.32 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-5- methylbenzofuran-3-one (D16)
IR (KBr) νmax (cm−1): 3063, 3025 (Aromatic C–H), 2981, 2864 (Aliphatic C–H), 1704, 1685 (C = O), 1642–1481 (C = C), 1256, 1258 (C–O). 1H-NMR δ (ppm): 5.68 (2H, s, OCH2), 6.88 (1H, s, = CH−), 7.11–7.17 (3H, m, Ar-H), 7.34 (2H, d, J: 8.64 Hz, Ar-H), 7.48 (2H, d, J: 7.54 Hz, Ar-H), 7.63–7.67 (2H, m, Ar-H), 8.08 (2H, d, J: 8.32 Hz, Ar-H). MS [M + 1]+: m/z 404.5.
2-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-6-methylbenzofuran-3-one (D17)
IR (KBr) νmax (cm−1): 3052, 3037 (Aromatic C–H), 2944, 2876 (Aliphatic C–H), 1696, 1667 (C = O), 1587–1469 (C = C), 1288, 1224 (C–O). 1H-NMR δ (ppm): 2.44 (3H, s, CH3), 5.67 (2H, s, OCH2), 6.88 (1H, s, = CH–), 7.12–7.15 (3H, m, Ar-H), 7.34 (2H, d, J: 7.85 Hz, Ar-H), 7.68 (2H, d, J: 7.88 Hz, Ar-H), 7.93–7.95 (3H, m, Ar-H), 8.08 (2H, d, J: 8.74 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6- methylbenzofuran-3-one (D18)
IR (KBr) νmax (cm−1): 3084, 3051 (Aromatic C–H), 2975, 2827 (Aliphatic C–H), 1694, 1661 (C = O), 1618–1478 (C = C), 1292,1219 (C–O). 1H-NMR δ (ppm): 2.44 (3H, s, CH3), 3.88 (3H, s, OCH3), 5.65 (2H, s, OCH2), 6.89 (1H, s, = CH−), 6.91 (1H, d, J: 8.42 Hz, Ar-H), 7.13–7.17 (3H, m, Ar-H), 7.35 (1H, s, Ar-H), 7.62–7.65 (2H, m, Ar-H), 7.75 (1H, t, J: 7.48 Hz, Ar-H), 7.94 (1H, d, J: 8.84 Hz, Ar-H), 8.07 (2H, d, J: 7.29 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6- methylbenzofuran-3-one (D19)
IR (KBr) νmax (cm−1): 3077, 3029 (Aromatic C–H), 2987, 2860 (Aliphatic C–H), 1705, 1681 (C = O), 1621–1476 (C = C), 1271, 1223 (C–O). 1H-NMR δ (ppm): 2.46 (3H, s, CH3), 5.68 (2H, s, OCH2), 6.89 (1H, s, = CH−), 7.12–7.14 (3H, d, J: 8.85 Hz, Ar-H), 7.36 (1H, s, Ar-H), 7.65–7.80 (3H, m, Ar-H), 7.94 (2H, d, J: 8.74 Hz, Ar-H), 8.05 (2H, d, J: 8.47 Hz, Ar-H). MS [M + 1]+: m/z 404.5.
2-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-6-methylbenzofuran-3-one (D20)
IR (KBr) νmax (cm−1): 3066, 3032 (Aromatic C–H), 2975, 2844 (Aliphatic C–H), 1698, 1659 (C = O), 1598–1475 (C = C), 1285, 1234 (C–O). 1H-NMR δ (ppm): 2.43 (3H, s, CH3), 5.69 (2H, s, OCH2), 6.86 (1H, s, = CH−), 7.09–7.13 (5H, m, Ar-H), 7.31 (1H, s, Ar-H), 7.65 (2H, d, J: 7.81 Hz, Ar-H), 7.93 (2H, d, J: 7.93 Hz, Ar-H), 8.02 (2H, d, J: 8.88 Hz, Ar-H). MS [M + 1]+: m/z 371.
