Abstract
This paper is a review of the work of my former academic group of research in the past 15 years, in the field of cognition enhancers (also called nootropics) that identified two very potent molecules: Unifiram and Sunifiram that for a variety of reasons were not protected by a patent. Some 12 years after their disclosure (2000) I casually found that on the web, there were dozens of sites offering Unifiram and Sunifiram as drugs that improve cognition in healthy individuals even if only few preclinical studies were done and their long-term toxicity was unknown.
Introduction: the back story
Three contextual aspects of this report deserve introduction before relaying the rest of the information; one is the initial laboratory approach, two is the laboratory findings, and the third is the ‘market’ inquiry.
Summary of lab findings: In 2000, my research group published two papers where two new compounds endowed with outstanding nootropic properties were described: Unifiram (DM232) and Sunifiram (DM235). Each presented a potency some 30 000 times higher than that of piracetam, the most representative of piracetam-like nootropics ().
‘Market’ inquiry: In March 2012, I received the following e-mail. In an effort to protect the individual who contacted me, I will refer to him as John Doe.
From: John Doe
Sent: Wednesday march 21,2012
Subject: DM235 human testing
Hello, my name is John Doe
I have read many papers on DM235 and other Nootropics. Currently I am a user of Oxiracetam to combat a premature aging syndrome, which causes me severe brain fatigue, loss of memory, etc. I am 34 years old
Before Oxiracetam, I used Piracetam, but after two years it was too weak to help my mitochondria, so recently I switched to Oxiracetam which works far better! Still Oxiracetam is not good enough, so I was happy to see the invention of DM235.
I have found 6 suppliers of DM235 who can provide 1000 mg sample amount for cheap-$88USD to $200 USD.
In the next few days I will place an order for 1000 mg DM235 so that I can test it on myself. I wonder, am I the first human to try it? Do you have any information about human use?
If I receive the sample and ingest it, I will email you details of my experience as a human Guinea Pig.
I weight 145 lbs and plan to take 1–5 mg total dose, but my digital scale is only accurate to 100 mg so I will need to dilute a solution or guess the dose from a few crystals.
Do you have any recommendations? Thanks.
Definition and approach
Definition and evaluation of cognition enhancing activity: For those who are not familiar with the topic, cognition enhancers are drugs that should restore normal conditions in individuals that have cognitive dysfunctions due to ageing or to age-related neurodegenerative diseases like Alzheimer’s, Parkinson’s and other neurological disorders. The word nootropics is generally used for piracetam like compounds. Both terms have also been used by street people to indicate drugs that stimulate normal mind, in this case, smart drugs, memory enhancers, and brain boosters, for their ability to produce positive effects on mental performance, are more frequently used.
The inherent complexity of neurological disorders, the uncertainty of animal models, and some unresolved questions in preclinical and clinical tests make the evaluation of nootropic activity a still difficult task, both in animals and in humans, for which satisfactory solutions have yet to be found for either.
One of the most used but much criticized assay is the mouse passive avoidance described by Jarvik and KoppCitation1. In our research, we used it (slightly modified) as a preliminary test to rank all our new compounds. In short, mice receive a shock when entering a dark room during a training session and remember it in the following day’s session unless their memory is impaired by the amnestic drugs. The parameter measured is the entry latency time (expressed in seconds) occurring between the time the mouse is placed in the light and the time it enters the dark room. In the first day, there is the training session, while in the second day, the mice are placed again in the light and the new latency time is measured on animals treated or untreated with the nootropic drug; comparison of the latency times of saline treated animals with those that received both the amnestic drug and the investigated one gives a measure of the cognitive activity of the compound tested. The nootropic activity is expressed as minimal effective dose (MED) usually in mg/kg.
The potency of the most active molecules that we have synthesized and evaluated is reported in Schemes 1–3. It is fair to say that the structure–activity relationships (SAR) that can be observed have to be considered with caution, as it is well known that, in in vivo experiments, the pharmacokinetic properties of any molecule may affect the amount of it that reaches the target and exert its action, making questionable the building of reliable SAR. After the initial experiments, any compounds that were considered more interesting were evaluated also in the Mondadori social learning testCitation2 and in the rat Morris water mazeCitation3, since we share the opinion that the conclusion on the nootropic activity of a drug should not rely on the results of one kind of test.
