Abstract
Spices are appreciated for their medicinal properties besides their use as food adjuncts to enhance the sensory quality of food. In this study, Crocus cancellatus subsp. damascenus was investigated for its antioxidant activities employing different in vitro systems. Stigma extract demonstrated a radical scavenging activity against both 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radicals with IC50 values of 34.6 and 21.6 µg/mL and a good ferric reducing ability (53.9 µM Fe(II)/g). In order to clarify the potential functional properties of this spice, the carbohydrate-hydrolysing enzymes and pancreatic lipase inhibitory properties were investigated. Crocus cancellatus subsp. damascenus extract inhibited α-amylase and α-glucosidase with IC50 values of 57.1 and 68.6 µg/mL, respectively. The bioactivity was discussed in terms of phytochemicals content. The obtained results may be of interest from a functional point of view or as food additive and to promote the revalorization of this species.
Introduction
Spices have been in use for thousands of years in cooking to enhance the sensory quality of food. In recent years, the physiological functionality of food spice used in traditional cooking has received much attention due to the increasing interest in human health and has been studied in vitro and in vivo by many research groupsCitation1–4.
Saffron spice is a member of the Iridaceae family. The stigmas must be hand-picked from the delicate blossoms upon opening to preserve the desirable volatile componentsCitation5. With its unmatched signature bitter-like taste, slightly metallic sub-notes and pungent hay-like aroma, saffron was used as both flavoring and coloring agent in foodCitation6. Interest in the impact of saffron on human health is growing due to their proved health propertiesCitation7. Iran has a long history in saffron production and there are a lot of established Iranian saffron populations, which are cultivated since ancient timesCitation8.
Among species distributed in Iranian country Crocus cancellatus subsp. damascenus was first described by W. Herbert but other names have been bestowed upon it over the years including Crocus edulis, which refer to its use as a foodCitation9.
Oxidative stress is initiated by reactive oxygen species (ROS). ROS can easily damaging various cellular macromolecules. However, human cells have developed a series of protecting mechanisms to prevent the production of free radicals and oxidative damage. Natural products have been proposed as a substitute of synthetic antioxidants since they are related to negative health effectsCitation10. Increasing evidence in both experimental and clinical studies suggests that oxidative stress plays a major role in the pathogenesis of both types of diabetes mellitus. Abnormally high levels of free radicals and the simultaneous decline of antioxidant defense mechanisms can lead to damage of cellular organelles and enzymes, increased lipid peroxidation and development of insulin resistanceCitation11. Three hundred and sixty-six million people had diabetes in 2011 and by 2030 this will have risen to 552 millionCitation12. Type 2 diabetes is a heterogeneous disease resulting from a dynamic interaction between defects in insulin secretion and insulin action. Patients with type 2 diabetes are insulin-resistant and often have a metabolic syndrome, a multifactorial intervention including arterial hypertension and dyslipidemia. One therapeutic approach for treating in the early stage diabetes is to decrease post-prandial hyperglycaemia by retarding the absorption of glucose through the inhibition of α-amylase and α-glucosidaseCitation13. However, these drugs have some adverse effects like causing hypoglycemia at higher doses and other side effects. For these reasons several research groups addressed their activity on the discovery of natural products with inhibitory potential on key enzymes related to type 2 diabetesCitation14.
Abnormalities in plasma lipid and lipoprotein concentrations in patients with diabetes are outlinedCitation15. Obesity rates increased to 82% globally in the past two decadesCitation16.
Pancreatic lipase is a key enzyme for the absorption of dietary triglycerides. Interference with fat hydrolysis results in the reduced utilization of ingested lipids, therefore, inhibition of lipases decreases fat absorption that is useful for obese patients.
The objective of the present study was to investigate the chemical composition of C. cancellatus subsp. damascenus stigmas extract and to correlate the chemical profile with the antioxidant activity, carbohydrate-hydrolyzing enzymes and pancreatic lipase inhibitory properties.
