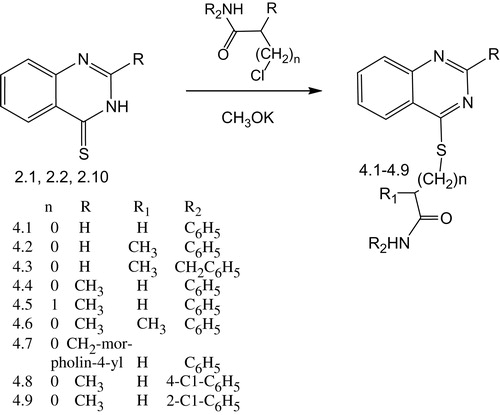
Abstract
In this study, a series of novel 2-alkyl(aryl)-quinazolin-4(3H)-thiones, 2-R-(quinazolin-4(3H)-ylthio)carboxylic acids and amides were synthesized and evaluated for antimicrobial and anticancer activities. Their structure was confirmed by elemental analysis and spectral data (FT-IR, LC-MS, 1H-NMR). Antimicrobial activity was tested in vitro against Staphylococcus aureus, Enterococcus faecalis, Enterobacter aerogenes, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Candida albicans and NCI in vitro preliminary anticancer activity against nine different cancer types. The most active antibacterial and antifungal compounds were: 2.1, 2.2 and 2.4. The introduction of the carboxylic acid or amide residue into the fourth position of quinazolin-4(3H)-thione resulted in the absence of antimicrobial activity. Substance 3.8 inhibited renal cancer UO-31 line and 2.18 – leukemia CCRF-CEM. The results of in silico molecular docking for DHFR and CK2 kinase had no correlation with in vitro properties, proposing the presence of other biological activity pathways.
Introduction
According to literature data, substituted quinazolines, i.e. condensed benzene–pyrimidine system, are intensively investigated for biological activities during several decades. In this paper, quinazoline-4(3H)-thione was chosen as the main structural fragment, as its derivatives have proven to show a broad spectrum of biological effects, such as anticonvulsantCitation1, antidiabeticCitation2, insecticidalCitation3, cardiotonic with myofibrillar Ca2+ sensitizing effectCitation4, inhibition of thymidylate synthaseCitation5, of tyrosine kinase domain of c-Src PleCitation6 and of phosphodiesteraseCitation7.
Given the constant and increasing threat of drug resistance, we were particularly interested in antimicrobial properties of quinazoline-4(3H)-thione’s derivatives. It was found that 4-(S-butylthio)quinazoline was more active than isoniazide, against atypical strains of mycobacteria, when investigated for antitubercular activity against Mycobacterium tuberculosis, M. kansasii, M. fortuitum, M. avium and M. intracellulareCitation8. The 4-ethylthio-6-fluoroquinazoline had potent antifungal activities against practically all the tested fungi (Gibberella zeae, Fusarium oxysporum, Cardamine mandshurica, Rhizoctonia solani, Thanatephorus cucumeris, Phytophthora infestans, Sclerotinia sclerotiorum, Botrytis cinerea, Colletotrichum gloeosporioides) and showed a broad-spectrum bioactivityCitation9. Also quinazoline-4(3H)-thione’s derivatives possessed antifungal properties against Sclerotium cepivorum and Botrytis alliiCitation10. Even bis-quinazolines showed antimicrobial activityCitation11.
Taking into consideration the above facts, we aimed to synthesize a series of novel 2-R-quinazolin-4(3H)-thiones in order to obtain the compounds with high antimicrobial and antifungal activity. Modification of thion pharmacophore with carboxyl or amide residue could influence the manifestation of pharmacological action of the synthesized compounds; especially since the 7-oxo-2-(trifluoromethyl)-4,7-dihydro-[1,2,4]triazolo[5,1-a]pyrimidine-6-carboxylic acids were reported to inhibit the growth of M. tuberculosis to 92%Citation12. Substituted 1-ethyl-6-fluorine-hydro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-quinoline-3-carboxylic acid had better antimicrobial properties against Xanthomonas oryzae, than norfloxacin and against Xanthomonas axonopodis, Erwinia aroideae and Rhizoctonia solan, than streptomycin sulfateCitation13. It was found that 2-(2-(4-(trifluoromethyl)benzylidene)hydrazinyl)-N-(4-(2-methyl-4-oxo-quinazolin-3(4H)-yl)-phenyl)acetamide was the most active compound in its series, when investigated for analgesic, anti-inflammatory and in vitro antimicrobial activitiesCitation14. Moreover, it is known that quinazoline derivatives are used in medicine as an effective anticancer agentsCitation15,Citation16. For example, Afatinib is a tyrosine kinase inhibitor (TKI) that also irreversibly inhibits human epidermal growth factor receptors (HER2 and HER4) and epidermal growth factor receptor (EGFR)Citation17.
Hence, the synthesis of (2-R-quinazolin-4(3H)-ylthio)carboxylic acids and amides was of undoubted interest from a synthetic and biological activity point of view.
Experimental
Chemistry
General
Melting points were determined in open capillary tubes in a Thiele's apparatus and were uncorrected. The elemental analyses (C, H, N, S) were performed using the ELEMENTAR vario EL cube analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). IR spectra (4000–600 cm−1) were recorded on a Bruker ALPHA FT-IR spectrometer (Bruker Bioscience, Germany) using a module ATR eco ZnSe. 1H NMR spectra (400 MHz) were recorded on a Varian-Mercury 400 spectrometer (Varian, Palo Alto, CA) with SiMe4 as internal standard in DMSO-d6 solution. LC-MS were recorded using chromatography/mass spectrometric system, which consists of high-performed liquid chromatograph “Agi1ent 1100 Series” (Agilent, Palo Alto, CA) equipped with diode-matrix and mass-selective detector “Agi1ent LC/MSD SL” (atmospheric pressure chemical ionization – APCI). The purity of all obtained compounds was checked by lH NMR and LC-MS.
2-R-4(3H)-Quinazolone derivatives were obtained as reported in literatureCitation18. Other starting materials and solvents were obtained from commercially available sources and used without additional purification.
Materials and methods
Preparation of 2-alkyl(aryl)-quinazolin-4-thiones (2.1–2.18)
2-Alkyl(aryl)-quinazolin-4(3H)-one (5 mmol) was added to the solution of 2,4-bis-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-disulfide (5 mmol, Lawesson’s reagent) in dioxane (20 ml) and refluxed for 1–2 h. The mixture was poured into the cold water. The precipitate was filtered and dried. Substances were recrystallized from a mixture of dioxane–water (2:1) (2.7, 2.9), 2-propanol–water (1:3) (2.4, 2.10), DMF–water (2.8, 2.12–2.14, 2.16, 2.17), acetone (2.5, 2.11), acetic acid (2.3, 2.6, 2.15, 2.18) or reprecipitated (2.2).
3H-Quinazoline-4-thione (2.1)
Yellow solid. Yield: 90.0%, m.p. 302–304 °C. IR (cm−1): 3039, 3010, 2982, 2830, 2792, 2735, 2699, 2662, 2626, 2489, 1815, 1685, 1621, 1595, 1566, 1517, 1463, 1441, 1396, 1341, 1302, 1261, 1243, 1197, 1107, 1026, 987, 954, 906, 864, 809, 771, 760, 682, 669, 639. 1H NMR: δ (ppm) 13.86 (br s, 1H, NH), 8.59 (d, J = 8.0 Hz, 1H, H5q.), 8.19 (s, 1H, Н2q.), 7.90 (t, J = 7.5 Hz, 1H, Н7q.), 7.74 (d, J = 8.0 Hz, 1H, Н8q.), 7.62 (t, J = 7.1 Hz, 1H, Н6q.). LC-MS: m/z = 163 [M + H]+. Anal. Calcd. for C8H6N2S C, 59.24; H, 3.73; N, 17.27; S, 19.77. Found: C, 59.22; H, 3.75; N, 17.26; S, 19.78.
2-Methyl-3H-quinazoline-4-thione (2.2)
Yellow solid. Yield: 73.9%, m.p. 218–220 °C. IR (cm−1): 3167, 3116, 3029, 2977, 2899, 2862, 2784, 2691, 2649, 1667, 1607, 1568, 1504, 1467, 1454, 1419, 1378, 1342, 1292, 1251, 1234, 1213, 1201, 1164, 1142, 1111, 1098, 1040, 1025, 999, 964, 880, 870, 805, 774, 762, 692, 660, 642. 1H NMR: δ (ppm) 13.72 (br s, 1H, NН), 8.55 (d, J = 8.1 Hz, 1H, НCitation5q.), 7.87 (t, J = 7.6 Hz, 1H, Н7q.), 7.64 (d, J = 8.0 Hz, 1H, Н8q.), 7.54 (t, J = 7.6 Hz, 1H, Н6q.), 2.48 (s, 3H, СН3). LC-MS: m/z = 177 [M + H]+. Anal. Calcd. for C9H8N2S C, 61.34; H, 4.58; N, 15.89; S, 18.19. Found: C, 61.38; H, 4.60; N, 15.84; S, 18.18.
2-Ethyl-3H-quinazoline-4-thione (2.3)
Yellow solid. Yield: 77.2%, m.p. 185–187 °C. IR (cm−1): 3187, 3138, 3094, 3073, 3042, 2979, 2918, 2875, 2848, 2729, 2693, 2660, 2629, 2598, 2568, 2508, 1959, 1855, 1770, 1743, 1714, 1660, 1617, 1603, 1566, 1505, 1464, 1452, 1417, 1371, 1343, 1312, 1259, 1227, 1185, 1148, 1124, 1076, 1034, 1013, 971, 917, 886, 854, 837, 802, 768, 642. 1H NMR: δ (ppm) 13.74 (br s, 1H, NН), 8.56 (d, J = 8.1 Hz, 1H, Н5q.), 7.87 (t, J = 7.6 Hz, 1H, Н7q.), 7.67 (d, J = 8.1 Hz, 1H, Н8q.), 7.55 (t, J = 7.6 Hz, 1H, Н6q.), 2.77 (q, J = 7.5 Hz, 2H, СН2СН3), 1.27 (t, J = 7.5 Hz, 3H, СН2СН3). LC-MS: m/z = 191 [M + H]+. Anal. Calcd. for C10H10N2S C, 63.13; H, 5.30; N, 14.72; S, 16.85. Found: C, 63.12; H, 5.34; N, 14.73; S, 16.82.
