Abstract
In this study, an alternative purification method for human paraoxonase 1 (hPON1) enzyme was developed using two-step procedures, namely, ammonium sulfate precipitation and Sepharose-4B-l-tyrosine-3-aminophenantrene hydrophobic interaction chromatography. SDS-polyacrylamide gel electrophoresis of the enzyme indicates a single band with an apparent MW of 43 kDa. The enzyme was purified 219-fold with a final specific activity of 4 408 400 U/mg and a yield of 10%. Furthermore, we examined the in vitro effects of some anabolic compounds, such as zeranol, 17 β-estradiol, diethylstilbestrol, oxytocin, and trenbolone on the enzyme activity to understand the better inhibitory properties of these molecules. The five anabolic compounds dose dependently decreased the activity of hPON1 with inhibition constants in the millimolar–micromolar range. The results show that these compounds exhibit inhibitory effects on hPON1 at low concentrations with IC50 values ranging from 0.064 to 16.900 µM.
Introduction
Paraoxonase 1 (PON1: EC 3.1.8.1) is a calcium-dependent serum esterase that is synthesized by the liver. In serum, it is closely associated with high-density lipoproteinsCitation1,Citation2. Paraoxonase hydrolyze organophosphate compounds which are widely used as insecticides and nerve gases. Therefore, it plays a major role in the detoxification of these compounds and other artificial substrates, so that it may alter significantly an individual’s susceptibility to the toxicity of these chemicals. In addition, paraoxonase is involved in lipid metabolism, since this enzyme probably hydrolyzes multiple oxygenated forms of polyunsaturated fatty acids of low-density lipoproteins associated phospholipids. For this reason, paraoxonase can be defined as an antioxidant enzymeCitation3,Citation4.
The use of anabolic steroids for growth promotion purposes in meat producing animals results in an improvement in muscle growth, more lean meat, and a higher feed efficiency. However, toxicological/epidemiological studies show that there are harmful effects to consumers; as a result, the public health is placed in risk. As a consequence, the use of anabolic steroids for fattening purposes has been banned in the European Union since 1986Citation5. These anabolic agents are used for increasing the rate of weight gain, improving the feed efficiency, storing protein, and decreasing fatnessCitation6–8. But, depending on the use of anabolic in animal feed, anabolic residues that may occur in meat and meat products present risks to human healthCitation9.
Zeranol is a resorcyclic acid lactone and a synthetic oestrogenic derivative of the mycotoxin zearalenone, which is produced by Fusarium moulds. It is a weak estrogen and is currently used to improve feed conversion efficiency and promote growth rates in livestock production. It has been widely used since 1969 as a growth promoter in the USA to improve the fattening rates of cattleCitation10.
Trenbolone acetate (TBA), a kind of 19-nortestesteron, is a synthetic steroid with anabolic propertiesCitation11–13. TBA decreases the rate of both protein synthesis and degradation, and when the rate of degradation is less than the rate of synthesis, muscle protein rate increasesCitation14.
Diethylstilbestrol (DES) is a synthetic estrogenic compound with carcinogenic and anabolic effects. Its most important effect is to improve the growth rate by increasing the quantity of digestible feed in livestock. As diethylstilbestrol is a carcinogenic compound, its use has been banned in animal production in European Union countriesCitation11.
Estradiol (ES) is a sort of natural anabolic steroid hormone. It exists as 17α- and 17β-isomers. Estradiol-17β has been widely applied in clinics for its most potent biological effects of the endogenous estrogensCitation5, and has also been used to promote unisexualization, improve feed conversion efficiency, and increase the rate of weight gain in aquaculture because of its protein synthesis stimulation and sex reversal effects. However, chronic exposure of humans to ES-17β through food chain can cause toxic effects as caused by other steroid hormones on public health, such as children precocious puberty, teratogenicity, and carcinogenesis. Ingestion of ES-17β residues in treated aquatic animal tissues may be potentially hazardous to consumers. Therefore, monitoring the residual content of this compound in fishery products is necessary for controlling the illegal use of such substances to ensure public health and trade contactsCitation15.