Materials and methods
Chemicals and reagents
Solvents analytical grade were purchased from VWR International s.r.l. (Milan, Italy).
Ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) solution, potassium persulphate, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), β-carotene, linoleic acid, Tween 20, propyl gallate, tripyridyltriazine (TPTZ), FeCl3, FeSO4, butylated hydroxytoluene (BHT), potato starch, sodium phosphate, sodium chloride, α-amylase from porcine pancreas (EC 3.2.1.1), α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20), maltose, sodium acetate, sodium potassium tartrate, 3,5-dinitrosalicylic acid, o-dianisidine color reagent (DIAN), glucose oxidase peroxidase enzyme solution (PGO), lipase Type II, crude from porcine pancreas (EC 3.1.1.3), 4-nitrophenyl octanoate, orlistat, catechin hydrate, caffeic acid, ferulic acid, diphenylborinic acid aminoethylester, Folin–Ciocalteu reagent, AlCl3 were purchased from Sigma-Aldrich S.p.a. (Milan, Italy). Acarbose was obtained from Serva (Heidelberg, Germany) and p-anisaldehyde from Alfa Aesar (Karlsruhe, Germany).
Samples and extraction procedures
Stigmas of C. cancellatus subsp. damascenus were bought in January 2006 in a local market in Mashhad (Iran) and were identified by Dr. Farsad Nadjafi, Ferdowsi University of Mashhad, Iran. A voucher specimen is deposited at the herbarium of School of Agriculture, Ferdowsi University of Mashhad. The dried stigmas of C. cancellatus subsp. damascenus (500 g) were extracted with ethanol 70% through maceration (48 h × 3 times). The resultant solutions were dried to give 28.9 g.
Determination of total phenol and flavonoid content
The amount of total phenols of C. cancellatus subsp. damascenus ethanol extract was determined by the Folin–Ciocalteu methodCitation17. Chlorogenic acid was used as a standard and the total phenol content was expressed as chlorogenic acid equivalents in mg per g of extract. The total flavonoid content was determined spectrophotometrically using a method based on the formation of a flavonoid–aluminium complexCitation17. Quercetin was chosen as a standard and the levels of total flavonoid content were determined in triplicate and expressed as quercetin equivalents in mg per g extract ().
Determination of total carotenoid content
The total carotenoid content was determined by measuring the absorption of the stigmas extract using the methodology proposed by Gao et alCitation18. β-Carotene was used as a standard. The total carotenoid content was determined in triplicate and expressed as β-carotene equivalents in mg per g of extract ().
Table 1. Extraction yield (%) and phytochemicals content of C. cancellatus subsp. damascenus stigmas extract.
Gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS) analyses
The C. cancellatus subsp. damascenus extract was analyzed by using a Hewlett–Packard 6890 gas chromatograph equipped with an HP-5 MS capillary column (30 m length, 0.25 mm i.d., 0.25 µm film thickness) and interfaced with a Hewlett Packard 5973 Mass Selective (Agilent Technologies, Cernasco sul Naviglio, Milan, Italy). Ionization of the sample components was performed in electron impact mode (EI, 70 eV). Helium was used as carrier gas. The analytical conditions were as follows: oven temperature was 5 min isothermal at 50 °C, then 50–250 °C at a rate of 5 °C/min; then held isothermal for 10 min. Constituents were tentatively identified by comparison of their retention times with those of the literature or with those of authentic compounds available in our laboratory. Further tentative identification was made by comparison of their mass spectra with those stored in Wiley 138 library. The extract was analyzed also by a Shimadzu GC17A gas chromatograph system (Shimadzu, Milan, Italy). An SE-52 capillary column (30 m with an internal diameter of 0.25 mm and a film thickness of 0.25 µm) was used with nitrogen as the carrier gas. GC oven temperature and conditions were as described above. The quantification of the components was performed on the basis of their GC peak areas and the percentages of the characterized components are as given in . Component relative concentrations were calculated based on GC peak areas without using correction factors.