Detailed lab findings
Unifi nootropics
Medicinal chemistry
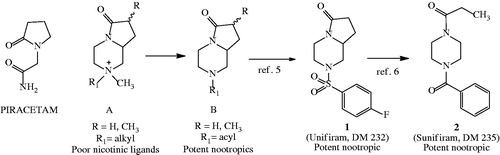
The first molecule identified was Unifiram (1). While studying the nicotinic activity of a series of bicyclic compounds (1,4-diazabicyclo[4.3.0]nonan-9-ones) such as ACitation4 that were in fact poor nicotinic agonists, it was clear that they shared the pyrrolidin-2-one nucleus with piracetam, one of the most studied and available nootropics.
We reasoned that proper modification of the structure to allow brain tissue to penetrate could result in new nootropic compounds. Therefore, we eliminated the charge on the NCitation4 nitrogen of piperazine, transforming it into an amide with different acid moieties (general structure B) and evaluated the compounds using the mouse passive avoidance test, obtaining very promising results. Thereafter, we optimized the lead to compound 1 that was active as nootropic at the dose of 0.001 mg/kg i.p., some thousand times lower than that of piracetam, 30 mg/kg, under the same conditionsCitation5. We named the molecule Unifiram from the acronym that identifies the University of Florence on the web. The compounds of this family present also some features of another class of nootropics, the ampakines, with the consequence that our compounds are considered on one hand, ampakines the other hand, they are piracetam-like, which creates confusion.
Schemes 1–3 illustrate the most productive chemical modulations that we have performed on Unifiram: they were aimed to (i) establish SAR, (ii) find more potent molecules, and (iii) help identification of the mechanism of action.
One of the first achievements of a classical chemical modulation was the discovery that opening the pyrrolidinone ring gave a new family of compounds among which 2 completely maintained the nootropic activity of Unifiram (Scheme 1). The compound was named Sunifiram, where “S” stands for seco [6].
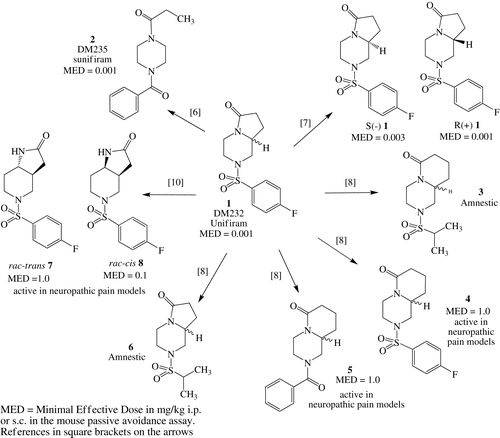
In Scheme 1, the main modulations made on Unifiram molecule are reported; the racemate has been resolved, finding that there is a modest enantioselectivity in favor of R-(+)-UnifiramCitation7. Then the consequences of pyrrolidinone ring expansion were evaluated and resulted in less potent nootropics; however, one of them, 3, showed an unexpected amnestic activity and, at the dose of 10 mg/kg, had the same effect of scopolamine. The same happened when the 4-F-benzenesulfonyl moiety of Unifiram was changed to isopropylsulfonyl to give 6Citation8. The use of isopropylsulfonic acid was suggested by its presence in a series of positive allosteric modulators of AMPA receptor that were active as cognition enhancersCitation9.
In the same series of expanded ring compounds, 4 and 5 were much less effective as nootropic (MED value =1 mg/kg) but presented interesting activity in neuropathic painCitation8.
Finally, the effect of extruding the nitrogen atom of piperazine involved in the pirrolidinone cycle was evaluated (that is, the piperazine moiety was substituted with a 4-aminopiperidine one). The rac-trans (7) and rac-cis (8) forms were prepared; the last one is the most active, showing good nootropic activity (MED value = 0.1 mg/kg). Both compounds were found active in two models of chronic neuropathic pain, originated from antitumor therapy and diabetes; in the latter model, rac-7 was found to be three times more active than rac-8. We resolved the racemates of both 7 and 8; enantioselectivity was not observed for the nootropic activity, while the antihyperalgesic activity was mainly due to the (+) forms: (+)-8 (oxaliplatin-induced neuropathy) or (+)-7 (streptozotocin-induced neuropathy)Citation10.
These modulations produced good (MED value = 0.1 mg/kg) or very good (MED value = 0.01 mg/kg) nootropics, however, only 2 (MED value = 0.001 mg/kg) reached the outstanding activity of 1.