2-Phenyl-3H-quinazoline-4-thione (2.4)
Yellow solid. Yield: 53.6%, m.p. 200–202 °C. IR (cm−1): 3140, 3115, 3029, 2975, 2905, 1662, 1599, 1565, 1492, 1463, 1447, 1429, 1345, 1316, 1299, 1284, 1252, 1241, 1220, 1145, 1132, 1080, 1029, 959, 945, 928, 889, 857, 808, 758, 701, 689, 658, 614. 1H NMR: δ (ppm) 13.93 (br s, 1H, NН), 8.64 (d, J = 7.6 Hz, 1H, Н5q.), 8.20 (d, J = 7.5 Hz, 2H, Н2, Н6ph.), 7.93 (t, J = 8.0 Hz, 1H, Н7q.), 7.79 (d, J = 7.5 Hz, 1H, Н8q.), 7.67–7.54 (m, 4H, Н6q., Н3–5ph.). LC-MS: m/z = 239 [M + H]+. Anal. Calcd. for C14H10N2S C, 70.56; H, 4.23; N, 11.75; S, 13.45. Found: C, 70.54; H, 4.23; N, 11.74; S, 13.43.
2-Benzyl-3H-quinazoline-4-thione (2.5)
Orange solid. Yield: 64.0%, m.p. 200–202 °C. IR (cm−1): 3139, 3117, 3087, 3062, 3031, 2969, 2915, 2892, 2875, 2497, 1674, 1651, 1618, 1598, 1572, 1507, 1494, 1464, 1454, 1435, 1414, 1342, 1318, 1254, 1232, 1181, 1155, 1122, 1072, 1028, 958, 919, 893, 865, 838, 827, 763, 729, 717, 695, 667, 658, 642, 610. 1H NMR: δ (ppm) 13.98 (br s, 1H, NН), 8.56 (d, J = 8.1 Hz, 1H, Н5q.), 7.87 (t, J = 7.5 Hz, 1H, Н7q.), 7.68 (d, J = 8.1 Hz, 1H, Н8q.), 7.57 (t, J = 7.6 Hz, 1H, Н6q.), 7.41 (d, J = 7.4 Hz, 2H, Н2, Н6ph.), 7.35 (t, J = 7.4 Hz, 2H, Н3, Н5ph.), 7.27 (t, J = 7.0 Hz, 1H, Н4ph.), 4.11 (s, 2H, СН2). LC-MS: m/z = 253 [M + H]+. Anal. Calcd. for C15H12N2S C, 71.40; H, 4.79; N, 11.10; S, 12.71. Found: C, 71.42; H, 4.80; N, 12.69; S, 11.12.
2-Phenethyl-3H-quinazoline-4-thione (2.6)
Yellow solid. Yield: 89.0%, m.p. 185–187 °C. IR (cm−1): 3138, 3079, 3026, 2971, 2919, 2723, 2502, 1977, 1955, 1938, 1850, 1738, 1714, 1654, 1603, 1567, 1505, 1494, 1464, 1449, 1430, 1346, 1322, 1270, 1231, 1212, 1146, 1073, 1031, 1020, 994, 969, 955, 902, 847, 766, 737, 691, 645, 619. 1H NMR: δ (ppm) 13.82 (br s, 1H, NН), 8.57 (d, J = 8.1 Hz, 1H, Н5q.), 7.88 (t, J = 7.6 Hz, 1H, Н7q.), 7.70 (d, J = 8.1 Hz, 1H, Н8q.), 7.57 (t, J = 7.6 Hz, 1H, Н6q.), 7.31 (d, J = 5.0 Hz, 4H, Н2, Н3, Н5, Н6ph), 7.21 (t, J = 5.1 Hz, 1H, Н4ph.), 3.13–3.01 (m, 4H, СН2СН2). LC-MS: m/z = 267 [M + H]+. Anal. Calcd. for C16H14N2S C, 72.15; H, 5.30; N, 10.52; S, 12.04. Found: C, 72.37; H, 5.28; N, 10.48; S, 12.01.
2-Styryl-3H-quinazoline-4-thione (2.7)
Yellow solid. Yield: 68.2%, m.p. 246–248 °C. IR (cm−1): 3158, 3100, 3025, 2966, 2917, 2849, 2505, 1739, 1666, 1643, 1608, 1568, 1503, 1469, 1446, 1434, 1346, 1328, 1255, 1222, 1192, 1161, 1148, 1069, 1033, 1016, 981, 962, 871, 842, 830, 779, 766, 747, 690, 676, 604. 1H NMR: δ (ppm) 13.83 (br s, 1H, NН), 8.58 (d, J = 8.0 Hz, 1H, Н5q.), 8.05 (d, J = 6.1 Hz, 1H, HetСН = ), 7.90 (t, J = 7.5 Hz, 1H, Н7q.), 7.76 (d, J = 8.2 Hz, 1H, Н8q.), 7.70 (d, J = 7.6 Hz, 2H, Н2, Н6ph.), 7.58 (t, J = 7.6 Hz, 1H, Н6q.), 7.54–7.42 (m, 3H, Н3–5ph.), 7.29 (d, J = 6.0 Hz, 1H, = СНC6H5). LC-MS: m/z = 219 [M + H]+. Anal. Calcd. for C16H12N2S C, 72.70; H, 4.58; N, 10.60; S, 12.13. Found: C, 72.69; H, 4.56; N, 10.64; S, 12.17.
2-Trifluoromethyl-3H-quinazoline-4-thione (2.8)
Yellow solid. Yield: 96.2%, m.p. 210–212 °C. IR (cm−1): 3152, 3112, 3085, 3056, 2983, 2922, 1668, 1631, 1596, 1573, 1516, 1464, 1360, 1317, 1218, 1206, 1161, 1130, 1034, 899, 846, 800, 765, 692, 624. 1H NMR: δ (ppm) 12.44 (br s, 1H, NН), 8.63 (d, J = 8.0 Hz, 1H, Н5q.), 7.99 (t, 1H, J = 6.7 Hz, Н7q.), 7.86 (d, J = 8.0 Hz, 1H, Н8q.), 7.76 (t, J = 6.9 Hz, 1H, Н6q.). LC-MS: m/z = 230 [M + H]+. Anal. Calcd. for C9H5F3N2S C, 46.96; H, 2.19; N, 12.17; S, 13.93. Found: C, 47.01; H, 2.22; N, 12.14; S, 13.93.
2-Morpholin-4-yl-3H-quinazoline-4-thione (2.9)
Yellow solid. Yield: 86.5%, m.p. 202–204 °C. IR (cm−1): 3245, 3197, 3137, 3055, 2959, 2900, 2851, 2571, 1620, 1573, 1490, 1456, 1434, 1406, 1369, 1336, 1303, 1279, 1254, 1227, 1178, 1153, 1131, 1109, 1063, 1028, 1019, 1000, 982, 918, 873, 859, 779, 755, 732, 713, 693, 633. 1H NMR: δ (ppm) 12.88 (br s, 1H, NН), 8.44 (d, J = 7.6 Hz, 1H, Н5q.), 7.67 (t, J = 6.4 Hz, 1H, Н7q.), 7.44 (d, J = 8.1 Hz, 1H, Н8q.), 7.26 (t, J = 6.6 Hz, 1H, Н6q.), 3.76–3.64 (m, 8H, morph.). LC-MS: m/z = 247 [M + H]+. Anal. Calcd. for C12H13N3OS C, 58.28; H, 5.30; N, 16.99; S, 12.96. Found: C, 58.31; H, 5.28; N, 17.01; S, 12.99.
2-(Morpholin-4-yl-methyl)-3H-quinazoline-4-thione (2.10)
Yellow solid. Yield: 58.5%, m.p. 180–182 °C. IR (cm−1): 3120, 3082, 3050, 2981, 2965, 2949, 2916, 2848, 2818, 2767, 2693, 2509, 1598, 1568, 1497, 1464, 1448, 1424, 1352, 1335, 1306, 1294, 1270, 1226, 1197, 1164, 1141, 1111, 1076, 1034, 1011, 987, 949, 910, 892, 870, 856, 839, 808, 782, 764, 683, 651, 604. 1H NMR: δ (ppm) 13.99–13.35 (br s, 1H, NН), 8.57 (d, J = 8.0 Hz, 1H, Н5q.), 7.87 (t, J = 7.4 Hz, 1H, Н7q.), 7.71 (d, J = 8.0 Hz, 1H, Н8q.), 7.58 (t, J = 7.5 Hz, 1H, Н6q.), 3.59 (s, 4H, N(CH2)2), 3.57 (s, 2H, СН2), 2.60–2.53 (m, 4H, O(CH2)2). LC-MS: m/z = 261 [M + H]+. Anal. Calcd. for C13H15N3OS C, 59.75; H, 5.79; N, 16.08; S, 12.27. Found: C, 59.72; H, 5.81; N, 16.07; S, 12.24.
2-(Thiophen-3-yl)-3H-quinazoline-4-thione (2.11)
Yellow solid. Yield: 80.3%, m.p. 215–219 °C. IR (cm−1): 3239, 3093, 3064, 2914, 2847, 1609, 1586, 1557, 1487, 1461, 1430, 1415, 1345, 1312, 1288, 1250, 1220, 1187, 1143, 1068, 1027, 1015, 968, 942, 884, 865, 843, 801, 765, 717, 686, 622. 1H NMR: δ (ppm) 13.80 (br s, 1H, NН), 8.80 (s, 1H, H2thioph.), 8.61 (d, J = 7.7 Hz, 1H, Н5q.), 7.96–7.86 (m, 2H, H5thioph., Н7q.), 7.75 (d, J = 8.0 Hz, 2H, H4thioph., Н8q.), 7.59 (t, J = 7.1 Hz, 1H, Н6q.). LC-MS: m/z = 246 [M + H]+. Anal. Calcd. for C12H8N2S2 C, 58.99; H, 3.30; N, 11.46; S, 26.25. Found: C, 59.07; H, 3.26; N, 11.43; S, 26.22.
2-(1H-Imidazol-1-yl)-3H-quinazoline-4-thione (2.12)
Yellow solid. Yield: 54.0%, m.p. 235–237 °C. IR (cm−1): 3111, 3065, 2980, 2921, 2853, 2760, 2638, 1677, 1626, 1604, 1580, 1516, 1465, 1431, 1396, 1356, 1340, 1317, 1288, 1255, 1227, 1183, 1121, 1102, 1074, 1034, 958, 920, 875, 862, 831, 761, 734, 690, 665, 646, 627, 604. LC-MS: m/z = 242 [M + H]+. Anal. Calcd. for C11H8N4S C, 57.88; H, 3.53; N, 24.54; S, 14.05. Found: C, 57.83; H, 3.52; N, 24.57; S, 14.00.