In this study, we developed an alternative purification method for the purification of the hPON1 enzyme. Specifically, human serum PON1 was purified by two-step procedures using ammonium sulfate precipitation and Sepharose-4B-l-tyrosine–3-aminophenantrene hydrophobic interaction chromatography which was specifically designed to the retained N-terminal hydrophobic signal peptide for PON1 enzyme.
In recent years, anabolisants are used for increasing the rate of weight gain, improving the feed efficiency, storing protein, and decreasing fatness. But, depending on the use of anabolic in animal feed, residues which may occur in meat and meat products present human health risks. Thus, the determination of the effects of these compounds on human PON1 activity is vital. However, to our knowledge, no study is available on the in vitro effects of anabolic compounds on PON1 activity. In this study, we aimed to determine any possible effect of some anabolic compounds on pure hPON1 activity.
Materials and methods
The materials used include Sepharose 4B, l-tyrosine, 3-aminophenantrene, paraoxon, protein assay reagents, and chemicals for electrophoresis were obtained from Sigma Chem. Co. (St. Louis, MO). All other chemicals used were of analytical grade. The anabolic compounds were provided by the local pharmacy.
Ammonium sulfate precipitation
Human serum was isolated from fresh human blood taken to dry tube. The blood samples were centrifuged at 1500 rpm for 15 min. and the serum was removed. First, serum paraoxonase was isolated by ammonium sulfate precipitation (60–80%)Citation16. The precipitate was collected by centrifugation at 15 000rpm for 20 min, and redissolved in 6.5 mL 100 mM Tris–HCl buffer (pH 8.0).
Hydrophobic interaction chromatography
The pooled precipitate obtained from human serum by using ammonium sulfate precipitation was subjected to hydrophobic interaction chromatography. The final saline concentration of precipitate was adjusted to 1 M ammonium sulfate, prior to that it was loaded onto the hydrophobic column prepared from Sepharose 4B-l-tyrosine-3-aminophenantrene. The preparation of hydrophobic column is as follows. About 10% CNBr was prepared in 1:1 dilution of Sepharose 4B and water. The mixture was titrated to pH 11 in an ice bath and maintained at that pH for 8–10 min. The reaction was stopped by filtering the gel on a Buchner funnel and washing with cold 0.1 M NaHCO3 buffer pH 10. l-Tyrosine was coupled to Sepharose-4B-l-tyrosine which was activated with CNBr by using saturated l-tyrosine solution in the same buffer. The reaction was completed by stirring with a magnet for 90 min. In order to remove excess of l-tyrosine from the Sepharose-4B-l-tyrosine gel, the mixture was washed with distilled water. The hydrophobic gel was obtained by diazotization of 3-aminophenantrene and coupling of this compound to the Sepharose-4B-l-tyrosine. The pH was adjusted to 9.5 with 1 M NaOH and, after gentle stirring for 3 h at room temperature; the coupled red Sepharose derivative was washed with 1 L of water and then 200 mL of 0.05 M Tris–sulfate buffer pH 7.5. The column was equilibrated with 0.1 M Na2HPO4 buffer pH 8.00 including 1 M ammonium sulfate. The paraoxonase was eluted with ammonium sulfate gradient using 0.1 M Na2HPO4 including 1 M ammonium sulfate buffer with and without ammonium sulfate pH 8.00. The purified hPON1 enzyme was stored in the presence of 2 mM CaCl2 at +4 °C, in order to maintain activity.
Total protein determination
The absorbance at 280 nm was used to monitor the protein in the column effluents and ammonium sulfate precipitation. Quantitative protein determination was achieved by absorbance measurements at 595 nm according to BradfordCitation17, with bovine serum albumin standard.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed in order to verify the purified enzyme. It was carried out in 12 and 3% acrylamide concentrations, containing 0.1% SDS, for the running and stacking gel, respectively, according to LaemmliCitation18.
Paraoxonase enzyme assay
Paraoxonase enzyme activity towards paraoxon was quantified spectrophotometrically by the method described by Gan et al.Citation19. The reaction was followed for 2 min at 37 °C by monitoring the appearance of p-nitrophenol at 412 nm in Biotek automated recording spectrophotometer (Biotek, Winooski, VT). A molar extinction coefficient (ɛ) of p-nitrophenol at pH 8.0 in 100 mM Tris–base buffer of 17 100M−1 cm−1 was used for the calculation. PON1 activity (1 U/L) was defined as 1 µmol of p-nitrophenol formed per minute.