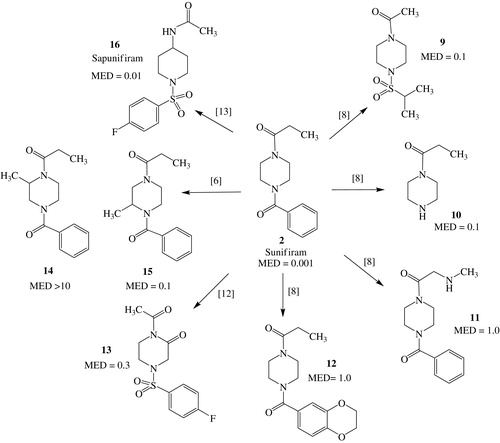
Similar modulation of Sunifiram (2, DM235) is reported in Scheme 2. Many acids were used to substitute the benzoyl and propionyl moieties of Sunifiram, with mixed results on nootropic activity. Compound 9, where the propionyl and benzoyl groups were substituted by acetyl and isopropylsulfonyl moieties, respectively, although still quite potent (MED value = 0.1 mg/kg), does not reach the potency of 2. The isopropylsulfonyl group had worked well in another family of nootropicsCitation9. In this instance, it was demonstrated, for the first time, that one of the nitrogen atoms of piperazine can remain unsubstituted (and, therefore, basic) as shown by the good activity of 108, a possible metabolite of 2. However, when we tried to exploit this finding by introducing an amino group in the chain of the propionate moiety, the result was disappointing as shown by the modest activity of 11 (MED value = 1.0 mg/kg)8. Since our compounds share also some features of ampakinesCitation11, we tried to hybridize the two families using acid moieties present in the ampakines but the results were not satisfactory as shown by the modest MED value of 12 (1.0 mg/kg)8.
Also the insertion of a carbonyl function into the piperazine cycle was not efficacious, since 13 (MED value = 0.3 mg/kg) is far from the potency of the parent compoundCitation12. The effects of alkyl substitution on the piperazine ring were also studied: positions 2 and 3 are clearly different as a compound substituted in position 2 (14 MED value > 10 mg/kg) is practically inactive whereas that substituted in position 3 (15, MED value = 0.1 mg/kg) maintains a good activityCitation6. The best result in this series was obtained when we substituted piperazine moiety with a 4-aminopiperidine one. In fact, compound 16, that has been named Sapunifiram, shows a very good activity (MED = 0.01 mg/kg)Citation13.
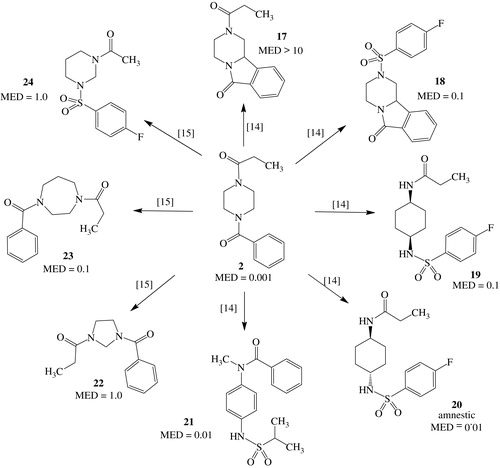
Further chemical modulations of 2 are reported: we transformed Sunifiram (2) in a less flexible tricyclic compound carrying the same number of heavy atoms, just linking the ortho position of the benzoyl group with position 2 of piperazine nucleus and the result was an inactive compound 17 (MED value>10 mg/kg) (Scheme 3). However, if the NCitation4 substituent of piperazine moiety was changed, as in 18, the compound showed good activity (MED value = 0.1 mg/kg) even if it did not reach the potency of parent drugCitation14. So compound 18 would represent a less flexible model useful to study the interaction of Sunifiram with its target.
Then, we tested the consequences of extruding both nitrogen atoms of piperazine and used both isomers of 1,4-cyclohexanediamine as a scaffold for new derivatives. The cis isomer gave nootropics like 19 with fairly good activity (MED value = 0.1 mg/kg), while the trans isomer gave, again unexpectedly, potent amnestic drugs, such as 20 (MED value = 0.01 mg/kg).
Playing the same game of scaffold hopping, we shifted to a 1,4-diaminobenzene scaffold obtaining some very interesting compounds like 21 (MED value = 0.01 mg/kg)Citation14. Also in the case of Sunifiram, we tried to change the size of the cycleCitation15: while reduction of the size-impaired nootropic activity (22, MED value = 1.0 mg/kg), its enlargement maintains a good activity (23, MED value = 0.1 mg/kg). Finally changing the relative position of the nitrogen atoms of piperazine was definitely detrimental for activity (24, MED value = 1.0 mg/kg).