2-(2-Chlorophenyl)-3H-quinazoline-4-thione (2.13)
Red solid. Yield: 65.1%, m.p. 145–146 °C. IR (cm−1): 2501, 1597, 1585, 1557, 1533, 1467, 1446, 1425, 1319, 1288, 1268, 1238, 1198, 1152, 1059, 1018, 966, 946, 936, 910, 876, 860, 838, 767, 754, 723, 707, 650, 635. 1H NMR: δ (ppm) 10.04 (br s, 1H, NH), 8.74 (d, J = 8.2 Hz, 1H, Н5q.), 8.10 (t, J = 7.5 Hz, 1H, Н7q.), 7.94 (d, J = 7.2 Hz, 1H, Н8q.), 7.84–7.76 (m, 2H, Н3, Н5ph.), 7.70 (d, J = 8.0 Hz, 1H, Н6ph.), 7.65 (t, J = 7.6 Hz, 1H, Н4ph.), 7.58 (t, J = 7.5 Hz, 1H, Н6q.). LC-MS: m/z = 273 [M + H]+. Anal. Calcd. for C14H9ClN2S C, 61.65; H, 3.33; N, 10.27; S, 11.76. Found: C, 61.67; H, 3.31; N, 10.26; S, 11.73.
2-(3-Chlorophenyl)-3H-quinazoline-4-thione (2.14)
Yellow solid. Yield: 62.5%, m.p. 223–224 °C. IR (cm−1): 3141, 3107, 3078, 3033, 2979, 2920, 1985, 1955, 1923, 1889, 1834, 1770, 1723, 1681, 1615, 1589, 1567, 1504, 1475, 1438, 1423, 1348, 1299, 1253, 1221, 1155, 1136, 1097, 1082, 1028, 998, 958, 908, 890, 883, 861, 800, 785, 765, 717, 696, 678, 650. 1H NMR: δ (ppm) 8.74 (d, J = 8.2 Hz, 1H, Н5q.), 8.10 (t, J = 8.3 Hz, 1H, Н7q.), 7.94 (d, J = 7.9 Hz, 1H, Н8q.), 7.84–7.76 (m, 2H, H2, Н5ph.), 7.70 (d, J = 8.0 Hz, 1H, Н6ph.), 7.65 (t, J = 6.9 Hz, 1H, Н4ph.), 7.58 (t, J = 7.5 Hz, 1H, Н6q.). LC-MS: m/z = 373 [M + H]+. Anal. Calcd. for C14H9ClN2S C, 61.65; H, 3.33; N, 10.27; S, 11.76. Found: C, 61.64; H, 3.32; N, 10.25; S, 11.74.
2-(4-Chlorophenyl)-3H-quinazoline-4-thione (2.15)
Yellow solid. Yield: 17.8%, m.p. 239–247 °C. IR (cm–1): 3139, 3110, 3079, 3048, 3029, 2980, 2913, 2850, 2497, 1963, 1935, 1909, 1709, 1596, 1571, 1563, 1504, 1488, 1464, 1433, 1400, 1345, 1316, 1298, 1276, 1251, 1221, 1181, 1152, 1131, 1115, 1090, 1075, 1027, 1011, 955, 888, 861, 828, 798, 770, 759, 747, 726, 694, 685, 665, 629. 1H NMR: δ (ppm) 10.24 (br s, 1H, NН), 8.73 (d, J = 8.1 Hz, 1H, Н5q.), 8.08 (t, 1H, J = 7.8 Hz, Н7q.), 7.93 (d, J = 8.0 Hz, 1H, Н8q.), 7.78 (d, J = 8.0 Hz, 2H, H2, H6ph.), 7.72–7.65 (m, 2H, H3, H5ph.), 7.60 (t, J = 7.5 Hz, 1H, Н6q.). LC-MS: m/z = 273 [M + H]+. Anal. Calcd. for C14H9ClN2S C, 61.65; H, 3.33; N, 10.27; S, 11.76. Found: C, 61.69; H, 3.35; N, 10.22; S, 11.76.
2-(3-Fluorophenyl)-3H-quinazoline-4-thione (2.16)
Yellow solid. Yield: 31.03%, m.p. 143–145 °C. IR (cm−1): 3082, 3061, 3012, 2954, 2918, 2850, 2497, 1940, 1868, 1799, 1746, 1682, 1655, 1601, 1589, 1563, 1533, 1482, 1456, 1441, 1325, 1312, 1297, 1266, 1238, 1214, 1169, 1159, 1087, 1040, 1021, 998, 972, 957, 918, 885, 866, 796, 781, 759, 679, 640. 1H NMR: δ (ppm) 13.95 (br s, 1H, NH), 8.68 (d, J = 8.1 Hz, 1H, Н5q.), 8.17 (m, 2H, Н2, Н5ph.), 8.06 (t, J = 7.1 Hz, 1H, Н7q.), 7.94 (d, J = 7.4 Hz, 1H, Н8q.), 7.73 (d, J = 7.2 Hz, 1H, Н6q.), 7.46 (dd, J1 = 17.7, J2 = 9.0 Hz, Н4, Н6ph.). LC-MS: m/z = 256 [M + H]+. Anal. Calcd. for C14H9FN2S C, 65.61; H, 3.54; N, 10.93; S, 12.51. Found: C, 65.65; H, 3.57; N, 11.01; S, 12.46.
2-(4-Trifluoromethylphenyl)-3H-quinazoline-4-thione (2.17)
Red solid. Yield: 46.7%, m.p. 122–123 °C. IR (cm−1): 3043, 3029, 3000, 2917, 2848, 2516, 1987, 1947, 1928, 1907, 1870, 1839, 1651, 1613, 1599, 1558, 1534, 1448, 1406, 1319, 1308, 1247, 1205, 1159, 1103, 1064, 1009, 936, 909, 839, 826, 769, 676, 644, 629, 615. 1H NMR: δ (ppm) 13.74 (br s, 1H, NН), 8.73 (d, J = 8.1 Hz, 1H, Н5q.), 8.30 (d, J = 8.1 Hz, 2H, H2, H6ph.), 8.00 (t, 1H, J = 7.5 Hz, Н7q.), 7.93 (d, J = 8.0 Hz, 1H, Н8q.), 7.89 (d, J = 8.2 Hz, 2H, H3, H5ph.), 7.68 (t, J = 7.5 Hz, 1H, Н6q.). LC-MS: m/z = 306 [M + H]+. Anal. Calcd. for C15H9F3N2S C, 58.82; H, 2.96; N, 9.15; S, 10.47. Found: C, 58.84; H, 3.01; N, 9.13; S, 10.44.
2-(3,4-Dimethoxyphenyl)-3H-quinazoline-4-thione (2.18)
Yellow solid. Yield: 75.5%, m.p. 216–217 °C. IR (cm−1): 3212, 3119, 3065, 2999, 2974, 2929, 2840, 2501, 1741, 1597, 1566, 1513, 1497, 1470, 1451, 1434, 1415, 1356, 1283, 1259, 1244, 1226, 1207, 1174, 151, 1076, 1022, 965, 935, 872, 861, 818, 770, 756, 711, 634, 616. 1H NMR: δ (ppm) 13.76 (br s, 1H, NH), 8.61 (d, J = 7.9 Hz, 1H, Н5q.), 7.93–7.83 (m, 3H, Н2, Н6ph., Н7q.), 7.76 (d, J = 8.0 Hz, 1H, Н8q.), 7.57 (t, J = 7.5 Hz, 1H, Н6q.), 7.14 (d, J = 8.5 Hz, 1H, Н5ph.), 3.92 (s, 3H, 3-ОСН3), 3.87 (s, 3H, 4-ОСН3). LC-MS: m/z = 299 [M + H]+. Anal. Calcd. for C16H14N2O2S C, 64.41; H, 4.73; N, 9.39; S, 10.75. Found: C, 64.43; H, 4.75; N. 9.37; S, 10.72.
Preparation of 2-R-(quinazolin-4-ylthio)carboxylic acids (3.1–3.8)
An appropriate amount of 2-R-quinazolin-4(3H)-thione (5 mmol) was added to methanol or ethanol (15 ml) with metallic potassium (0.39 g, 10 mmol). After the dissolution, the appropriate halogenocarboxylic acid (5 mmol) was added. The resulting mixture was refluxed for 1–5 h. After cooling to room temperature the hydrochloric acid was added to reach pH 4–5. The crystalline precipitate was filtered and reprecipitated.
(Quinazolin-4-ylthio)acetic acid (3.1)
Orange solid. Yield: 63.6%, m.p. 192–194 °C. IR (cm−1): 3115, 3059, 2982, 2930, 2786, 2427, 2319, 1865, 1697, 1661, 1610, 1571, 1537, 1489, 1469, 1406, 1373, 1320, 1300, 1263, 1235, 1199, 1165, 1114, 1021, 1001, 970, 921, 907, 895, 880, 858, 815, 766, 681, 654. 1H NMR: δ (ppm) 12.90 (br s, 1Н, СООН), 9.04 (s, 1Н, Н2q.), 8.13 (d, J = 7.9 Hz, 1Н, Н5q.), 8.08–7.98 (m, 2Н, Н7, Н8q.), 7.79 (t, J = 7.5 Hz, 1Н, Н6q.), 4.30 (s, 2Н, SСН2). LC-MS: m/z = 221 [M + H]+. Anal. Calcd. for C10H8N2O2S C, 54.53; H, 3.66; N, 12.72; S, 14.56. Found: C, 54.52; H, 3.64; N, 12.75; S, 14.57.
2-Methyl-(quinazolin-4-ylthio)acetic acid (3.2)
Orange solid. Yield: 62.8%, m.p. 178–180 °C. IR (cm−1): 3167, 3110, 3030, 2978, 2917, 2850, 2395, 2312, 1909, 1715, 1673, 1611, 1572, 1553, 1500, 1486, 1466, 1393, 1339, 1291, 1252, 1219, 1173, 1158, 1029, 998, 930, 901, 859, 770, 752, 689, 665, 645, 604. 1H NMR: δ (ppm) 12.87 (br s, 1Н, СООН), 8.08 (d, J = 7.9 Hz, 1Н, Н5q.), 7.89 (d, J = 7.6 Hz, 1Н, Н8q.), 7.97 (t, J = 7.6 Hz, 1Н, Н7q.), 7.68 (t, J = 7.4 Hz, 1Н, Н6q.), 4.19 (s, 2Н, SСН2), 2.68 (s, 3Н, СН3). LC-MS: m/z = 235 [M + H]+. Anal. Calcd. for C11H10N2O2S C, 56.40; H, 4.30; N, 11.96; S, 13.69. Found: C, 56.39; H, 4.31; N, 12.00; S, 13.64.