Table 2. Main constituents tentatively identified in C. cancellatus subsp. damascenus stigmas extract.
HPTLC analysis
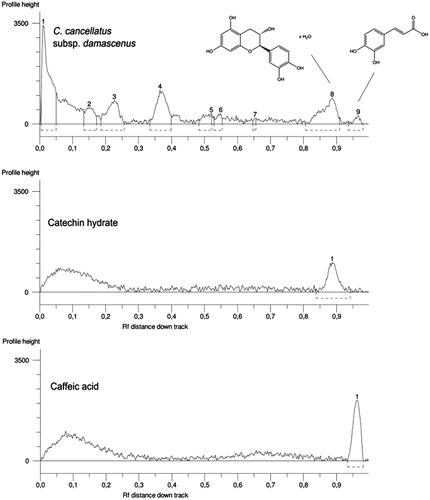
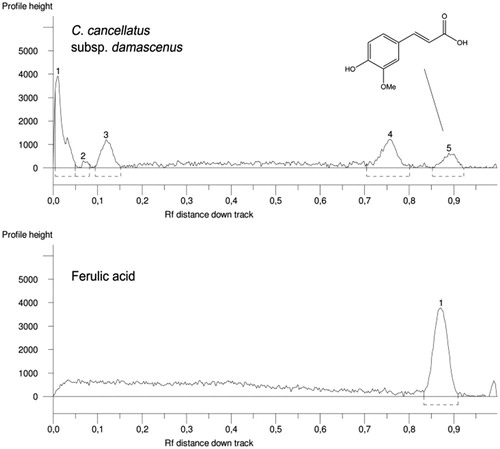
The high performance thin layer chromatography (HPTLC) system (CAMAG, Muttenz, Switzerland) consisted of a Linomat 5 sample applicator using 100 µL syringes and connected to a nitrogen tank and a Camag TLC Visualizer linked to winCATS software. Analyses were conducted by means of normal phase glass plates 20 cm × 10 cm (VWR International s.r.l., Milan, Italy) with glass-backed layers silica gel 60 (2 µm thickness) prewashed with methanol and carefully dried for 3 min at 100 °C. The syringe delivery speed was 150 nL/s and the other operating conditions were: injection volume, 1 μL; band width, 8 mm; distance from bottom, 15 mm; solvent front position, 90 mm. The HPTLC plates were developed with the mobile phase ethyl acetate/dichloromethane/acetic acid/formic acid/water (100:25:10:10:11; v/v/v/v). For the identification of ferulic acid the mobile phase ethyl acetate/dichloromethane/acetic acid/formic acid/water (100:31.25:1.25:1.25:1.25; v/v/v/v) was instead utilized. The developed layers were allowed to dry and then derivatized with Natural Product Reagent (NPR) (1 g diphenylborinic acid aminoethylester in 200 mL of ethyl acetate) and anhysaldehyde (1.5 mL p-anisaldehyde, 2.5 mL H2SO4, 1 mL AcOH in 37 mL EtOH). All plates were inspected under a UV light at 254 or 366 nm or under white light upper and lower (WRT) before and after derivatization by means of a Camag TLC visualizer. Band stability was checked by keeping the resolved peaks and inspecting at intervals of 12, 24 and 48 h. Repeatability was determined by running a minimum of three analyses. Rf (retardation factor) values for main selected compounds varied less than 0.02%.
The quantification of catechin hydrate and ferulic and caffeic acids was done. Working stock solutions were prepared by dilution with methanol (or ethanol for caffeic acid) to give final concentrations ranging from 0.5 to 10 mg/mL. Standard solutions of each compound were spotted on HPTLC plate to give absolute amounts in the range 0.5–10 µg/band. The calibration curves were prepared using absolute amount (µg/band) as independent variable (X) and the peak area of standards as dependent variable (Y). Regression analyses test of the compound were performed using GraphPad Prism Software (GraphPad Software, San Diego, CA). The curves confirmed linear relationship between the working concentration and the peak areas.