2-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6- methylbenzofuran-3-one (D21)
IR (KBr) νmax (cm−1): 3098, 3045 (Aromatic C–H), 2976, 2834 (Aliphatic C–H), 1698, 1659 (C = O), 1611–1477 (C = C), 1288,1217 (C–O). 1H-NMR δ (ppm): 2.45 (3H, s, CH3), 3.87 (3H, s, OCH3), 5.64 (2H, s, OCH2), 6.88 (1H, s, = CH−), 7.03 (1H, d, J: 8.02 Hz, Ar-H), 7.12 (2H, d, J: 8.85 Hz, Ar-H), 7.31 (1H, s, Ar-H), 7.57–7.60 (2H, m, Ar-H), 7.71 (1H, t, J: 7.45 Hz, Ar-H), 7.92 (2H, d, J: 8.84 Hz, Ar-H), 8.08 (2H, d, J: 7.28 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6-methylbenzofuran-3-one (D22)
IR (KBr) νmax (cm−1): 3077, 3029 (Aromatic C–H), 2987, 2860 (Aliphatic C–H), 1705, 1681 (C = O), 1621–1476 (C = C), 1271, 1223 (C–O). 1H-NMR δ (ppm): 2.46 (3H, s, Ar–CH3), 5.68 (2H, s, OCH2), 6.89 (1H, s, = CH–), 7.12–7.14 (3H, d, J: 8.85 Hz, Ar-H), 7.36 (1H, s, Ar-H), 7.65–7.80 (3H, m, Ar-H), 7.94 (2H, d, J: 8.74 Hz, Ar-H), 8.05 (2H, d, J: 8.47 Hz, Ar-H). MS [M + 1]+: m/z 404.5.
2-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-6-methoxybenzofuran-3-one (D23)
(KBr) νmax (cm−1): 3074, 3056 (Aromatic C–H), 2968, 2845 (Aliphatic C–H), 1696, 1661 (C = O), 1605–1475 (C = C), 1269,1224 (C–O). 1H-NMR δ (ppm): 3.90 (3H, s, OCH3), 5.66 (2H, s, OCH2), 6.80 (1H, s, = CH−), 6.85–6.89 (2H, m, Ar-H), 7.07–7.15 (2H, m, Ar-H), 7.35 (1H, t, J: 7.74 Hz, Ar-H), 7.42 (2H, d, J: 8.05 Hz, Ar-H), 7.58–7.64 (2H, m, Ar-H), 7.67–7.71 (1H, m, Ar-H), 8.05 (2H, d, J: 8.04 Hz, Ar-H). MS [M + 1]+: m/z 387.
2-[3-(2-(4-Methylphenyl)-2-oxoethoxy)benzylidene]-6-methoxybenzofuran-3-one (D24)
(KBr) νmax (cm−1): 3085, 3034 (Aromatic C–H), 2967, 2879 (Aliphatic C–H), 1701, 1669 (C = O), 1597–1473 (C = C), 1267, 1234 (C–O). 1H-NMR δ (ppm): 2.47 (3H, s, CH3), 3.90 (3H, s, OCH3), 5.77 (2H, s, OCH2), 6.84 (1H, s, = CH−), 6.88 (1H, d, J:7.85 Hz, Ar-H), 6.95–7.02 (2H, m, Ar-H), 7.21 (2H, d, J: 7.86 Hz, Ar-H), 7.48–7.52 (2H, m, Ar-H), 7.91 (2H, d, J: 8.14 Hz, Ar-H), 8.15 (2H, d, J: 8.69 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D25)
(KBr) νmax (cm−1): 3095, 3030 (Aromatic C–H), 2961, 2857 (Aliphatic C–H), 1702, 1668 (C = O), 1585–1484 (C = C), 1274,1232 (C–O). 1H-NMR δ (ppm): 3.89 (3H, s, OCH3), 5.76 (2H, s, OCH2), 6.83 (1H, s, = CH−), 6.86 (1H, dd, J: 2.02 ve 1.95 Hz, J: 8.55 Hz, Ar-H), 6.77–6.98 (1H, m, Ar-H), 7.15–7.21 (3H, m, Ar-H), 7.45 (1H, t, J: 8.05 ve 8.24 Hz, Ar-H), 7.59–7.61 (2H, m, Ar-H), 7.88–8.01 (2H, m, Ar-H), 8.22 (2H, d, J: 8.68 Hz, Ar-H). MS [M + 1]+: m/z 417.1.
2-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D26)
(KBr) νmax (cm−1): 3074, 3038 (Aromatic C–H), 2954, 2887 (Aliphatic C–H), 1702, 1667 (C = O), 1585–1454 (C = C), 1268, 1252 (C–O). 1H–NMR δ (ppm): 3.91 (3H, s, OCH3), 5.78 (2H, s, OCH2), 6.85 (1H, s, = CH−), 6.94–7.05 (2H, m, Ar-H), 7.12–7.18 (3H, m, Ar-H), 7.45 (2H, d, J: 7.85 Hz, Ar-H), 7.88 (2H, d, J: 8.22 Hz, Ar-H), 8.13 (2H, d, J: 8.53 Hz, Ar-H). MS [M + 1]+: m/z 420.6.