2-Ethyl-(quinazolin-4-ylthio)acetic acid (3.3)
Yellow solid. Yield: 37.7%, m.p. 175–180 °C. IR (cm−1): 2971, 2928, 2422, 2309, 1916, 1711, 1612, 1567, 1547, 1486, 1468, 1350, 1298, 1260, 1217, 1187, 1164, 1152, 1057, 1028, 988, 904, 889, 856, 763, 706, 668, 642, 624. 1H NMR: δ (ppm) 14.72 (br s, 1Н, СООН), 8.07 (d, J = 8.2 Hz, 1H, Н5q.), 7.98–7.91 (m, 1H, Н7q.), 7.88 (d, J = 8.2 Hz, 1H, Н8q.), 7.66 (t, J = 7.5 Hz, 1H, Н6q.), 4.17 (s, 2H, SСН2), 2.93 (q, J = 7.5 Hz, 2H, СН2СН3), 1.38–1.28 (m, 3H, СН2СН3). LC-MS: m/z = 249 [M + H]+. Anal. Calcd. for C12H12N2O2S C, 58.05; H, 4.87; N, 11.28; S, 12.91. Found: C, 12.91; H, 4.82; N, 11.31; S, 12.93.
2-Phenyl-(quinazolin-4-ylthio)acetic acid (3.4)
Yellow solid. Yield: 59.0%, m.p. 168–170 °C. IR (cm−1): 3117, 3053, 3027, 2953, 2917, 2852, 1667, 1601, 1565, 1540, 1505, 1483, 1469, 1450, 1435, 1345, 1298, 1285, 1253, 1242, 1222, 189, 1147, 1104, 1081, 1030, 944, 926, 889, 858, 812, 760, 699, 687, 659, 619. 1H NMR: δ (ppm) 12.95 (br s, 1Н, СООН), 8.55 (d, J = 8.04 Hz, 2Н, Н2, Н6ph.), 8.13 (d, J = 8.15 Hz, 1Н, Н5q.), 8.03–7.94 (m, 2Н, Н7, Н8q.), 7.71 (t, J = 7.56 Hz, 1Н, Н6q.), 7.58–7.51 (m, 3Н, Н3–5ph.), 4.28 (s, 2Н, SСН2). LC-MS: m/z = 297 [M + H]+. Anal. Calcd. for C15H11N2O2S C, 63.59; H, 3.91; N, 9.89; S, 11.32. Found: C, 63.57; H, 3.94; N, 9.89; S, 11.30.
2-Benzyl-(quinazolin-4-ylthio)acetic acid (3.5)
White solid. Yield: 62.1%, m.p. 175–178 °C. IR (cm−1): 3140, 3089, 3060, 3031, 2972, 2914, 2893, 2871, 1672, 1652, 1619, 1599, 1572, 1508, 1495, 1465, 1453, 1415, 1338, 1319, 1254, 1233, 1183, 1156, 1123, 1073, 1029, 999, 959, 920, 887, 867, 842, 828, 765, 731, 715, 696, 669, 657, 644, 611. LC-MS: m/z = 311 [M + H]+. Anal. Calcd. for C17H14N2O2S C, 65.79; H, 4.55; N, 9.03; S, 10.33. Found: C, 66.01; H, 4.53; N, 9.00; S, 10.35.
2-Phenethyl-(quinazolin-4-ylthio)acetic acid (3.6)
Pink solid. Yield: 15.0%, m.p. 171–173 °C. IR (cm−1): 3028, 2989, 2927, 2858, 2761, 2657, 2499, 1942, 1713, 1614, 1569, 1553, 1488, 1458, 1382, 1358, 1295, 1188, 1162, 1077, 1030, 1011, 998, 937, 907, 887, 861, 788, 775, 761, 740, 698, 655, 625. 1H NMR: δ (ppm) 12.97–12.77 (br s, 1H, СООН), 8.09 (d, J = 8.0 Hz, 1H, Н5q.), 7.95 (t, J = 7.1 Hz, 1H, Н7q.), 7.89 (d, J = 8.2 Hz, 1H, Н8q.), 7.68 (t, J = 6.8 Hz, 1H, Н6q.), 7.30–7.19 (m, 4H, Н2, Н3, Н5, Н6ph.), 7.17 (t, J = 6.0 Hz, 1H, Н4ph.), 4.18 (s, 2H, SСН2), 3.20 (m, 4H, СН2СН2). LC-MS: m/z = 325 [M + H]+. Anal. Calcd. for C18H16N2O2S C, 66.65; H, 4.97; N, 8.64; S, 9.88. Found: C, 66.64; H, 5.00; N, 8.64; S, 9.87.
2-Styryl-(quinazolin-4-ylthio)acetic acid (3.7)
Red solid. Yield: 63.9%, m.p. 208–210 °C. IR (cm−1): 3062, 3027, 2929, 1714, 1671, 1635, 1609, 1580, 1563, 1536, 1485, 1454, 1382, 1347, 1298, 1263, 1204, 1146, 1105, 1072, 1027, 997, 974, 944, 908, 869, 846, 753, 697, 679, 649, 621, 611. 1H NMR: δ (ppm) 12.99 (br s, 1H, СООН), 8.16 (d, J = 7.6 Hz, 1Н, HetСН=), 8.08 (d, J = 8.0 Hz, 1H, Н5q.), 7.98–7.87 (m, 2Н, Н7, Н8q.), 7.80–7.71 (m, 3Н, Н2, Н6ph., Н6q.), 7.53–7.42 (m, 3Н, Н3–5ph.), 7.33 (d, J = 7.6 Hz, 1Н, = СНC6H5), 4.23 (s, 2Н, SСН2). LC-MS: m/z = 323 [M + H]+. Anal. Calcd. for C18H14N2O2S C, 67.06; H, 4.38; N, 8.69; S, 9.95. Found: C, 67.05; H, 4.38; N, 8.72; S, 9.99.
2-Trifluoromethyl-(quinazolin-4-ylthio)acetic acid (3.8)
White solid. Yield: 92.6%, m.p. 240–242 °C. IR (cm−1): 2997, 2920, 2712, 2595, 1712, 1612, 1566, 1488, 1416, 1394, 1381, 1352, 1303, 1264, 1248, 1223, 1187, 1149, 1112, 1026, 1003, 963, 915, 883, 850, 801, 769, 756, 734, 693, 673, 629, 610. 1H NMR: δ (ppm) 12.97 (br s, 1H, СООН), 8.25 (d, J = 8.2 Hz, 1H, Н5q.), 8.13–8.04 (m, 2Н, Н7, Н8q.), 7.90 (t, J = 8.1 Hz, 1Н, Н6q.), 4.23 (s, 2Н, SСН2). LC-MS: m/z = 289 [M + H]+. Anal. Calcd. for C11H7F3N2O2S C, 45.84; H, 2.45; N, 9.72; S, 11.12. Found: C, 45.85; H, 2.43; N, 9.72; S, 11.13.
2-Morpholin-(quinazolin-4-ylthio)acetic acid (3.9)
Yellow solid. Yield: 29.5%, m.p. 120–121 °C. IR (cm−1): 2965, 2926, 2861, 2836, 1624, 1582, 1559, 1513, 1488, 1472, 1440, 1410, 1374, 1360, 1338, 1303, 1260, 1222, 1162, 1151, 1113, 1101, 1070, 1020, 996, 931, 915, 862, 834, 794, 769, 736, 682, 666, 642. 1H NMR: δ (ppm) 12.95 (br s, 1H, СООН), 8.06 (d, J = 8.2 Hz, 1H, Н5q.), 8.00 (d, J = 7.2 Hz, 1H, Н8q.), 7.87 (t, J = 7.5 Hz, 1Н, Н7q.), 7.43 (t, J = 8.0 Hz, 1Н, Н6q.), 4.23 (s, 2Н, SСН2), 4.05 (s, 4Н, N(CH2)2), 3.71 (s, 4Н, O(CH2)2). LC-MS: m/z = 306 [M + H]+. Anal. Calcd. for C14H15N3O3S C, 55.07; H, 4.95; N, 13.76; S, 10.50. Found: C, 55.02; H, 4.96; N, 13.78; S, 10.51.
Preparation of 2-R-(quinazolin-4-ylthio)carboxylic acids amides (4.1–4.9)
An appropriate amount of 2-R-quinazolin-4(3H)-thione (5 mmol) was added to ethanol or methanol (20 ml) with metallic potassium (0.39 g, 10 mmol).
Then the appropriate amide of halogenocarboxylic acid (10 mmol) was added and refluxed for 1–2 h. After cooling to room temperature the mixture was poured into the cold water. The crystalline precipitate was filtered. Substances were recrystallized from a mixture of 2-propanol–water (1:3) or from 2-propanol.
N-Phenyl-2-(quinazolin-4-ylthio)acetamide (4.1)
Yellow solid. Yield: 88.0%, m.p. 121–124 °C. IR (cm−1): 3298, 3194, 3144, 3058, 2954, 2922, 2850, 1662, 1598, 1535, 1497, 1485, 1444, 1421, 1358, 1319, 1279, 1242, 1180, 1164, 1147, 1113, 1079, 1020, 996, 956, 904, 886, 869, 857, 836, 791, 757, 692, 677, 652, 616. 1H NMR: δ (ppm) 10.49 (br s, 1H, NH), 8.99 (s, 1H, Н2q.), 8.19 (d, J = 8.1 Hz, 1H, Н5q.), 8.01 (t, J = 7.4 Hz, 2H, Н7, Н8q.), 7.77 (d, J = 7.4 Hz, 1H, Н6q.), 7.67–7.57 (m, 2H, Н2, Н4ph.), 7.31 (t, J = 7.2 Hz, 2H, Н3, Н5ph.), 7.25–7.19 (m, 1H, Н4ph.), 4.41 (s, 2H, SCH2). LC-MS: m/z = 296 [M + H]+. Anal. Calcd. for C16H13N3OS C, 65.06; H, 4.44; N, 14.23; S, 10.86. Found: C, 65.03; H, 4.45; N, 14.23; S, 10.81.