In vitro inhibition kinetic studies
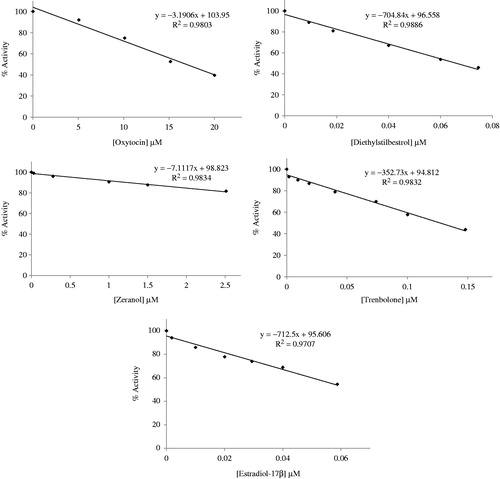
For the inhibition studies, in the presence of purified hPON1, different concentrations of anabolic compounds were added to the cuvette. Paraoxonase activity with anabolic compounds was assayed by following the hydration of paraoxon. Activity % values of paraoxonase for five different concentrations for each of the anabolic compounds were determined by regression analysis using the Microsoft Office 2000 Excel. Paraoxonase activity without an anabolic compound was accepted as 100% activity. The inhibitor concentration causing up to 50% inhibition (IC50 values) on the hPON1 enzyme activity were determined from the graphs.
Result and discussion
Paraoxonase has been purified so far from different sources with different yields and purification foldsCitation19–24. However, most of these previous studies either included many steps or had low purification yields. For these reasons, we focused in this study to develop a new and simpler chromatographic method for the purification of hPON1. In this study, a new strategy for the purification of the PON1 enzyme was developed. Human serum paraoxonase was purified by two sequential procedures, ammonium sulfate precipitation followed by hydrophobic interaction chromatography specifically designed for PON1 enzyme.
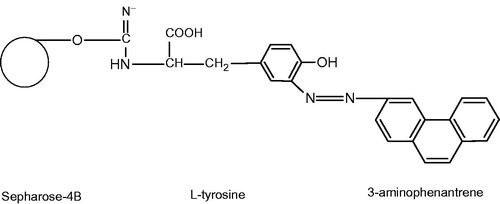
Subsequently, prior to loading onto hydrophobic interaction column; the precipitate was saturated with 1 M ammonium sulfate in order to improve its efficiency for binding to hydrophobic gel of the column. A new hydrophobic gel has been synthesized in order to reduce the number of the purification steps of paraoxonase enzyme. The hydrophobic gel was designated based on the retained N-terminal hydrophobic signal peptide for PON1 enzyme. 3-Aminophenantrene, which is a hydrophobic group, was added to Sepharose-4B gel matrix with the extension of l-tyrosine arm ().
Figure 1. Schematic representation of the Sepharose-4B-l-tyrosine-3-aminophenantrene hydrophobic gel. l-Tyrosine by using saturated l-tyrosine solution in the same buffer was coupled to Sepharose-4B-l-tyrosine activated with CNBr. The functional group of l-tyrosine (–NH2) was covalently bound with Sepharose 4B by means of an amide bond. After that, l-tyrosine was attached to the activated gel as a spacer arm, and finally diazotized 3-aminophenantrene was clamped to the meta position of l-tyrosine molecule as ligand. In this way, Sepharose-4B-l-tyrosine-3-aminophenantrene hydrophobic interaction gel was obtained. The hydrophobic interaction chromatography column was equilibrated with 0.1 M Na2HPO4 buffer pH 8.00 including 1 M (NH4)2SO4.
shows the typical elution pattern of the enzyme activity on hydrophobic column. The enzyme activity and the total protein concentration were determined from all fractions collected from each of the purification steps. The fractions with the highest paraoxonase activity and the lowest protein contents, i.e., 21, 22m and 23 tubes, were pooled. Finally, PON1 was purified 219.14-fold. In another study, paraoxonase activity from pooled plasma of Q and R phenotypes shows quite variation 122.7 and 737 units, respectivelyCitation19. As seen in , each purification step yielded excellent results compared with the final specific activity and purification values reported for other purification proceduresCitation20,Citation24.