2-[3-(2-(4-Nitrophenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D27)
(KBr) νmax (cm−1): 3088, 3055 (Aromatic C–H), 2972, 2831 (Aliphatic C–H), 1697, 1659 (C = O), 1612–1477 (C = C, N = O), 1288,1217 (C–O). 1H-NMR δ (ppm): 3.89 (3H, s, OCH3), 5.64 (2H, s, OCH2), 6.81 (1H, s, = CH−), 6.85–6.89 (2H, d, J: 8.85 Hz, Ar-H), 7.07 (1H, dd, J: 1.37 Hz, J: 8.23 Hz, Ar-H), 7.32 (1H, t, J: 7.67, Hz, Ar-H), 7.38–7.43 (2H, m, Ar-H), 7.52–7.53 (2H, m, Ar-H), 7.67–7.71 (1H, m, Ar-H), 7.98 (2H, d, J: 8.03 Hz, Ar-H). MS [M + 1]+: m/z 432.1.
2-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D28)
(KBr) νmax (cm−1): 3048, 3063 (Aromatic C–H), 2948, 2838 (Aliphatic C–H), 1697, 1663 (C = O), 1606–1454 (C = C), 1252,1228 (C–O). 1H-NMR δ (ppm): 3.91 (3H, s, OCH3), 5.67 (2H, s, OCH2), 6.81 (1H, s, = CH−), 6.94–7.02 (3H, m, Ar-H), 7.35 (2H, d, J: 7.78 Hz, Ar-H), 7.51 (2H, d, J: 8.05 Hz, Ar-H), 7.62–7.67 (2H, m, Ar-H), 7.67 (1H, s, Ar-H), 8.08 (2H, d, J: 8.06 Hz, Ar-H). MS [M + 1]+: m/z 387.
2-[4-(2 -(4-Methylphenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D29)
(KBr) νmax (cm−1): 3072, 3045 (Aromatic C–H), 2977, 2885 (Aliphatic C–H), 1702, 1668 (C = O), 1585–1456 (C = C), 1255, 1241 (C–O). 1H-NMR δ (ppm): 2.48 (3H, s, CH3), 3.91 (3H, s, OCH3), 5.76 (2H, s, OCH2), 6.85 (1H, s, = CH−), 6.98–7.05 (2H, m, Ar-H), 7.24 (2H, d, J: 7.86 Hz, Ar-H), 7.49–7.53 (3H, m, Ar-H), 7.94 (2H, d, J: 8.24 Hz, Ar-H), 8.16 (2H, d, J: 8.28 Hz, Ar-H). MS [M + 1]+: m/z 401.
2-[4-(2 -(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D30)
(KBr) νmax (cm−1): 3098, 3032 (Aromatic C–H), 2965, 2844 (Aliphatic C–H), 1704, 1665 (C = O), 1598–1475 (C = C), 1285,1227 (C–O). 1H-NMR δ (ppm): 3.91 (3H, s, OCH3), 5.75 (2H, s, OCH2), 6.82 (1H, s, = CH−), 6.86 (1H, dd, J: 2.02, J: 8.55 Hz, Ar-H), 6.95–6.97 (1H, m, Ar-H), 7.11–7.14 (1H, m, Ar-H), 7.43 (1H, t, J: 8.05 ve 8.24 Hz, Ar-H), 7.59–7.61 (2H, m, Ar-H), 7.69 (1H, d, J: 8.56, Hz, Ar-H), 8.30 (2H, d, J: 8.73 Hz, Ar-H), 8.41 (2H, d, J: 8.68 Hz, Ar-H). MS [M + 1]+: m/z 417.1.
2-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6- methoxybenzofuran-3-one (D31)
(KBr) νmax (cm−1): 3041, 3027 (Aromatic C–H), 2932, 2873 (Aliphatic C–H), 1701, 1668 (C = O), 1580–1451 (C = C), 1253, 1242 (C–O). 1H-NMR δ (ppm): 3.90 (3H, s, OCH3), 5.77 (2H, s, OCH2), 6.86 (1H, s, = CH−), 6.94 (1H, d, J:7.85 Hz, Ar-H), 7.14 (1H, d, J:7.89 Hz, Ar-H), 7.19–7.24 (2H, m, Ar-H), 7.45 (2H, d, J: 7.85 Hz, Ar-H), 7.54 (1H, d, J: 8.02 Hz, Ar-H), 7.88 (2H, d, J: 8.22 Hz, Ar-H), 8.13 (2H, d, J: 8.53 Hz, Ar-H). MS [M + 1]+: m/z 420.6.
2-[4-(2-(4-Nitrophenyl)-2-oxoethoxy)benzylidene]-6-methoxybenzofuran-3-one (D32)
(KBr) νmax (cm−1): 3084, 3057 (Aromatic C–H), 2952, 2868 (Aliphatic C–H), 1698, 1658 (C = O), 1603–1474 (C = C, N = O), 1275,1226 (C–O). 1H-NMR δ (ppm): 3.89 (3H, s, OCH3), 5.64 (2H, s, OCH2), 6.81 (1H, s, = CH−), 7.10–7.16 (2H, m, Ar-H), 7.38 (2H, d, J: 8.23 Hz, Ar-H), 7.77 (2H, d, J: 7.68, Ar-H), 7.85–7.89 (2H, m, Ar-H), 7.94–7.96 (1H, m, Ar-H), 8.31 (1H, d, J: 8.03 Hz, Ar-H). MS [M + 1]+: m/z 432.1.