N-Phenyl-2-(quinazolin-4-ylthio)propionamide (4.2)
Yellow solid. Yield: 80.8%, m.p. 138–140 °C. IR (cm−1): 3237, 3191, 3131, 3061, 3043, 2979, 2928, 2871, 2854, 1658, 1602, 1560, 1545, 1485, 1445, 1376, 1334, 1320, 1294, 1280, 1254, 1229, 1188, 1163, 1089, 1075, 1029, 990, 958, 931, 900, 869, 835, 790, 758, 713, 696, 679, 652. 1H NMR: δ (ppm) 10.47 (br s, 1H, NH), 9.05 (s, 1H, Н2q.), 8.14 (d, J = 8.2 Hz, 1H, Н5q.), 8.06–7.95 (m, 2H, Н7, Н8q.), 7.75 (t, J = 7.4 Hz, 1H, Н6q.), 7.63 (d, J = 8.2 Hz, 2H, Н2, Н6ph.), 7.33 (t, J = 7.5 Hz, 2H, Н3, Н5ph.), 7.08 (t, J = 7.0 Hz, 1H, Н4ph.), 5.06 (q, J = 6.6 Hz, 1H, SСН), 1.69 (d, J = 6.9 Hz, 3H, SСНСН3). LC-MS: m/z = 310 [M + H]+. Anal. Calcd. for C17H15N3OS C, 66.00; H, 4.89; N, 13.58; S, 10.36. Found: C, 66.01; H, 4.87; N, 13.57; S, 10.38.
N-Benzyl-2-(quinazolin-4-ylthio)propionamide (4.3)
Yellow solid. Yield: 74.2%, m.p. 100–160 °C. IR (cm−1): 3320, 3270, 3063, 3032, 2972, 2921, 2853, 1645, 1615, 1597, 1551, 1496, 1483, 1451, 1420, 1396, 1375, 1360, 1331, 1319, 1303, 1278, 1257, 1237, 1219, 1187, 1162, 1150, 1099, 1078, 1045, 1022, 995, 959, 909, 867, 835, 810, 790, 758, 732, 695, 679, 651, 618. 1H NMR: δ (ppm) 8.98 (s, 1H, Н2q.), 8.80 (br s, 1H, NH), 8.12 (d, J = 8.2 Hz, 1H, Н5q.), 8.06–7.95 (m, 2H, Н7, Н8q.), 7.74 (t, J = 7.8 Hz, 1H, Н6q.), 7.38–7.20 (m, 5H, Н2–6ph.), 4.97–4.88 (m, 1H, SСН), 4.33 (d, J = 5.8 Hz, 2H, СН2), 1.63 (d, J = 7.0 Hz, 3H, SСНСН3). LC-MS: m/z = 324 [M + H]+. Anal. Calcd. for C18H17N3OS C, 66.85; H, 5.30; N, 12.99; S, 9.91. Found: C, 66.85; H, 5.32; N, 12.96; S, 9.87.
2-(2-Methyl-quinazolin-4-ylthio)-N-phenylacetamide (4.4)
Yellow solid. Yield: 64.6%, m.p. 178–180 °C. IR (cm−1): 3271, 3189, 3130, 3055, 3030, 2977, 2907, 2849, 1652, 1597, 1548, 1531, 1495, 1479, 1443, 1396, 1382, 1365, 1330, 1318, 1292, 1244, 1208, 1162, 1148, 1075, 1027, 1015, 991, 972, 921, 906, 872, 842, 775, 752, 719, 687, 666, 638. 1H NMR: δ (ppm) 10.43 (br s, 1H, NH), 8.11 (d, J = 8.1 Hz, 1H, Н5q.), 7.95 (t, J = 7.6 Hz, 1H, Н7q.), 7.86 (d, J = 8.2 Hz, 1H, Н8q.), 7.67 (t, J = 7.5 Hz, 1H, Н6q.), 7.61 (d, J = 7.8 Hz, 2H, Н2, Н6ph.), 7.33 (t, J = 7.8 Hz, 2H, Н3, Н5ph.), 7.07 (t, J = 7.3 Hz, 1H, Н4ph.), 4.32 (s, 2H, SСН2), 2.67 (s, 3H, CH3). LC-MS: m/z = 310 [M + H]+. Anal. Calcd. for C17H15N3OS C, 66.00; H, 4.89; N, 13.58; S, 10.36. Found: C, 66.03; H, 4.91; N, 13.64; S, 10.32.
3-(2-Methyl-quinazolin-4-ylthio)-N-phenylpropionamide (4.5)
Yellow solid. Yield: 46.4%, m.p. 199–201 °C. IR (cm−1): 3246, 3186, 3121, 3060, 3023, 2971, 2917, 2849, 1682, 1605, 1546, 1480, 1443, 1370, 1336, 1306, 1282, 1253, 1209, 1175, 1146, 1080, 1029, 993, 965, 921, 906, 866, 846, 787, 753, 695, 667, 640. 1H NMR: δ (ppm) 10.00 (br s, 1H, NH), 8.04 (d, J = 8.2 Hz, 1H, Н5q.), 7.96–7.89 (m, 1H, Н7q.), 7.86 (d, J = 8.3 Hz, 1H, Н8q.), 7.68–7.57 (m, 3H, Н2, Н6ph., Н6q.), 7.30 (t, J = 7.3 Hz, 2H, Н3, Н5ph.), 7.04 (t, J = 7.0 Hz, 1H, Н4ph.), 3.63 (t, J = 6.2 Hz, 2H, SCH2), 2.89 (t, J = 6.2 Hz, 2H, SCH2CH2), 2.70 (s, 3H, СН3). LC-MS: m/z = 324 [M + H]+. Anal. Calcd. for C18H17N3OS C, 66.85; H, 5.30; N, 12.99; S, 9.91. Found: C, 66.84; H, 5.31; N, 13.02; S, 9.94.
2-(2-Methyl-quinazolin-4-ylthio)-N-phenylpropionamide (4.6)
Yellow solid. Yield: 72.4%, m.p. 119–130 °C. IR (cm−1): 3455, 3260, 3199, 3139, 3083, 3030, 2978, 2928, 2869, 1669, 1623, 1600, 1550, 1503, 1482, 1444, 1375, 1339, 1325, 1294, 1251, 1229, 1210, 1186, 1155, 1076, 1057, 1029, 991, 919, 869, 844, 799, 755, 716, 694, 669, 641. 1H NMR: δ (ppm) 10.41 (br s, 1H, NH), 8.06 (d, J = 8.1 Hz, 1H, Н5q.), 7.95 (t, J = 7.9 Hz, 1H, Н7q.), 7.86 (d, J = 8.2 Hz, 1H, Н8q.), 7.72–7.60 (m, 3H, Н6q., Н2, Н6ph.), 7.33 (t, J = 7.8 Hz, 2H, Н3, Н5ph.), 7.08 (t, J = 7.3 Hz, 1H, Н4ph.), 4.93 (q, J = 6.9 Hz, 1H, SCH), 2.68 (s, 3H, CH3), 1.68 (d, J = 6.0 Hz, 3H, SCHCH3). LC-MS: m/z = 368 [M + H]+. Anal. Calcd. for C18H17N3OS C, 66.85; H, 5.30; N, 12.99; S, 9.91. Found: C, 66.83; H, 5.32; N, 13.00; S, 9.91.
2-(2-Morpholin-4-ylmethyl-quinazolin-4-ylthio)-N-phenylacetamide (4.7)
Yellow solid. Yield: 73.5%, m.p. 184–185 °C. IR (cm−1): 3306, 3053, 2964, 2952, 2941, 2921, 2883, 2840, 2826, 1676, 1597, 1561, 1547, 1525, 1497, 1481, 1452, 1439, 1419, 1366, 1353, 1325, 1308, 1284, 1258, 1240, 1189, 1170, 1135, 1112, 1079, 1069, 1030, 1005, 984, 969, 957, 922, 896, 887, 874, 858, 837, 821, 774, 758, 704, 691, 650, 637, 605. 1H NMR: δ (ppm) 10.38 (br s, 1H, NH), 8.15 (d, J = 8.1 Hz, 1H, Н5q.), 8.04–7.92 (m, 2H, Н7, Н8q.), 7.76 (t, J = 7.8 Hz, 1H, Н6q.), 7.61 (d, J = 7.6 Hz, 2H, Н2, Н6ph.), 7.32 (d, J = 6.0 Hz, 2H, Н3, Н5ph.), 7.06 (t, J = 6.3 Hz, 1H, Н4ph.), 4.32 (s, 2H, SCH2), 3.75 (s, 2H, СН2N), 3.40 (s, 4H, N(CH2)2), 2.55 (s, 4H, O(CH2)2). LC-MS: m/z = 395 [M + H]+. Anal. Calcd. for C21H22N4O2S C, 63.94; H, 5.62; N, 14.20; S, 8.13. Found: C, 63.94; H, 5.60; N, 14.27; S, 8.11.
N-(2-Chlorophenyl)-2-(2-methyl-quinazolin-4-ylthio)acetamide (4.8)
Yellow solid. Yield: 85.5%, m.p. 188–190 °C. IR (cm−1): 3329, 2895, 1680, 1611, 1585, 1552, 1530, 1479, 1470, 1456, 1436, 1417, 1380, 1318, 294, 1281, 1255, 1241, 1197, 1149, 1055, 1032, 989, 960, 935, 917, 898, 871, 857, 839, 789, 765, 753, 735, 680, 667, 640. 1H NMR: δ (ppm) 9.89 (br s, 1H, NH), 8.12 (d, J = 8.1 Hz, 1H, Н5q.), 7.97 (t, J = 7.3 Hz, 1H, Н7q.), 7.88 (d, J = 8.3 Hz, 1H, Н8q.), 7.76 (d, J = 7.8 Hz, 1H, Н6ph.), 7.68 (t, J = 7.5 Hz, 1H, Н6q.), 7.50 (d, J = 7.9 Hz, 1H, Н3ph.), 7.34 (t, J = 7.6 Hz, 1H, Н4ph.), 7.20 (t, J = 7.3 Hz, 1H, Н5ph.), 4.39 (s, 2H, SCH2), 2.70 (s, 3H, CH3). LC-MS: m/z = 344 [M + H]+. Anal. Calcd. for C17H14ClN3OS C, 59.39; H, 4.10; N, 12.22; S, 9.33. Found: C, 59.38; H, 4.13; N, 12.21; S, 9.36.