DPPH radical scavenging activity assay
Radical scavenging capacity was determined using DPPH assayCitation3. The DPPH radicals scavenging activity was calculated according to the following equation: Scavenging activity = [(A0−A1/A0) × 100], where A0 is the absorbance of the control (blank, without extract) and A1 is the absorbance in the presence of the extract.
Antioxidant capacity determined by radical cation ABTS+
This assay was based on the method previously describedCitation17. The scavenging ability of sample was calculated according to the following equation: ABTS scavenging activity (%) = [(A0−A)/A0] × 100, where A0 is the absorbance of the control reaction and A is the absorbance in the presence of samples.
β-Carotene bleaching test
The bleaching β-carotene was measured and expressed as antioxidant activity (AA)Citation17: [1−(A0−At)/( −
)] × 100, where A0 and
are the absorbance values measured at the incubation t = 0 min for samples and control, respectively, while At and
are the absorbance values measured in the samples and control, respectively, at t = 30 min and t = 60 min.
FRAP assay
The ferric-reducing ability power (FRAP) method measures the absorption change that appears when the TPTZ (2,4,6-tripyridyl-s-triazine)–Fe3+ complex is reduced to the TPTZ–Fe2+ form in the presence of antioxidant compoundsCitation19. The FRAP value represents the ratio between the slope of the linear plot for reducing Fe3+–TPTZ reagent by extracts compared to the slope of the plot for FeSO4.
Fe2+ chelating activity assay
The chelating activity of C. cancellatus subsp. damascenus extract for ferrous ions Fe2+ was measured according to the method previously describedCitation20. The chelating activity of the extract for Fe2+ was calculated using equation: chelating rate = (A0−A1)/A0 × 100, where A0 was the absorbance of the blank and A1 was the absorbance in the presence of the extract.
Carbohydrate-hydrolyzing enzymes inhibitory activity
The α-amylase bioassay method adopted was previously reportedCitation4. The α-amylase inhibition was expressed as percentage of inhibition and calculated by the following equations: % reaction = [(maltose) test/(maltose) control] × 100; % inhibition = 100 − % reaction ± SD.
The α-glucosidase inhibition was measured through a modified Sigma-Aldrich bioassay methodCitation4. The enzyme inhibition was expressed as percentage of inhibition and calculated by the following equations: % reaction = [(glucose) test/(glucose) control] × 100; % inhibition = 100 − % reaction ± SD.
Measurement of pancreatic lipase activity
The inhibition of pancreatic lipase was determined as previously describedCitation21. A water solution of type II crude porcine pancreatic lipase was prepared. A reaction mixture (100 mL of 5 mM 4-nitrophenyl octanoate, 4 mL of Tris-HCl buffer (pH 8.5), 100 mL of extract and 100 mL of enzyme solution) was prepared and incubated at 37 °C for 25 min before the substrate was added. The absorbance was measured at 412 nm. Orlistat (20 µg/mL) was used as positive control.
Statistical analysis
The concentration giving 50% inhibition (IC50) was calculated by nonlinear regression with the use of Prism GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). The concentration–response curve was obtained by plotting the percentage inhibition versus concentration. Differences within and between groups were evaluated by one-way analysis of variance test (ANOVA) followed by a multicomparison Dunnett’s test compared with the positive controls.
Results and discussion
Even though spices by themselves do not significantly contribute to the nutritive value of diet, by virtue of their health benefits they have been extensively investigatedCitation1–4,Citation21,Citation22. In this context, we decided to investigate the radical scavenging, the inhibition of lipid oxidation under accelerated conditions, the metal chelating and reducing ability of C. cancellatus subsp. damascenus dried stigmas.
Chemical composition
The ethanol extract of C. cancellatus subsp. damascenus showed a total amount of phenols with a value of 146.8 mg chlorogenic acid equivalent per g of extract and a total flavonoid content of 57.5 mg quercetin equivalent per g of extract.