2-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D33)
IR (KBr) νmax (cm−1): 3071, 3063 (Aromatic C–H), 2982, 2943, 2832 (Aliphatic C–H), 1701, 1665 (C = O), 1605–1458 (C = C), 1254,1285 (C–O). 1H-NMR δ (ppm): 5.67 (2H, s, OCH2), 6.88 (1H, s, = CH−), 7.14 (1H, d, J: 8.18 Hz, Ar-H), 7.29 (1H, d, J: 8.75 Hz, Ar-H), 7.46 (2H, t, J: 8.10 Hz, Ar-H), 7.58–7.65 (3H, m, Ar-H), 7.74 (1H, t, J: 7.40 Hz, Ar-H), 7.89 (2H, d, J: 8.15 Hz, Ar-H), 8.12 (2H, d, J: 7.59 Hz, Ar-H). MS [M + 1]+: m/z 390.5.
2-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D34)
IR (KBr) νmax (cm−1): 3072, 3035 (Aromatic C–H), 2953, 2839 (Aliphatic C–H), 1686, 1657 (C = O), 1623–1475 (C = C), 1284,1242 (C–O). 1H-NMR δ (ppm): 3.93 (3H, s, OCH3), 5.62 (2H, s, OCH2), 6.90 (1H, s, = CH−), 7.14 (2H, d, J: 8.42 Hz, Ar-H), 7.16 (2H, d, J: 8.51 Hz, Ar-H), 7.35 (2H, d, J: 8.19 Hz, Ar-H), 7.45 (1H, t, J: 8.24 Hz, Ar-H), 7.58–7.62 (2H, m, Ar-H), 8.14 (2H, d, J: 7.18 Hz, Ar-H). MS [M + 1]+: m/z 420.5.
2-[3-(2 -(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D35)
IR (KBr) νmax (cm−1): 3039, 3028 (Aromatic C–H), 2976, 2884 (Aliphatic C–H), 1701, 1683 (C = O), 1634–1482 (C = C), 1252, 1225 (C–O). 1H-NMR δ (ppm): 5.67 (2H, s, OCH2), 6.87 (1H, s, = CH−), 7.25 (1H, d, J: 8.14 Hz, Ar-H), 7.35 (2H, d, J: 8.74 Hz, Ar-H), 7.63 (1H, t, J: 7.46 Hz, Ar-H), 7.64–7.68 (3H, m, Ar-H), 7.76 (2H, d, J: 8.23 Hz, Ar-H), 8.24 (2H, d, J: 8.56 Hz, Ar-H). MS [M + 1]+: m/z 425.
2-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D36)
IR (KBr) νmax (cm−1): 3055, 3027 (Aromatic C–H), 2977, 2951, 2846 (Aliphatic C–H), 1702, 1667 (C = O), 1606–1463 (C = C), 1265, 1294 (C–O). 1H-NMR δ (ppm): 5.68 (2H, s, OCH2), 6.89 (1H, s, = CH−), 7.15 (1H, d, J: 8.20 Hz, Ar-H), 7.33 (1H, d, J: 8.84 Hz, Ar-H), 7.52 (2H, t, J: 8.24 Hz, Ar-H), 7.67–7.76 (3H, m, Ar-H), 7.86 (1H, t, J: 7.85 Hz, Ar-H), 7.94 (2H, d, J: 8.21 Hz, Ar-H), 8.11 (2H, d, J: 7.63 Hz, Ar-H). MS [M + 1]+: m/z 390.5.
2-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D37)
IR (KBr) νmax (cm−1): 3072, 3035 (Aromatic C–H), 2953, 2839 (Aliphatic C–H), 1686, 1657 (C = O), 1623–1475 (C = C), 1284,1242 (C–O). 1H–NMR δ (ppm): 3.93 (3H, s, OCH3), 5.62 (2H, s, OCH2), 6.90 (1H, s, = CH–), 7.14 (2H, d, J: 8.42 Hz, Ar-H), 7.16 (2H, d, J: 8.51 Hz, Ar-H), 7.35 (2H, d, J: 8.19 Hz, Ar-H), 7.45 (1H, t, J: 8.24 Hz, Ar-H), 7.58–7.62 (2H, m, Ar-H), 8.14 (2H, d, J: 7.18 Hz, Ar-H). MS [M + 1]+: m/z 420.5.