N-(4-Chlorophenyl)-2-(2-methyl-quinazolin-4-ylthio)acetamide (4.9)
Yellow solid. Yield: 52.5%, m.p. 162–164 °C. IR (cm−1): 3264, 3186, 3119, 3054, 2920, 2889, 2850, 1660, 1612, 1594, 1549, 1521, 1491, 1481, 1457, 1400, 1379, 1330, 1293, 1274, 1245, 1207, 1168, 1151, 1094, 1076, 1014, 991, 970, 920, 892, 841, 823, 765, 755, 734, 708, 697, 667, 639. 1H NMR: δ (ppm) 10.57 (br s, 1H, NH), 8.11 (d, J = 8.1 Hz, 1H, Н5q.), 7.96 (t, J = 7.6 Hz, 1H, Н7q.), 7.87 (d, J = 8.2 Hz, 1H, Н8q.), 7.71–7.60 (m, 3H, Н6q., Н2, Н6ph.), 7.39 (t, J = 6.8 Hz, 2H, Н3, Н5ph.), 4.31 (s, 2H, SCH2), 2.62 (s, 3H, CH3). LC-MS: m/z = 344 [M + H]+. Anal. Calcd. for C17H14ClN3OS C, 59.39; H, 4.10; N, 12.22; S, 9.33. Found: C, 58.41; H, 4.11; N, 12.25; S, 9.33.
Docking, scoring and visual inspection of synthesized substances into the enzymes binding site
Flexible molecular docking was carried out using the software package OpenEye, including related utilities: Fred Receptor2.2.5, Vida4.1.1, Flipper, Babel3, Omega2.4.3 and Fred2.2.5Citation19,Citation20. The crystal structures of the enzymes were obtained from the protein data bankCitation21.
The methodology of research consisted of the following steps:
generation of R-, S- and cis-, trans-isomers of ligands (the studied compounds and relevant drugs, program Flipper), which allowed the production of studied compounds’ isomer’s range;
molecular modeling (Hyper Chem 7.5) by generation of the obtained isomeric forms’ 3D-structures – using the method of molecular mechanics (MM +) and semi-empirical quantum mechanical method with Polak–Ribiere algorithm (PM3);
generation of ligands conformations (Omega2.4.3). The number of obtained conformations was not significant due to the further selection of the most optimal conformers using the program Fred2.2.5;
carrying out molecular docking (Fred2.2.5).
Scoring functions (Shapegauss, PLP, Chemgauss2, Chemgauss3, Chemscore, OEChemscore, Screenscore, CGO, CGT, Zapbind, Consensus Score) were obtained as a result of studies, the values of which assess specific characteristics of the ligand–protein complex, indicating the possibility of their matching.
Antimicrobial and antifungal activity in vitro testing
Materials and methods
The in vitro antibacterial activity of all newly synthesized compounds were tested in the Zaporozhye Regional Hospital Bacterial Laboratory against Gram positive bacteria (Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212), Gram negative bacteria (Enterobacter aerogenes 12, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Klebsiella pneumonia 68) and antifungal properties against Candida albicans ATCC 885653. The amount of microbial cells was 1.5 × 108 c.f.u./mL. Incubation period of bacteria was 24 h at 35 °C, yeast – 48–72 h at 28–30 °С. The agar-diffusion method was used for determination of the preliminary antibacterial and antifungal activity. Standard sterilized filter paper discs (6 mm diameter) were impregnated with a solution of the test compound in DMSO (100 µg/disk) and placed it on an agar (Müller–Hinton Broth (Oxoid)) plate seeded with the appropriate test organism in triplicates. DMSO alone was used as a control at the same above-mentioned concentration. Gatifloxacin, Gemifloxacin, Moxifloxacin, Ceftriaxone and Nystatin were used as reference drugs. The results were recorded for each of the tested compound as the average diameter of inhibition zones diameters (IZD) of bacterial or fungal growth around the discs in mm.
Anticancer activity testing
Materials and methods
Primary anticancer assay was performed against human tumor cell lines panel derived from nine neoplastic diseases, in accordance with the protocol of the Drug Evaluation Branch, National Cancer Institute, BethesdaCitation22. The human tumor cell lines of the cancer screening panel were grown in RPMI 1640 medium containing 5% fetal bovine serum and 2 mM l-glutamine. For a typical screening experiment, cells were inoculated in 96-well microtiter plates in 100 mL assay volume, at plating densities ranging from 5000 to 40 000 cell/well. After cell inoculation, the microtiter plates were incubated at 37 °C, under an atmosphere of 5:95 CO2:air (v/v) at 100% relative humidity, for 24 h prior to addition of drugs under assessment. Following drug additions (1 µM), the plates were incubated for an additional 48 h, under the same conditions. Sulforhodamine B (SRB) solution (100 µL, 0–4% w/v in 1% aq. acetic acid) was added to each well and plates were incubated for 10 min at room temperature. The percentage growth was evaluated spectrophotometrically versus controls not treated with test agents.
Results and discussion
Chemistry
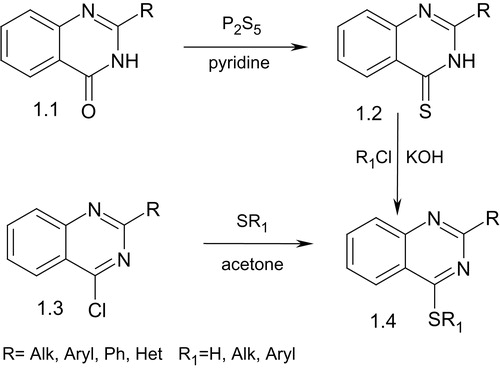
Among known methods of 2-R-quinazolin-4(3H)-thiones (1.2) synthesis are thionation of 2-R-quinazolin-4(3H)-ones (1.1) by phosphorus pentasulfide in acetone in the presence of potassium acetate or the alkylation of 4-chloroquinazoline (1.3) with appropriate thions (Scheme 1)Citation9.
Alkylation of 2-R-quinazolin-4(3H)-thiones (1.2) by the halogenoalkanes in an alkaline medium with the presence of tetrabutylammonium bromide leads to the corresponding S-derivatives (1.4) (Scheme 1)Citation23.
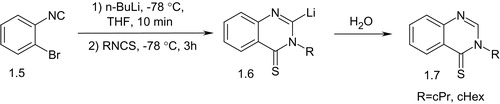
When isothiocyanates were used as electrophiles to react with ortho-bromophenyl isocyanides (1.5), appropriate cyclic 2-R-quinazolin-4(3H)-thiones (1.7) were formed in high yields (Scheme 2)Citation24. The reaction mixture with isothiocyanates was gradually warmed to 40 °C before quenching with water to form intermediate 1.6.
Thus, the variety of the known 2-R-quinazolin-4(3H)-thiones is quite narrow, so synthetic and biological investigation in this field is still novel and promising.
Synthesis of novel 2-R-quinazolin-4(3H)-thiones
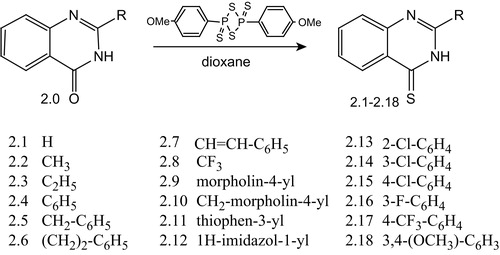
Thionation of 2-R-4(3H)-quinazolones was carried out with the help of a known reagent, namely Lawesson's (2,4-bis(p-methoxyphenyl)-1,3-dithiadiphosphetane-2,4-disulfide)Citation25,Citation26, which reduced the reaction time and increased the yield of the required 2-R-quinazolin-4(3H)-thiones (2.1–2.18) (Scheme 3).
The structure of all synthesized compounds was evaluated by elemental analysis and their spectral data (IR, LC-MS, 1H-NMR spectra).
The data obtained by LC-MS spectra confirmed the purity of all obtained substances, demonstrating their appropriately protonated molecular ions [M + H]+.
In the IR-spectra of compounds 2.1–2.18, the aromatic rings’ stretchings were presented as the peaks of the weak and medium intensity absorption at 1620–1428 cm−1. Furthermore, the existence of aromatic system was confirmed by C–H stretchings at 3025–3148 cm−1. Out-of-plane vibrations of =C–H bond were observed in the range from 871 to 690 cm−1. Methylene groups of 2.3, 2.5, 2.6, 2.9, 2.10, 2.12 were characterized by deformational vibrations at 1465–1456 cm−1, and methyl groups of 2.2, 2.3, 2.18 at 1378–1356 cm−1. Methoxy group of compound 2.18 resonated as two intense stretchings: asymmetric at 1258–1243 cm−1 and symmetric at 1021 cm−1. Signals of aliphatic C–N bond (2.9, 2.10) were presented in the range from 1369 to 1000 cm−1, whereas the C=N bond (2.1–2.18) absorbed approximately at the same frequencies as the C=C bond at 1685 to 1613 cm−1, but broadened and more intensive. The vibration of chlorine substituent of compounds 2.13–2.15 was observed between 1097 and 1027 cm−1, and the vibrations of fluorine substituents (2.16) were observed at 1266 to 1087 cm−1. Multiplet of strong peaks in the range from 1360 to 1103 cm−1 was typical for CF3-group for compounds 2.8 and 2.17. The broadened and very weak absorption at 2571–2489 cm−1 only partially confirmed the presence of SH-groups of compounds, as vibrations overlapped with signals of other groups. The stretching of C=S bond of compound 2.11 was observed at 1250–1015 cm−1.
1H NMR-spectra data characterized substances 2.1–2.18 according to their proposed structures, and quinazoline's signals were found in the next range of ppm: one-proton doublets of H5-quin. and H8-quin. at the 8.74–8.44 and at the 7.94–7.44 respectively; H7-quin. and H6-quin. as one-proton triplets at the 8.10–7.67 and at the 7.76–7.26, and for unsubstituted quinazolin-4-(3H)-thione (2.1) H2-quin. was observed at 8.19 ppm. The one-proton broadened singlet of the NH-group was detected at the 13.98–13.72 ppm, and for compounds 2.8, 2.9 2.13 it was shifted to stronger field – the 12.44 ppm, the 12.88 ppm and the 10.04 ppm, respectively. Signals of phenyl protons were presented at 8.30–7.14 ppm with proper multiplicity. The SCH = CH signal of compound 2.7 was recorded at 7.29 ppm. Aliphatic morpholyl protons for 2.9 and 2.10 were observed in strong field at 3.76–2.53 ppm. The alkyl and methoxy groups were found at 3.92–1.27 ppm.