Our results about the total flavonoid content are in agreement with those reported by Acar et al.Citation23 for Colchicum baytopiorum, Crocus flavus subsp. dissectus and Cenchrus biflorus (values of 36, 71 and 32 mg quercetin equivalent per g of extract). Karimi et al.Citation24 determined the total phenol and flavonoid content of C. sativus from Iran founding for the ethanol extract a value of 6.0 mg gallic acid equivalent per g dried weight (DW) and 2.9 mg rutin equivalent per g DW, respectively, while a total phenols content of 3.42 mg gallic acid per g DW phenolics was found in the methanolic extract of saffron petalCitation25. The total carotenoid content of our sample was estimated as 93.5 mg β-carotene equivalent per g of extract. GC–MS analysis identified in the extract of C. cancellatus subsp. damascenus a total of 11 compounds among them 5-(hydroxymethyl)-2-furancarboxaldehyde, (29.5%), methyl oleate (10.7%), methyl stearate (5.5%) and osthol (5.4%) are the most abundant.
Catechin hydrate, caffeic acid and ferulic acid were identified by comparing their Rf values obtained from the peaks with those of standards ( and ).
Quantification of catechin hydrate, caffeic and ferulic acids was performed using regression equations. The correlation coefficients (R2) were found to be >0.98. Among identified compounds, the most abundant one was catechin hydrate, with an amount of 55.2 mg per g of extract. The contents of ferulic and caffeic acids were found to be 13.9 and 10.01 mg per g of extract, respectively.
Antioxidant activity
The extract of C. cancellatus subsp. damascenus was tested for its antioxidant activities employing various established in vitro systems (). Stigmas extract exhibited concentration–response relationship in all tests. DPPH is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant activity of food. Stigmas ethanol extract demonstrated DPPH radical scavenging activity with an IC50 value of 34.6 µg/mL. This activity is 6.2-fold lower than the positive control ascorbic acid (IC50 value of 5.0 µg/mL).
Table 3. Radical scavenging activity, antioxidant property, metal reducing and chelating activity of C. cancellatus subsp. damascenus stigmas extract.
To confirm the radical scavenging activity, ABTS test was applied founding an IC50 value of 21.6 µg/mL. The extract was also able to inhibit the discoloration of β-carotene with IC50 values of 104.5 and 41.0 µg/mL after 30 and 60 min of incubation, respectively. The sample was also tested to observe its influence on metal transition ion iron. In FRAP assay that measures the reducing ability of antioxidant compound on ferric tripyridyltriazine (Fe3+–TPTZ) complex, C. cancellatus subsp. damascenus showed a reducing potency of 53.9 µM Fe(II)/g that is greater than positive control BHT (62.2 µM Fe(II)/g). The reducing ability of the sample could be correlated with the phenolic level as reported in literature since Soong and BarlowCitation26 demonstrated that phenolics could act as reducing agents, hydrogen donators and singlet oxygen quenchers. Moreover, stigmas extract is able also to chelate iron. In fact, in Fe2+-chelating activity assay stigmas extract showed an IC50 value of 24.6 μg/mL. The obtained results clearly evidenced that this spice can inhibit the iron-mediated lipid peroxidation of low-density lipoprotein, an effect ascribed to their capacity to form complexes with reduced metals and act as hydrogen donors.