Figure 2. Elution graphic of PON1 with hydrophobic interaction chromatography. Purification of human serum PON1 by Sepharose 4B-l-tyrosine-3-aminophenantrene hydrophobic interaction chromatography with ammonium sulfate gradient. Fractions from the ammonium sulfate extraction were pooled as described in Methods section. This material was eluted by increasing the ammonium sulfate concentration. Protein concentration was determined by measuring an absorbance of 280 nm and PON1 activities of fractions were assayed activity using paraoxon substrate. 1 unit = 1 μmol min−1 per ml. U, units.
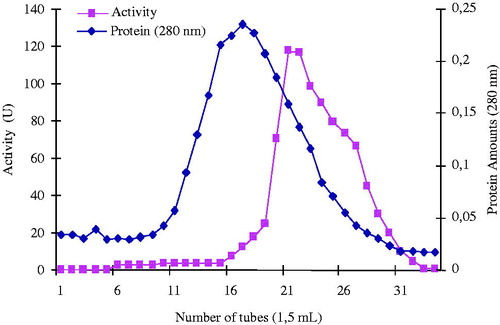
Table 1. Purification of human serum paraoxonase 1.
Different purification protocols have been used for PON enzyme from different sources. Furlong et al.Citation20 reported 62.1-fold PON purification from human serum using four-step purification protocols, namely, Agarose Blue, Sephadex G-200, and DEAE–Trisacryl M Sephadex G-75. Sheep serum paraoxonase was purified in 330–385-fold using ethanol, pH, and ionic strength fractionationCitation25. Rodrigo et al. purified the liver paraoxonase in 415-fold by hydroxyapatite adsorption, chromatography on DEAE–Sepharose CL-6B, non-specific affinity chromatography on Cibacron Blue 3 GA, and anion exchange on Mono Q HR 5/5. In addition, liver PON3 has been purified in 177-fold using a protocol consisting of seven stepsCitation26. Colak and Gencer purified human PON1 enzyme using two-step procedures, namely ammonium sulfate precipitation and Sepharose-4B-l-tyrosine-9-aminophenantrene hydrophobic interaction chromatography. Overall purification rate was found 526-foldCitation27. In another work, the effect of ammonium sulfate on the activities of Paraoxonase isoenzymes Q and R was researched. For this purpose, ammonium sulfate precipitation was performed before the Q and R isoenzymes. After ammonium sulfate precipitation, the specific activity of R isoform is 20.7 mU/mg. However, after ammonium sulfate precipitation, the specific activity of Q isoform is 6.6 mU/mgCitation28. Paraoxonase was purified shark Scyliorhinus canicula serum by Sayın et al. Purification of shark serum Paraoxonase was performed using the following methods: ammonium sulfate fractionation (60–80%) and Sepharose 4B-l-yrosine-1-naphtylamine hydrophobic interaction chromatography. A 37-fold purification with a yield of 1.127% was foundCitation29. PON was purified and characterized from the Merino and Kivircik sheep’s blood serums by a two-step procedure using ammonium sulfate precipitation and Sepharose-4B-l-tyrosine-1-napthylamine hydrophobic interaction chromatography for the first time by Erol et al. On SDS polyacyrilamide gel electrophoresis, purified human serum paraoxonase yielded a single band of 66 kDa on SDS-PAGECitation30.
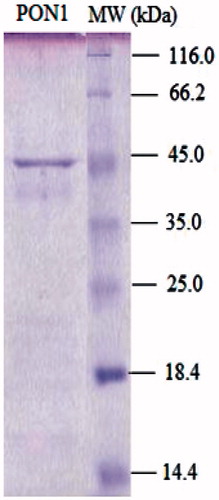
illustrates the final purification patterns determined by SDS gel electrophoresis. The purified human serum A-esterase gives a single band on SDS-PAGE with a weight of 43 kDa. This corresponds to the previous studiesCitation19,Citation20,Citation31. Some purification studies indicate the differences of the migration of PON bands in SDS gel electrophoresisCitation19. Furlong et al.Citation25 demonstrated two PON bands purified from rabbit serum. Gan et al.Citation19 report that human paraoxonase contains 15.8% carbohydrate. Sequence analysisCitation32 indicates five potential N-glycosylation sites in rabbit paraoxonase and four in humans. Therefore, a minimum molecular weight of human PON1 was 43 kDaCitation32. Moreover, a molecular weight of PON enzyme may increase up to 47–54 kDa in case of the contamination of albumin and ApoA1Citation33. However, our purification protocol yielded a single 43 kDa band suggesting the purification free from contaminants.