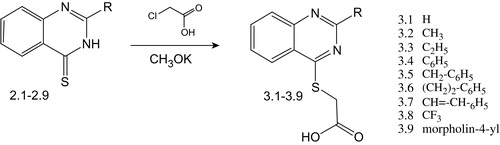
Synthesis of (2-R-quinazolin-4(3H)-ylthio)acetic acids
The (2-R-quinazolin-4(3H)-ylthio)acetic acids (3.1–3.9) were obtained by refluxing the appropriate 2-R-quinazolin-4(3H)-thiones (2.1–2.9) with chloroacetic acid in potassium alkoxide (Scheme 4).
The IR spectra of (2-R-quinazolin-4(3H)-ylthio)acetic acids (3.1–3.9) were characterized by high intensity bands of carbonyl group stretchings at 1715–1624 cm−1, and for compounds 3.1, 3.2 and 3.5 the signal was observed as a doublet. The hydroxy group was characterized by a broad peak at 2964–2914 cm−1 (overlapping with CH vibrations). The weak intensity absorption band at 1635 cm−1 and medium at 1614–1416 cm−1 corresponds to double bonds and aromatic ring of compounds. Aromatic and vinyl CH group vibrations were observed at 3167–2965 cm−1. Methylene groups of 3.1–3.9 had characteristic absorption peak at 1488–1458 cm−1, and methyl groups of 3.2, 3.3 absorbed at 1392–1345 cm−1. The stretching vibrations of =CH group appeared at 3062–3027 cm−1 and out-of-plane vibrations from 908 to 697 cm−1. In compound 3.8, the CF bond had a characteristic multiplet with strong bands in the range from 1352 to 1112 cm−1. The C=N bond was characterized by broadened vibrations of variable intensity at 1667–1610 cm−1.
In the 1H NMR spectra, the substances 3.1–3.9 were characterized by broadened one-proton singlet of carboxylic group proton at 14.72–12.87 ppm. For 3.1, the H2-quin. signal was found at 9.04 ppm. The one-proton doublets of H5-quin. and H8-quin. appeared at 8.25–8.06 and 8.13–7.97 ppm; one-proton signals H7-quin. and H6-quin. were shown as triplets at 8.09–7.89 and 7.90–7.43 ppm, respectively. The signals of phenyl protons were also observed in the low field at 8.55–7.15 ppm. The cis CH=CH fragment of 3.7 was detected at 7.33 and 8.16 ppm, deshielded by substituents. Characteristic two-proton singlet of SCH2-group was detected at 4.30–4.17 ppm. Aliphatic morpholyl protons of 3.9 were observed at 4.05–3.71 ppm as singlets, and protons of alkyl substituents of compounds 3.2–3.3, 3.5–3.6, 3.9 were observed in a strong field at 3.20–1.28 ppm with corresponding multiplicity.
Synthesis of (2-R-quinazolin-4 (3H)-ylthio)carboxylic acids amides
The next step of synthesis was refluxing the 2-R-quinazolin-4(3H)-thiones (2.1, 2.2, 2.10) with halogenocarboxylic acids amides in potassium methylate to obtain the appropriate (2-R-quinazolin-4(3H)-ylthio)carboxylic acids amides (4.1–4.9) (Scheme 5).
In the IR-spectra, the amide NH-proton of compounds 4.1–4.9 was characterized by broadened and medium intensive signal at 3245–3455 cm−1. The carbonyl vibrations were observed at 1682–1645 cm−1. Double bonds of aromatic rings showed medium strength absorption peaks at 1615–1439 cm−1. Aromatic and vinyl CH-groups occurred at 3329–3023 cm−1, and aliphatic CH for compounds 4.2, 4.3, 4.6 at 2979–2853 cm−1. Methylene groups of compounds 4.1, 4.3–4.5, 4.7–4.9 had characteristic absorption peak at 1485–1479 cm−1, and methyl groups of compounds 4.2–4.6, 4.8, 4.9 at 1380–1370 cm−1. The signals of CN-bond appeared in the range from 1353 to 1005 cm−1, and C=N bond at 1682–1611 cm−1. Chlorine substituted phenyls 4.8 and 4.9 had a characteristic valence vibrations at 1094–1032 cm−1.
In the 1H NMR spectra of (2-R-quinazolin-4(3H)-ylthio)carboxylic acids amides (4.1–4.9), NH-proton was shown almost for all amides as broadened one-proton singlet at 10.57–9.89 ppm, except the compound 4.3 (8.80 ppm). The H5 and H8 quin. signals were found at 8.06–7.89 and 8.01–7.90 ppm, H7 and H6 at 8.01–7.90 and 7.77–7.62 ppm; H2 quin. for 4.1–4.3 at 9.05–8.98 ppm. The SCH- of 4.2, 4.3, 4.6 resonated as one-proton quadruplet at 5.06–4.85 ppm. The SCH2-group appeared as a triplet for 4.5 at 3.63 ppm and two-proton singlet at 4.41–4.31 ppm for all other compounds. The signals of phenyl protons were presented at 7.61–7.04 ppm. Aliphatic signals were observed in the strong field (2.89–1.63 ppm).
Pharmacology
Docking studies to Candida albicans dihydrofolate reductase
In continuation of our potential antimicrobials investigation, the dihydrofolate reductase (DHFR) was used as a model to study compounds’ affinity to it. DHFR is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as electron donorCitation27. A variety of drugs act as its inhibitors: the antibiotic trimethoprim and its derivatives, the antimalarial drugs pyrimethamine and proguanil, the chemotherapeutic agent methotrexateCitation28. Thus, we were aimed to perform the docking studies, as an approach to find the molecules with affinity to a specific biological target, namely, synthesized compounds into the active site of DHFR to predict the possible presence of the antimicrobial activity.
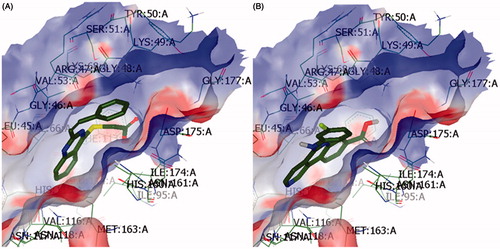
Investigation was conducted by flexible molecular docking using the software package “OpenEye”, including related utilities: Fred Receptor2.2.5, Vida4.1.1, Flipper, Babel3, Omega2.4.3 and Fred2.2.5Citation19,Citation20. The crystal structure of the enzyme DHFR (1AOE.pdb) was obtained from the protein data bankCitation21. The 7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine (GW345) was used as a reference ()Citation27.
Figure 1. Interaction of 2-(2-methyl-quinazolin-4-ylthio)-N-phenylpropionamide (4.6) (A) and reference (7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine) (B) into the DHFR binding site.
![Figure 1. Interaction of 2-(2-methyl-quinazolin-4-ylthio)-N-phenylpropionamide (4.6) (A) and reference (7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine) (B) into the DHFR binding site.](/cms/asset/bf5cfeb7-7302-4cad-8d08-43ee51cbc577/ienz_a_1018243_f0001_c.jpg)
The obtained scoring functions (Shapegauss, PLP, Chemgauss2, Chemgauss3, Chemscore, OEChemscore, Screenscore, CGO, CGT, Zapbind, Consensus Score) indicate the best possibility of the matching into the ligand–protein complex.
The analysis of docking interactions between DHFR and investigated compounds revealed the best results for 2-(2-methylquinazolin-4-ylthio)-N-phenylacetamide (4.6) (). Also, the Consensus Score of 2-(2-morpholin-4-ylmethylquinazolin-4-ylthio)-N-phenylacetamide (4.7) was at the same level as the reference ().
Table 1. The obtained scoring functions of the investigated compounds and (7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine) into DHFR binding site.
By generalizing obtained results, amides had better affinity to DHFR. The more planar substituents the molecules had the better was the affinity. Introduction of simple alkyl radicals worsens it. Therefore, to prove the presence of antimicrobial activity, the next step of investigation was in vitro screening.
Antimicrobial and antifungal activity study
All the newly synthesized compounds were evaluated for in vitro antibacterial activity against Gram positive bacteria (Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212), Gram negative bacteria (Enterobacter aerogenes 12, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Klebsiella pneumonia 68) and antifungal properties against Candida albicans ATCC 885653 ().
Table 2. Microorganisms’ growth inhibition zones (in mm).
As a result, it was found that Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa were insensitive to all the synthesized compounds. Moreover, compounds 2.5–2.7, 2.12–2.14, 2.16–2.18, 3.1–3.9, 4.1–4.9 had no antimicrobial and antifungal effects on all studied microorganisms.
Substances 2.2–2.4, 2.9–2.11, 2.15 had the most significant influence on the growth of C. albicans, showing the antifungal activity. 2-Methylquinazolin-4-(3H)-thione (2.2) possessed the best results of inhibition zone diameter, 23 mm, exceeding 21 mm of Nystatin (). The growth of S. aureus and E. aeruginosa were slightly suppressed by compounds 2.1, 2.4, 2.8 and 2.9. Interestingly, the most active among them appeared to be unsubstituted quinazolin-4(3H)-thione (2.1), which delayed their growth at 15 and 11 mm, respectively. The latter one (2.1) and 2-phenylquinazolin-4(3H)-thione (2.4) also moderately inhibited the growth of K. pneumonia at 11 and 10 mm. But, unfortunately, they did not suppress the level of any of the references even at a concentration of 100 µg/disk.
Hence, the studies have shown that the most active antimicrobial and antifungal compounds were: unsubstituted (2.1), 2-methyl-(2.2) and 2-phenylquinazolin-4(3H)-thione (2.4).
Structure–antimicrobial activity correlation
Some regularity in the appearance of biological activity could be traced. The introduction of larger substituent in the second position of quinazolin-4(3H)-thione gives a significant weakening of the antibacterial action against S. aureus, E. faecalis, K. pneumoniae and results in a moderate antifungal action against C. albicans. The modification of forth position with carboxylic acids and amides residues surprisingly leads to the disappearance of any antimicrobial activity. Analyzing the molecular docking results, there was no correlation between affinity to DHFR and antimicrobial data. Thus, we could suppose that synthesized compounds had other mechanism of antimicrobial activity.