Previously, Assimopoulou et al.Citation27 reported the strong radical scavenging activity (above 2000 ppm) of C. sativus growing in Greece while Gismondi et al.Citation28 described the antioxidant properties of Civitaretenga C. sativus from Italy. In DPPH test stigmas extract showed an IC50 value of 3.76 mg DW while FRAP assay indicated that one gram of dried sample presented the same antioxidant power of 2.5 mM FeSO4. Iranian C. sativus ethanol extract demonstrated an IC50 value of 299.4 µg/mL at tested concentration of 300 µg/mL against DPPH radical while a ferric reducing power of 53.1% was obtained testing the same concentrationCitation24. Crocus flavus subsp. dissectus at 2 mg/mL exhibited antioxidant activity measured by DPPH and β-carotene bleaching test equal to in the concentration of 0.8 mg/mL of BHA used as standard antioxidantCitation23. Moreover, C. cancellatus subsp. damascenus showed also a high flavonoids content that could contribute to the antioxidant activity. In particular, several studies demonstrated that diet flavonoids are absorbed from the gut after consumption and significantly increase the antioxidant capacity of the blood. These beneficial effects of increased antioxidant capacity in the body may be the reduction of oxidative damage to important moleculesCitation29,Citation30.
Carbohydrate-hydrolyzing enzyme inhibitory activity
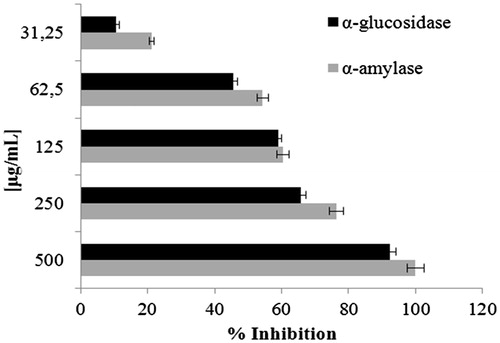
Recently, several studies demonstrated the hypoglycemic properties of spice and their constituentsCitation14. α-Amylase and α-glucosidase hydrolyzed glucosidic bonds in order to convert food containing starch into digestible carbohydrate food so inhibition of these enzymes could reduce the high post-prandial blood glucose peaks in diabetics. Crocus cancellatus subsp. damascenus showed a concentration–response relationship in both test with IC50 values of 57.1 and 68.6 µg/mL for α-amylase and α-glucosidase, respectively (). Acarbose was used as positive control (IC50 values of 50.0 and 35.5 µg/mL against α-amylase and α-glucosidase, respectively).
Figure 3. Concentration–response curve of C. cancellatus subsp. damascenus against α-glucosidase and α-amylase enzymes. Data are mean ± SD (n = 3).
A perusal analysis of literature revealed that C. sativus injected in alloxan-diabetic rats determined a significant increase of fasting blood glucose and HbA1c levels, but a decrease of blood insulin levelsCitation31. The influence of saffron on glucose metabolism was confirmed by Kang et al.Citation32 that demonstrated how this spice increases glucose uptake and insulin sensitivity in muscle cells via multi-pathway mechanisms.
Pancreatic lipase inhibitory activity
Lipase inhibitory activity was measured by monitoring the hydrolysis of p-NPC. Orlistat showed an IC50 value of 0.018 mg/mL. Crocus cancellatus subsp. damascenus extract induced 50.39% of inhibition of lipase activity at 5 mg/mL. The potential of natural products for the treatment of obesity is still largely unexploredCitation33. Natural sources could represent a starting point for further investigation in developing functional food and isolation of active compoundsCitation34.
Conclusion
This study investigated the phytochemical composition and the potential biological activity for the treatment of dysmetabolic disorders, such as type 2 diabetes and obesity, of C. cancellatus subsp. damascenus extract. Based on the experimental results reported herein, C. cancellatus subsp. damascenus possess high levels of phenols and flavonoids.
Catechin hydrate, caffeic acid and ferulic acid were identified as main constituents. The investigation of biological properties was accomplished by four standard antioxidant assay procedures and α-amylase, α-glucosidase and pancreatic lipase inhibitory assays. Collectively, the results emphasize the role of this spice as a potential dietary nutraceutical supplement to keep human beings healthy. Furthermore, it holds promise for becoming a natural food additive as an antioxidant agent.