2-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-5-chlorobenzofuran-3-one (D38)
IR (KBr) νmax (cm−1): 3056, 3038 (Aromatic C–H), 2984, 2897 (Aliphatic C–H), 1702, 1685 (C = O), 1635–1474 (C = C), 1236, 1227 (C–O). 1H-NMR δ (ppm): 5.68 (2H, s, OCH2), 6.88 (1H, s, = CH−), 7.29 (2H, d, J: 8.20 Hz, Ar-H), 7.55–7.64 (3H, m, Ar-H), 7.75 (2H, d, J: 8.12 Hz, Ar-H), 7.86 (2H, d, J: 8.12 Hz, Ar-H), 8.18 (2H, d, J: 8.27 Hz, Ar-H). MS [M + 1]+: m/z 425.
Anti-cancer activity
The cytotoxic and/or growth inhibitory effects of the compounds were evaluated in vitro against approximately sixty human tumor cell lines derived from nine neoplastic diseases namely; leukemia (L, 4 or 6 cell lines), non-small cell lung cancer (NSCLC, 9 cell lines), colon cancer (CC, 7 cell lines), central nervous system cancer (CNSC, 6 cell lines), melanoma (M, 8 or 9 cell lines), ovarian cancer (OC, 6 or 7 cell lines), renal cancer (RC, 8 cell lines), prostate cancer (PC, 2 cell lines), breast cancer (BC, 6 or 8 cell lines). The evaluation of anti-cancer activity was performed at the National Cancer Institute (NCI) of Bethesda, USA, following the in vitro screening program at 10-fold dilutions of five concentrations ranging from 10−4 to 10−8 M. The percentage growth was evaluated spectrophotometrically versus controls not treated with test agents. A 48 h continuous drug exposure protocol was followed and a sulforhodamine B (SRB) protein assay was used to estimate cell viability of growth. Three dose response parameters (GI50, TGI and LC50) were calculated for each experimental agentCitation47,Citation48.
Results and discussion
Chemistry
New aurone derivatives (D1–D38) were synthesized by an extensive synthetic procedure. Synthetic way for the compounds was outlined inSchemes 1,2 and3. Aurone synthesis is usually carried out with condensation reaction of benzofuranones and benzaldehydes and cyclization of various 2′-hydroxy aryl compoundsCitation49. In this study, we also used condensation reaction and obtained 10 different aurone derivatives (C1–C10) from five benzofuranone derivatives (A1–A4, B) and final 38 compounds were gathered from these aurones derivatives. Benzofuranone derivatives (A1–A4, B) were synthesized in two different ways. Compounds A1–A4 were acquired at the end of an extended and laborious synthetic procedure (Scheme 1). Initially, 2′-hydroxyacetophenone derivative was obtained and used as starting material. For this, phenole derivative was primarily acetylated with acetic anhydride or acetyl chloride in pyridine, then the obtained acetyloxyphenol (I) derivative was heated at 160–170 °C in Fries conditions to get 2′-hydroxyacetophenone (II). This compound (II) was acetylated to protect phenolic hydroxyl group. Bromination of the obtained acetyloxyacetophenone (III) compound in acetic acid gave 2′-acetyloxy-2-bromoacetophenone (IV), which was then hydrolized with 10% HCl acid to afford 2′-hydroxy-2-bromoacetophenone (V). Eventually 5/6-substituted benzofuranone derivatives (A1–A4) were reached with the reaction of compound V and sodium acetate in ethanol via Williamson ether synthesis reaction.
Scheme 1. The synthesis of the benzofuran-3-one derivatives (A1–A4). Reactants/reagents and reaction conditions. a: (CH3CO)2O, reflux, 30 min; b: AlCl3, 160–170 °C; c: (CH3CO)2O, 50–100 °C, 3–4 h; d: Br2, HBr, diethyl ether or acetic acid; e: 10% HCl, reflux; f: CH3COONa, EtOH, reflux.
Scheme 2. The synthesis of 6-methoxybenzofuranone (B). Reactants/reagents and reaction conditions. a: (CH3)2SO4, NaOH, acetone, reflux, 4 h; b: AlCl3, CS2, dichloromethane, reflux, 30 min; c: CH3COONa, EtOH, reflux.
Scheme 3. The synthesis of the final compounds (D1–D38). Reactants/reagents and reaction conditions. a: n-butanol/isobutanol, catalytic HCl, reflux, 1 h; b: acetone, K2CO3, reflux, 6 h.
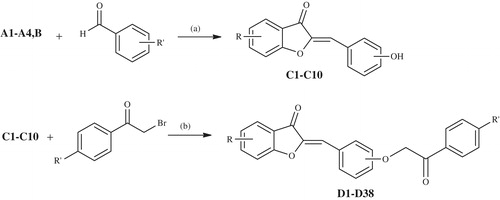
As for 6-methoxybenzofuranone (B) compound, it was obtained by a three step reaction using resorcinol as a starting material (Scheme 2). In first step, 1,3-dihydroxybenzene was methylated with dimethylsulfate in basic medium to get 1,3-dimethoxybenzene. Second, according to Friedel–Crafts reaction, chloro acetyl group was linked to the aromatic ring when methoxy group on the ortho position of the carbonyl group including carbon loss of methyl radical with the effect of aluminium chloride and turn into phenolic hydroxyl. 6-Methoxybenzofuranone was gained from the obtained 2′-hydroxy-4’-methoxy-2-chloroacetophenone compound with ring closure reaction in Williamson ether synthesis conditions in third step.