Docking studies of CK2 kinase
Most interesting target for anticancer investigations is protein kinase CK2 (casein kinase II), a constitutively active serine/threonine kinase, that is involved in a variety of roles essential to the maintenance of cellular homeostasis. It regulates multiple processes that play important roles in the sensitivity of cancer to epidermal growth factor receptor targeting therapeutics, including PI3K-Akt-mTOR signaling, Hsp90 activity and inhibition of apoptosisCitation27. Hence, we conducted docking studies of synthesized substances into the active site of protein kinase CK2 (3NSZ)Citation21. Silmitasertib (CX-4945) was used as the reference ()Citation29.
Figure 2. Interaction of 2-styryl-(quinazolin-4-ylthio)acetic acid (3.7) (A) and Silmitasertib (CX-4945) (B) into the binding site of protein kinase CK2.
According to docking results, 11 substances had better Consensus Scores than Silmitasertib. Among them, the best two were 2-styryl-(quinazolin-4-ylthio)acetic acid (3.7) and N-(4-chlorophenyl)-2-(2-methyl-quinazolin-4-ylthio)acetamide (4.9) with Consensus Scores three times less than that of Silmitasertib, representing their anticancer potential (, ).
Table 3. The obtained scoring functions of the investigated compounds and Silmitasertib into CK2 kinase binding site.
Thus, introduction of the amide residues again facilitates substances affinity. So, substances structures were sent to the National Cancer Institute (NCI) to be in vitro investigated for anticancer properties.
Preliminary in vitro anticancer testing
The activity of the compounds was measured according to a value of 100 that meant no growth inhibition. A value of 30 would mean 70% growth inhibition. A value of 0 meant no net growth over the course of the experiment. A value of −30 would mean 70% lethality. A value of −100 meant all cells were dead ().
Table 4. Percentage of in vitro tumor cell lines growth with investigated substances in 10 µM.
Hence, 2-trifluoromethyl-(quinazolin-4-ylthio)acetic acid (3.8) was the most active among all, inhibiting growth of UO-31 of renal cancer to 17.01% and A498 to 54.81%. While it had promoted the growth of CNS cancer SF-268 line, leukemia CAKI-1 and SR lines, and ovarian cancer OVCAR-3. The best anticancer compound against leukemia CCRF-CEM cell line appeared to be 2-(3,4-dimethoxyphenyl)-3H-quinazoline-4-thione (2.18) with 18.75% of cancer line growth. 2-Benzyl-3H-quinazoline-4-thione (2.5) was practically ineffective against all cancer cell lines, except the light inhibition of UO-31 and A498 of renal cancer with 75.05 and 80.24%.
2-Trifluoromethyl-3H-quinazoline-4-thione (2.8) also showed light anticancer effect against ovarian IGROV1 to 71.97% and renal cancer CAKI-1 to 73.23%, still promoting the growth of ovarian OVCAR-3 and prostate DU-145 cancer lines. Therefore, investigated substances have not undergone the predetermined threshold inhibition criteria to be progressed to the next 5-dose screen.
Structure–anticancer activity correlation
The best anticancer modification was synthesis of 2-(3,4-dimethoxyphenyl)-substituted 3H-quinazoline-4-thione, because introduction of carboxylic acid fragment and the halogen not only increased the anticancer properties, but procancer as well. Comparing docking results with in vitro one, substances 2.5, 2.8 and 2.18 had low affinity to CK2 kinase. So, their anticancer activity mechanism should be of other inhibition pathway.
Conclusions
A number of novel 2-alkyl(aryl)-quinazolin-4-thiones, 2-R-(quinazolin-4-ylthio)carboxylic acids and amides were synthesized and characterized by their structure, in silico DHFR and CK2 affinity and in vitro biological activity. The antibacterial and antifungal screening against S. aureus, E. faecalis, E. aerogenes, P. aeruginosa, E. coli, K. pneumonia and C. albicans has found the most active unsubstituted (2.1), 2-methyl-(2.2) and 2-phenylquinazolin-4(3H)-thione (2.4). The modification of forth position with carboxylic acids and amides residues resulted in disappearance of any antimicrobial activity. The NCI anticancer study revealed 2-trifluoromethyl-(quinazolin-4-ylthio)acetic acid (3.8) and 2-(3,4-dimethoxyphenyl)-3H-quinazoline-4-thione (2.18) to have anticancer properties against renal cancer UO-31 and leukemia CCRF-CEM cell lines. Comparison of the in silico molecular docking for DHFR and CK2 kinase inhibition and in vitro biological activities speculatively had proven other antimicrobial and anticancer action mechanisms for synthesized substances. Thus, library of the antimicrobial and anticancer substances among 2-R-quinazolin-4(3H)-thione derivatives was enlarged to be used for future effective drug modifications. And, taking into the account their other non-investigated biological activities, studies of the novel synthesized compounds will be continued.
Supplementary material available online.
Supplementary Information
GENZ_1018243_Supplementary_data.pdf
Download PDF (765.6 KB)Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
The authors gratefully acknowledge “Enamine Ltd.” (Kiev, Ukraine) for financial support of this work.
References
- Zheng Y, Bian M, Deng X-Q, et al. Synthesis and anticonvulsant activity evaluation of 5-phenyl-[1,2,4]triazolo[4,3-c]quinazolin-3-amines. Arch Pharm 2013;346:119–26
- Lee NK, Lee JW, LEE S404-904 J Apt., et al. SK Chemicals Co., Ltd.; Leadgenex Inc; Industry Academic Cooperation Foundation of Kyunghee University, Eur Pat EP 1844023 A1, 2007
- Wu J, Bai S, Yue M, et al. Synthesis and insecticidal activity of 6,8-dichloroquinazoline derivatives containing a sulfide substructure. Chem Pap 2014;68:969–75
- Nomoto T, Ohno K. Studies on cardiotonic agents. VII. Potent cardiotonic agent KF15232 with myofibrillar Ca2+ sensitizing effect. Chem Pharm Bull 1991;39:900–10
- Thornton TJ, Jones TR, Jackman AL, et al. Quinazoline antifolates inhibiting thymidylate synthase: 4-thio-substituted analogs. J Med Chem 1991;34:978–84
- Patrick A, Green TP, Hennequin LF, et al. Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J Med Chem 2004;47:871–87
- Sanchez AI, Martinez-Barrasa V, Burgos C, et al. Synthesis and evaluation of quinazoline derivatives as phosphodiesterase 7 inhibitors. Bioorg Med Chem 2013;21:2370–8
- Kune JI, Jaroslav B, Pour M, et al. Quinazoline derivatives with antitubercular activity. Farmaco 2000;55:725–9
- Xu G-F, Song B-A, Bhadury PS, et al. Synthesis and antifungal activity of novel s-substituted 6-fluoro-4-alkyl(aryl)thioquinazoline derivatives. Bioorg Med Chem 2007;15:3768–74
- Shalaby AA, El-Khamry AMA, Shiba A, et al. Synthesis and antifungal activity of some new quinazoline and benzoxazinone derivatives. Arch Pharm 2000;333:365–72
- Shiba SH, El-Rhamry AA, Shaban ME, Atia KS. Synthesis and antimicrobial activity of some bis-quinazoline derivatives. Pharmazie 1997;52:189–94
- Abdel-Rahman HM, El-Koussi NA, Hassan HY. Fluorinated 1,2,4-triazolo[1,5-a]pyrimidine-6-carboxylic acid derivatives as antimycobacterial agents. Arch Pharm 2009;342:94–9
- Yu Z, Shi G, Sun Q, et al. Design, synthesis and in vitro antibacterial/antifungal evaluation of novel 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7(1-piperazinyl)quinoline-3-carboxylic acid derivatives. Eur J Med Chem 2009;44:4726–33
- Saravanan G, Alagarsamy V, Prakash CR. Synthesis, analgesic, anti-inflammatory and in vitro antimicrobial activities of some novel isoxazole coupled quinazolin-4(3H)-one derivatives. Med Chem Res 2013;22:340–50
- El-Azab AS, Al-Omar MA, Abdel-Aziz Al A-M, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur J Med Chem 2010;45:4188–98
- Alanazi AM, Abdel-Aziz Al A-M, Al-Suwaidan IA, et al. Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Eur J Med Chem 2014;79:446–54
- Kumar S, Agrawal R. Next generation tyrosine kinase inhibitor (TKI): Afatinib. Recent Pat Anticancer Drug Discov 2014;9:382–93
- Tomisek AJ, Christensen BT. Quinazolines. VI. Syntheses of certain 2-methyl-4-substituted quinazolines. J Am Chem Soc 1948;70:2423–5
- Alvarez J, Shoichet B. Virtual screening in drug discovery. Boca Raton, FL: CRC Press, Taylor & Francis; 2005
- Fred Receptor2.2.5, Vida4.1.1, Flipper, Babel3, Omega2.4.3 and Fred2.2.5: OpenEye Sci. Soft Inc Santa Fe, NM, USA, 2011. Available from: http://www.eyesopen.com
- Protein Data Bank, pdb. Available from: http://www.pdb.org
- Boyd MR. The NCI in vitro anticancer drug discovery screen: concept, implementation and operation. Totowa, NJ: Humana Press; 1997
- Kunes J, Bazant J, Pour M, et al. Quinazoline derivatives with antitubercular activity. Farmaco 2000;55:725–9
- Lygin AV, Meijere A. Ortho-lithiophenyl isocyanide: a versatile precursor for 3H-quinazolin-4-ones and 3H-quinazolin-4-thiones. Org Lett 2009;11:389–92
- Kovalenko SM, Vlasov SV, Silin OV, Chernykh VP. Recyclization of 2-imino-2H-1-benzopyrans under the action of nucleophilic reagents: the novel approach for 2-(coumarin-3-yl)-3H-quinazolin-4-thiones. J Sulf Chem 2009;30:53–63
- Salah SI, Ali MA-H, Yassien G, et al. Synthesis and biological evaluation of some new fused quinazoline derivatives. J Chem Res 1997:154–5
- Whitlow W, Howard AJ, Stewart D, et al. X-ray crystallographic studies of Candida albicans dihydrofolate reductase. High resolution structures of the holoenzyme and an inhibited ternary complex. J Biol Chem 1997;272:30289–98
- Hawser S, Lociuro S, Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol 2006;71:941–8
- Bliesath J, Huser N, Omoric M, et al. Combined inhibition of EGFR and CK2 augments the attenuation of PI3K-Akt-mTOR signaling and the killing of cancer cells. Cancer Lett 2012;322:113–18