Declaration of interest
This work was supported by European Community POR (Programmi Operativi Regionali) Calabria FSE (Fondo Sociale Europeo) 2007/2013. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Loizzo MR, Tundis R, Menichini F, et al. Influence of ripening stage on health benefits properties of Capsicum annuum var. acuminatum L.: in vitro studies. J Med Food 2008;11:184–9
- Chohan M, Forster-Wilkins G, Opara EI. Determination of the antioxidant capacity of culinary herbs subjected to various cooking and storage processes using the ABTS(*+) radical cation assay. Plant Foods Hum Nutr 2008;63:47–52
- Loizzo MR, Menichini F, Conforti F, et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem 2009;117:174–80
- Iauk L, Acquaviva R, Mastrojeni S, et al. Antibacterial, antioxidant and hypoglycaemic effects of Thymus capitatus (L.) Hoffmanns. et Link leaves' fractions. J Enzyme Inhib Med Chem 2014;17:1–6
- Hill T. The contemporary encyclopedia of herbs and spices: seasonings for the global kitchen. Hoboken: John Wiley & Sons, Inc.; 2004
- Moraga AR, Rambla JL, Ahrazem O, et al. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009;70:1009–16
- Kumar V, Bhat ZA, Kumar D, et al. Pharmacological profile of Crocus sativus – a comprehensive review. Pharmacologyonline 2011;3:799–11
- Ghorbani M. The economics of saffron in Iran. Acta Hortic 2007;739:321–31
- Ghaffari SM, Djavadi SB. Chromosome study on Crocus cancellatus subsp. damascenus from Iran. Iran J Bot 2007;13:1–3
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 1994;344:721–4
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17:24–38
- IDF. Diabetes. Available from: www.idf.org [last accessed 14 Jun 2014]
- Chen X, Zheng Y, Shen Y. Voglibose (Basen, AO-128), one of the most important α-glucosidase inhibitors. Curr Med Chem 2006;13:109−16
- Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini-Rev Med Chem 2010;10:315–31
- Aronson D, Rayfield EJ. Diabetes and obesity. In: Fuster V, Ross R, Topol EJ, eds. Atherosclerosis and coronary artery disease. Philadelphia (PA): Lippincott-Raven; 1996
- EU Commission. Strategy for Europe on nutrition, overweight and obesity related health issues. Brussels, Belgium: EU Commission; 2010
- Loizzo MR, Tundis R, Bonesi M, et al. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J Food Comp Anal 2012;25:179–84
- Gao X, Ohlander M, Jeppsson N, et al. Changes in antioxidant effects and their relationship to phytonutrients in fruits of Sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 2000;48:1485–90
- Benzie IF, Strains JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6
- Dinis CP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9
- Marrelli M, Loizzo MR, Nicoletti M, et al. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J Sci Food Agric 2014;94:2217–24
- Srinivasan K. Dietary spices as beneficial modulators of lipid profile in conditions of metabolic disorders and diseases. Food Funct 2013;4:503–21
- Acar G, Dogan NM, Duru ME, Kıvrak I. Phenolic profiles, antimicrobial and antioxidant activity of the various extracts of Crocus species in Anatolia. Afr J Microbiol Res 2010;4:1154–61
- Karimi E, Oskoueian E, Hendra R, Jaafar HZ. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010;15:6244–56
- Goli SAH, Mokhtari F, Rahimmalek M. Phenolic compounds and antioxidant activity from saffron (Crocus sativus L.) petal. J Agr Sci 2012;4:175–81
- Soong Y, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 2004;88:411–17
- Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res 2005;19:997–1000
- Gismondi A, Serio M, Canuti L, Canini A. Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am J Plant Sci 2012;3:1573–80
- Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr 2003;133:3285S−92S
- Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, et al. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem 2006;54:8956–61
- Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants 2011;10:82–9
- Kang C, Lee H, Jung E-S, et al. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem 2012;135:2350−8
- Bhutani KK, Birari RB, Kapat K. Potential antiobesity and lipid lowering natural products: a review. Nat Prod Commun 2007;2:331–48
- Slanc P, Doliak B, Kreft S, et al. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother Res 2009;23:874–7