2-(3- or 4-Hydroxybenzylidene)benzofuran-3-one derivatives (C1–C10) were formed with the reaction of ketone compounds (A1–A4, B) and 3-/4-hydroxybenzaldehydes according to Claisen-Schmidt condensation. Finally, final 2-[3- or 4-(2-aryl-2-oxoethoxy)arylidene]benzofuran-3-one compounds (D1–D38) were achieved with the reaction of brominated acetophenones and 2-(3- or 4-hydroxybenzylidene)benzofuran-3-one derivatives (C1–C10) according to SN2 nucleophilic substitution reaction (Scheme 3).
The melting points were determined and uncorrected for all new compounds. The reactions were carried out in 55–75% yield. The structures of the final compounds (D1–D38) were elucidated by using IR, 1H-NMR and MS spectral data. In the IR spectra of the compounds, characteristic stretching bands were observed at 1649–1712, 1456–1647 and 1217–1288 cm−1 due to C = O, C = C and C–O bonds. In double bond region, two bands were seen belonging to two carbonyl group which are on third position of benzofuranone ring and on benzoyl moiety. In the 1H-NMR spectra, protons of acetyl residue were observed at about 5.62–5.79 ppm. The azomethine protons, −N = CH−, were resonated as singlets at about 6.81–6.99 ppm. All other aliphatic and aromatic protons were observed at expected regions. The aromatic protons were seen at 6.85–8.43 ppm. The aurone derivatives are expected to be found mostly in both of E and Z isomerism and β-hydrogen atoms resonated ranged from 6.7 to 7.1 ppm. According to reported values for 1H chemical shifts for (Z)-aurones (ca. 6.7 ppm) and (E)-aurones (ca. 7.2 ppm)Citation50, therefore the obtained final compounds are thought to be in (Z) isomerism. ES–MS spectra of the compounds gave confirmative molecular peaks.
All final compounds (D1–D38) were offered to NCI, USA for testing their anti-cancer activity according to drug screening protocol of the institute. Compounds D2, D3, D5, D8, D9, D10, D14, D17, D23, D27, D28, D30, D32 and D37 were selected by NCI for 60 human tumor cell lines’ anti-cancer screening test at single dose assay. In vitro single dose anti-cancer assay was performed in full NCI 60 cell panel representing leukemia (L), non-small cell lung cancer (NSCLC), colon cancer (CC), central nervous system cancer (CNSC), melanoma (M), ovarian cancer (OC), renal cancer (RC), prostate cancer (PC), and breast cancer (BC). Results were given as percentage growth of the cancer diseases which were treated with selected compounds (, Supplementary material). As can be seen from , according to mean values, most of the compounds have affected primarily leukemia cell lines and compound D37 exhibited the highest anti-tumor activity on leukemia cancer type with the growth percentage of 49.47%. Same compound showed the lowest mean growth percentage among all tested compounds and it can be claimed as the most effective compound. HOP-92 (NSCLC) with 48.94%, HCT-116 (CC) with 23.58%, MCF7 (BC) with 47.13%, IGROV-1 (OC) with 32.85%, MOLT-4 (L) with −17.79% and SR (L) with −22.38% growth percentages were determined as the most susceptible cell lines against compound D37. Among all tumor cell lines, UO-31 which is renal cancer cell, has detected as the most sensitive one with growth percentages −37.07% against D2, −44.36% against D8, −29.24% against D28. Compound D17 has also caused −34.25% growth percentage against MOLT-4 which is a leukemia cell line.
Table 3. Sixty human tumor cell lines’ anti-cancer screening data at single dose assay as percent cell growth promotion of selected compounds.
Conclusion
In this work, the synthesis of 2-[3- or 4 -(2-aryl-2-oxoethoxy)arylidene]benzofuran-3-one derivatives (D1–D38) and anti-cancer activity of the selected compounds were investigated. The final compounds were obtained in a multistep synthetic procedure and the structures of the final compounds were proved using IR, 1H-NMR and MS spectral data. According to anti-cancer activity results, compound D8 exhibited the highest activity causing a growth percentage −44.36% on UO-31 cell line.
Supplementary material available online
Supplemental Material.pdf
Download PDF (1,023.2 KB)Acknowledgements
The authors present their thanks to NCI (USA) and Anadolu University BIBAM (Türkiye) for anti-cancer test results and NMR spectra, respectively.