Figure 3. SDS-PAGE of human serum paraoxonase. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction chromatography were analyzed by SDS-PAGE (12 and 3%) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 2 contained 3 µg of various molecular mass standards: β-galactosidase (116,0), bovine serum albumin (66.0), ovalbumin (45.0), carbonic anhydrase, (33,0), ∞-lactoglobulin (25.0), lysozyme (19.5). Thirty microgram of purified hPON (lane 1) migrated with a mobility corresponding to an apparent Mr 43.0 kDa.
The kinetic parameters for the various anabolic compounds are presented in . The IC50 values obtained for each of the anabolic compounds are significantly different (). We have determined the IC50 values of 0.064–16.900 µM for the inhibition of hPON1 activity. As it understood, these anabolic compounds were effective inhibitors on purified human serum PON1 activity. Paraoxon was used as a substrate at this work. Relatively studies have not reported on investigations of the inhibition of paraoxonase as substrate using paraoxon.
Table 2. The IC50 values of anabolisant compounds.
Many chemical species influence metabolism at low concentrations by decreasing or increasing the normal enzyme activity, especially by inhibiting enzymes with critical functionCitation34, being thus drug targetsCitation35. PON is important in the metabolism as prganophosphates (OP) hydrolyzer. OP are pesticides that inhibit cholinesterase. They cause poisonings and deathsCitation36–38. Paraoxonase, a member of the A-oxonase family, breaks down acetylcholinesterase inhibitors before they bind to the cholinesterases, and thus protects people from harmful effects caused by exposure to low doses of OP pesticidesCitation35,Citation39. Yet, it is estimated that worldwide 220 000 people are killed each year from such exposures. This is one reason why inhibitors of paraoxonase must be well investigated. PON is also a drug targetCitation40–43. We have performed a number of studies regarding the interactions of different inhibitors with several such enzymes, including PON1Citation44–50. Sinan et al. showed that gentamycin sulfate and cefazolin sodium salt inhibited human serum PON1 dose and time dependently, with IC50 values of 0.887 and 0.0084 mM, respectively, but did not affect liver PON1 activity in human hepatoma HepG2 cellsCitation16. In another work, human serum paraoxonase (hPON1) was purified and the in vitro effects of commonly used antibiotics, namely clarithromycin and chloram_phenicol, on purified human serum, paraoxonase enzyme activity (serum hPON1) and human hepatoma (HepG2) cellparaoxonase enzyme activity (liver hPON1) were determined. And they were determined to inhibit serum hPON1 and liver hPON1Citation51. It was determined that commonly used antibiotics, namely sodium ampicillin, ciprofloxacin, and clindamycin phosphate, were effective inhibitors on human serum PON1Citation52. Kıranoglu et al. showed that while mouse liver PON activity showed a statistically significant decrease for ethinyl estradiol in combination with desogestrel and levonorgestrel all three drugs, serum PON activity increasedCitation53. In another work, in vitro inhibitory effects of oxytocin, dexamethasone, atropine sulfate, gentamicin sulfate, sulfadoxine-trimethoprim, furosemid, metamizole sodium, and toldimfos sodium were investigated. The IC50 values obtained varied markedly from 0.014 to 507.72 mg/mL. According to these findings, most potent and significant inhibition was displayed by dexamethasone, atropine sulfate, and furosemidCitation54.
However, it is thought that more extensive inhibition studies are necessary for a better understanding of the protective role of PONs against the toxic effects of xenobiotics, including environmental heavy metals and oxidative stress by-productsCitation16,Citation55. But, there are only few studies regarding effects of drugs on PON1 activity in the literature. Considering these, we report in the present study, the effects of some anabolic compounds against purified hPON1.
Declaration of interest
This work was supported by Balikesir University Research Project (2012/12).