Declaration of interest
This work was supported by Anadolu University, Turkey (BAP Project No: 050301). The authors report no conflicts of interest.
References
- Singh M, Kaur M, Silakari O. Flavones: an important scaffold for medicinal chemistry. Eur J Med Chem 2014;84:206–39
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005;26:343–56
- Detsi A, Majdalani M, Kontogiorgis CA, et al. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem 2009;17:8073–85
- Lewin G, Aubert G, Thoret S, et al. Influence of the skeleton on the cytotoxicity of flavonoids. Bioorg Med Chem 2012;20:1231–9
- Fan R, Ban SR, Feng X, et al. Synthesis and anti-VSMC vegetation activities of new aurone derivatives. Chem Res Chinese Universities 2012;28:438–42
- Venkateswarlu S, Panchagnula GK, Gottumukkala AD, Subbaraju GV. Synthesis, structural revision, and biological activities of 40-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron 2007;63:6909–14
- Mcalpine JB, Chu W-l A, Ratnayake S, et al. Antimicrobial aurone derivatives. WO 1997026873 A1, 1997
- Kumar R, Dubey RK, Dixit P, Arya S. Naturally occurring aurones and chromones- potential organic therapeutic agents improvising nutritional security. Int J Innov Res Sci Eng Technol 2014;3:8141–8
- Kayser O, Kiderlen AF. Leishmanicidal activity of aurones. Tokai J Exp Clin Med 1999;23:423–6
- Kayser O, Chen M, Kharazmi A, Kiderlen AFZ. Aurones interfere with Leishmania major mitochondrial fumarate reductase. Naturforsch 2002;57:717–20
- Roussaki M, Lima SC, Kypreou AM, et al. Aurones: a promising heterocyclic scaffold for the development of potent antileishmanial agents. Int J Med Chem 2012;2012:1–8
- Wang J, Wang N, Yao X, Kitanaka S. Structures and anti-histamine activity of chalcones & aurones compounds from Bidens parviflora Willd. Asian J Trad Med 2007;2:23–9
- Bandgar BP, Patil SA, Korbad BL, et al. Synthesis and biological evaluation of a novel series of 2,2-bisaminomethylated aurone analogues as anti-inflammatory and antimicrobial agents. Eur Med Chem 2010;45:3223–7
- Jardosh H, Patel MP. Antimicrobial and antioxidant evaluation of new quinolone based aurone analogs. Arabian J Chem 2014. (in press)
- Morimoto M, Fukumoto H, Nozoe T, et al. Synthesis and insect antifeedant activity of aurones against spodoptera litura larvae. J Agric Food Chem 2007;55:700–5
- Zhang M, Xu XH, Cui Y, et al. Synthesis and herbicidal potential of substituted aurones. Pest Manag Sci 2012;68:1512–22
- Gao X, Yang L, Shu L, et al. Aurone constituents from the flowers of rosa rugosa and their biological activities. Heterocycles 2012;85:1925–31
- Gao X-M, Yang L-Y, Huang X-Z, et al. Aurones and isoaurones from the flowers of rosa damascena and their biological activities. Heterocycles 2013;87:583–9
- Meguellati A, Ahmed Belkacem A, Yi W, et al. B-ring modified aurones as promising allosteric inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Eur Med Chem 2014;80:579–92
- Haudecoeur R, Ahmed Belkacem A, Yi W, et al. Discovery of naturally occurring aurones that are potent allosteric inhibitors of hepatitis C virus RNA-dependent RNA polymerase. J Med Chem 2011;54:5395–402
- Gupta AK, Singh P, Sabarwal N. Rationalization of molecular descriptors of aurone analogs toward antimalarial activity. Asian J Pharm Clin Res 2014;7:186–92
- Carrasco MP, Newton AS, Gonçalves L, et al. Probing the aurone scaffold against Plasmodium falciparum: design, synthesis and antimalarial activity. Eur J Med Chem 2014;80:523–34
- Di Giovanni S, Borloz A, Urbain A, et al. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: thin layer chromatography versus microplate methods. Eur J Pharm Sci 2008;33:109–19
- Sheng R, Xu Y, Hu C, et al. Design, synthesis and AChE inhibitory activity of indanone and aurone derivatives. Eur J Med Chem 2009;44:7–17
- Geldenhuys WJ, Funk MO, Van der Schyf CJ, Carroll RT. A scaffold hopping approach to identify novel monoamine oxidase B inhibitors. Bioorg Med Chem Lett 2012;22:1380–3
- Nenadis N, Sigalas MP. A DFT study on the radical scavenging activity of maritimetin and related aurones. J Phys Chem 2008;112:12196–202