References
- Aldridge WN. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J 1953;53:110–17
- Aldridge WN. Serum esterases. 2. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E 600) and its identity with the A-esterase of mammalian sera. Biochem J 1953;53:117–24
- Watson AD, Berliner JA, Hama SY, et al. Protective effect of high-density lipoprotein associated PON: inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995;96:2882–91
- Watson AD, Navah M, Hama SY, et al. Effect of platelet activating factor-acetyihydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 1995;95:774–82
- European Union (1986) – Council Directive 86/469/EEC of 16 September 1986 concerning the examination of animals and fresh meat for the presence of residues. Off J Eur Union, L 275 of 26.9.1986, 36–45
- Cecave MJ, Hancock DL. Effects of anabolic steroids on nitrogen metabolism and growth of steers fed corn silage and corn-based diets supplemented with urea or combinations of soybean meal and feather meal. J Anim Sci 1994;72:515–22
- Moran C, Ourke JF, Prendiville DJ, et al. The effect of estradiol trenbolonee acetate, or zeranol on growth rate, mammary development, carcass traits, and plasma estradiol concentrations of beef heifers. J Anim Sci 1991;69:4249–58
- Sawaya W, Lone KP, Hasain A, et al. Screening for estrogenic steroids in sheep and chicken by the application of enzyme-linked immunosorbent assay and a comparison with analysis by gas chromatography-mass spectrometry. Food Chem 1998;63:563–9
- Stan HJ, Abraham B. Determination of residues of anabolic drugs in meat by gas chromatography-mass spectrometry. J Chromatogr 1980;195:231–41
- Metzler M. Metabolism of some anabolic agents: toxicological and analytical aspects. J Chromatogr 1989;489:11–21
- European Commission, Unit B3-management of scientific committees II: opinion of the scientific committee on veterinary measures relating to public health. Assessment of potential risks to human health from hormone residues in bovine meat and meat products 30 April 1999. Available from: http://europa.eu.int/comm/food/fs/him/himindexen.html
- Hsu S, Hsu H, Eckerlin RH, Henion JD. Identification and quantitation of trenbolone in bovine tissue by gas chromatography-mass spectrometry. J Chromatogr 1988;424:219–29
- Laitem L, Gaspar P, Bello I. Detection of trenbolonee residues in meat and organs of slaughtered animals by thin-layer chromatography. J Chromatogr 1978;147:538–9
- Apple JK, Dikeman ME, Simms DD. Effects of synthetic hormone implants, singularly or in combinations, on performance carcass traits and longissimus muscle palatability of Holstein steers. J Anim Sci 1991;69:4437–48
- Hu W, Huang RB, Chen XJ, Jin HM. Study on derivation method of dihydrotheelin by gas chromatography. Anal Test Technol Instrum 2001;7:41–4
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–74
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51
- Laemmli UK. Cleavage of structual proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–6
- Furlong CE, Costa LG, Hasett C, et al. Human and rabbit paraoxonases: purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxification. Chem Biol İnteract 1993;87:35–48
- Alici HA, Ekinci D, Beydemir S. Intravenous anesthetics inhibit human Paraoxonase-I (PON1) activity in vitro and in vivo. Clin Biochem 2008;41:1384–90
- Pla A, Rodrigo L, Hernandez AF, et al. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact 2007;167:63–70
- Rodrigo L, Gil F, Hernandez AF, et al. Purification and characterization of paraoxon hydrolase from rat liver. Biochem J 1997;321:595–601
- Beltowski J, Wojcicka G, Jamroz A. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) or tissue paraoxonase 1 and plasma platelet activating factor acetylhydrolase activities. J Cardiovasc Pharmacol 2004;43:121–7
- Main AR. The role of A-esterase in the acute toxicity of paraoxon, TEPP and parathion. Can J Biochem Physiol 1956;34:197–216
- Rodrigo L, Gil F, Hernandez FA, et al. Identification of paraoxonase 3 in rat liver microsomes, Purification and biochemical properties. Biochem J Imm Pub 2003;15:261–8