- Shin SY, Shin MC, Shin JS, et al. Synthesis of aurones and their inhibitory effects on nitric oxide and PGE2 productions in LPS-induced RAW 264.7 cells. Bioorg Med Chem Lett 2011;21:4520–3
- Lee YR, Hwang JK, Koh HW, et al. Sulfuretin, a major flavonoid isolated from Rhus verniciflua, ameliorates experimental arthritis in mice. Life Sci 2012;90:799–807
- Huang W, Liu MZ, Li Y, et al. Design, synthesis, and antitumor activity of novel chromone and aurone derivatives. Bioorg Med Chem 2007;15:5191–7
- Schoepfer J, Fretz H, Chaudhuri B, et al. Structure-based design and synthesis of 2-benzylidene-benzofuran-3-ones as flavopiridol mimics. J Med Chem 2002;25:1741–7
- Boumendjel A. Aurones: a subclass of flavones with promising biological potential. Curr Med Chem 2003;10:2621–30
- Dubois C, Haudecoeur R, Orio M, et al. Versatile effects of aurone structure on mushroom tyrosinase activity. Chem Bio Chem 2012;13:559–65
- Parate A, Sharma R, Maheshwari I. Structural indents for 5-hydroxyaurone derivatives as potent anticancer agents against HUVEC cancer cell lines-kNN MFA approach. Der Pharmacia Sinica 2013;4:121–9
- Zwergel C, Valente S, Salvato A, et al. Novel benzofuran–chromone and –coumarin derivatives: synthesis and biological activity in K562 human leukemia cells. Med Chem Commun 2013;4:1571–9
- Cheng H, Zhang L, Liu Y, et al. The total design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur J Med Chem 2010;45:5950–7
- Okombi S, Rival D, Bonnet S, et al. Discovery of benzylidenebenzofuran-3(2h)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 2006;49:329–33
- Lawrence NJ, Rennison D, McGown AT, Hadfield JA. The total synthesis of an aurone isolated from uvaria hamiltonii: aurones and flavones as anticancer agents. Bioorg Med Chem Lett 2003;13:3759–63
- Zheng X, Wang H, Liu YM, et al. Synthesis, characterization, and anticancer effect of trifluoromethylated aurone derivatives. J Heterocyclic Chem 2014. (in press)
- Ballinari D, Bonomini L, Ermoli A, et al. Chem. Abst. CA2441274A1. WO2002083123 A1, 2002
- Pratt WB, Ruddon RW, Ensminger WD. The anticancer drugs. New York: Oxford University Press; 1994:69–198
- Rajski SC, Williams RM. DNA cross-linking agents as antitumor drugs. Chem Rev 1998;98:2723–96
- Demirayak S, Yurttaş L. Synthesis and anticancer activity of some 1,2,3-trisubstituted pyrazinobenzimidazole derivatives. J Enzyme Inhib Med Chem 2014. (in press)
- Gundogdu Karaburun N, Karaburun AC, Demirayak S, et al. Synthesis and anticancer activity of some 2-[3/4-(2-substituted phenyl-2-oxoethoxy)benzylidene]-6-substituted-2,3-dihydro-1H-inden-1-one derivatives. Lett Drug Des Discov 2014;11:578–85
- Sousa CM, Berthet J, Delbaere S, Coelho PJ. One pot synthesis of aryl substituted aurones. Dyes Pigments 2011;92:537–41
- Thakkar K, Cushman M. A novel oxidative cyclization of 2'-hydroxychalcones to 4,5-dialkoxyaurones by thallium(III) nitrate. J Org Chem 1995;60:6499--6510
- Bose AK, Manhas MS, Ghosh M, et al. Microwave-induced organic reaction enhancement chemistry. 2. Simplified techniques. J Org Chem 1991;56:6968–70
- Higginbotham L, Stephen H. Coumaraone Series. I. The preparation of 4-, 5- and 6-methylcoumaran-2-ones and some derivate of o-, m- and p-tolyloxyacetic acids. J Am Chem Soc 1920;117:1534–42
- Balakrishna KJ, Rao NP, Seshadri TR. A method of distinguishing between flavones and flavonols. Proc Indian Natl Sci Acad A 1949;29A:394–403
- Geissman TA, Harborne JB. Anthochlor pigments. XIII. The ultraviolet absorption spectra of phenolic plant pigments. polyhydroxyaurones. J Am Chem Soc 1956;78:832–7
- Dean FM, Podimuang V. The course of the Algar-Flynn-Oyamada (A.F.O.) reaction. J. Chem Soc 1965:3978–87
- Goldsack RJ, Shannon JS. Proximity effects in the electron impact mass spectra of aurones and related compounds. Org Mass Spectrometr 1980;15:545–55
- Boyd MR. Status of the NCI preclinical antitumor drug discovery screen, principles and practice of oncology. Princip Prac Oncol 1989;3:1–42
- Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109