- Colak U, Gençer N. Immobilization of paraoxonase onto chitosan and its characterization. Artif Cells Blood Substit Immobil Biotechnol 2012;40:290–5
- Gençer N, Arslan O. Effect of ammonium sulphate on the activities of paraoxonase isoenzymes Q and R. Asian J Chem 2011;23:3657–9
- Sayın D, Çakır DT, Gençer N, Arslan O. Effects of some metals on paraoxonase activity from shark Scyliorhinus canicula. J Enzyme Inhib Med Chem 2012;27:595–8
- Erol K, Gençer N, Arslan M, Arslan O. Purification, characterization, and investigation of in vitro inhibition by metals of paraoxonase from different sheep breeds. Artif Cells Nanomed Biotechnol 2013;41:125–30
- Furlong CE, Richter RJ, Chapline C, Crabb JW. Purification of rabbit and human serum paraoxonase. Biochemistry 1991;30:10133–40
- Hassett C, Richter RJ, Humbert R, et al. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry 1991;30:10141
- Zimmerman JK, Grothusen JR, Bryson PK, Brown TM. Partial purification and characterization of paraoxonase from rabbit serum. In: Reiner E, Aldridge WN, Hoskin FCG, eds. Enzymes hydrolyzing organophosphorus compounds. Chichester, UK: Ellis Horwood Ltd; 1989:128–42
- Senturk M, Talaz O, Ekinci D, et al. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3
- Robertson JG. Enzymes as a special class of therapeutic target: clinical drugs and modes of action. Curr Opin Struct Biol 2007;17:674–9
- Abdollahi M, Jafari A, Jalali N, et al. A new approach to the efficacy of oximes in the management of acute organophosphate poisoning. Iranian J Med Sci 1995;20:105–9
- Shadnia S, Azizi E, Hosseini R, et al. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol 2005;9:439–45
- Ashani Y, Pistinner S. Estimation of the upper limit of human butyrylcholinesterase dose required for protection against organophosphates toxicity: a mathematically based toxicokinetic model. Toxicol Sci 2004;77:358–67
- Ekinci D, Beydemir S, Kufrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–50
- Akgur SA, Ozturk P, Solak I, et al. Human serum paraoxonase (PON1) activity in acute organophosphorus insecticide poisoning. Forensic Sci Int 2003;133:136–40
- Coban TA, Beydemir S, Gulcin I, Ekinci D. The inhibitory effect of ethanol on Carbonic Anhydrase isoenzymes: an in vivo and in vitro study. J Enzyme Inhib Med Chem 2008;23:266–70
- Ekinci D, Beydemir S, Alim Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–7
- Gulcin I, Beydemir S, Coban A, Ekinci D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresen Environ Bull 2008;17:1283–7
- Sayın D, Çakır DT, Gençer N, Arslan O. Effects of some metals on paraoxonase activity from shark Scyliorhinus canicula. J Enzyme Inhib Med Chem 2012;27:595–8
- Arslan M, Gençer N, Arslan O, Guler OO. In vitro efficacy of some cattle drugs on bovine serum paraoxonase 1 (PON1) activity. J Enzyme Inhib Med Chem 2012;27:722–9
- Gençer N, Arslan O. The effect of ammonium sulphate on the activities of paraoxonase isoenzymes Q and R. Asian J Chem 2011;23:3657–9
- Gençer N, Arslan O. In vitro effects of some pesticides on PON1Q192 and PON1R192 isoenzymes from human serum. Fresenius Environ Bull 2011;20:590–6
- Gençer N, Arslan O. Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:134–40
- Sinan S, Köçkar F, Gençer N, et al. Effect of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull 2006;29:1559–63
- Sınan S, Kockar F, Gencer N, et al. Amphenicol and makrolid derived antibiotics inhibits paraoxonase enzyme activity in human serum and human hepatoma cells (Hepg2) in vitro. Biochemistry (Moscow) 2006;71:59–64
- Sinan S, Kockar F, Gencer N, et al. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma cells (HepG2) in vitro. Biochemistry (Moscow) 2006;71:46–50
- Sinan S, Kockar F, Gencer N, et al. Effects of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull 2006;29:1559–63
- Kıranoglu S, Sinan S, Gencer N, et al. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull 2007;30:1048–51
- Arslan M, Gençer N, Arslan O, Guler OO. In vitro efficacy of some cattle drugs on bovine serum paraoxonase 1 (PON1) activity. J Enzyme Inhib Med Chem 2012;27:722–9
- Pla A, Rodrigo L, Hernandez AF, et al. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact 2007;167:63–70