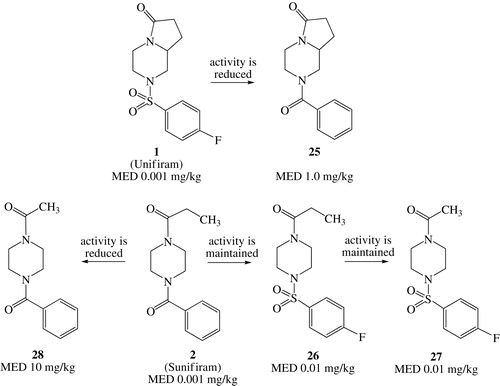
As it can be seen from these data, at certain point of our research we dedicated ourselves to make changes on the scaffold, while keeping the propionyl/benzoyl or acetyl/4-fluorobenzenesulfonyl substituents. The reason was that some structural modification gave puzzling results; some, not exhaustive, examples, are shown in Scheme 4. When decorating the diazabicyclo[4.3.0]nonan-9-one scaffold of Unifiram (MED value = 0.001 mg/kg), the benzoyl group, which conferred high potency to Sunifiram (MED value = 0.001 mg/kg), gave a large drop in activity (25, MED value = 1.0 mg/kg). On the contrary, the substitution of the benzoyl group with the 4-fluorobenzenesulfonyl moiety on the piperazine scaffold gave only a small reduction in activity (from 2, MED value = 0.001 mg/kg, to 26, MED value = 0.01 mg/kg). Changing the propionyl group on 26 with an acetyl moiety maintained activity (27, ME value = D 0.01 mg/kg); the same modification on 2 gave a large drop in potency (28, MED value = 10 mg/kg).
Scheme 4. Effect on nootropic activity of benzoyl and 4-F-benzenesulfonyl groups on the scaffolds of Unifiram and Sunifiram.
From the data reported in these schemes, it can be realized that SAR are inconsistent, supporting the hypothesis that pharmacokinetic properties of each molecule play a major role on the level of nootropic activity found. In this case, Unifiram and Sunifiram would represent the best compromise between the binding to the target and their pharmacokinetic properties. More reliable information for optimization would require the knowledge of the biological target to perform in vitro studies, such as binding, that at the moment are not possible.
Pharmacology
The most potent compounds of the series, Unifiram and Sunifiram, were selected for further studies aimed to find their pharmacological properties and possibly their mechanism of action. Summarizing the results reported in our papers, where the interested scientists are addressed for details in methods and proceduresCitation5–8,Citation10,Citation12–18, the following were the main findings:
Unifiram and Sunifiram antagonized memory disruption is produced not only by the muscarinic antagonist scopolamine but also by mecamylamine (nicotinic antagonist), baclofen (GABA agonist), clonidine (alpha-2 agonist), and NBQX (AMPA antagonist)Citation16,Citation18. The MEDs varied according to the amnestic drug and administration way.
Unifiram and Sunifiram at the dose of 0.001 mg/kg i.p. were able to revert the memory impairment induced by scopolamine (mouse passive avoidance test); when administered alone they were able to ameliorate unimpaired memory processes (procognitive effect) at higher dosesCitation14.
Unifiram and Sunifiram at the dose of 0.1 mg/kg i.p. were able to prevent amnesia induced by scopolamine in the rat Morris water maze; however, when administered alone, they were inactive. (lack of procognitive effect)Citation16,Citation17.
Unifiram and Sunifiram at the dose of 0.1 mg/kg i.p. were active in the rat social-learning test (procognitive effect)Citation6,Citation16.
Unifiram and Sunifiram at the dose of 0.1 mg/kg i.p. significantly reduced the total sleeping time induced by pentobarbital in miceCitation16,Citation17.
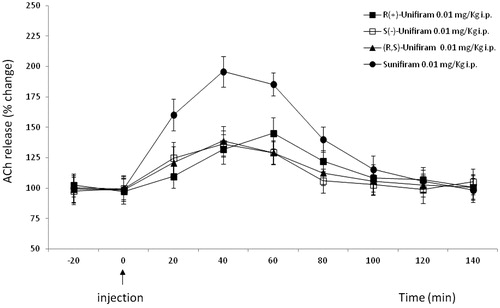
Unifiram slightly increased the release of ACh from parietal cortex in freely moving rats while Sunifiram was definitely more efficient. As a consequence of cholinergic potentiation, Unifiram and Sunifiram show analgesic activity in the mouse hot-plate test, where they increase the pain threshold after i.p. administration in a dose-dependent manner, but with a bell-shaped curveCitation6. The enantiomers of Unifiram showed some enantioselectivity also in this test: while both (S)-(−)-1 and (R)-(+)-1 at the dose of 0.01 mg/kg showed a similar efficacy (), (R)-(+)-1 was able to increase ACh release also at 0.001 mg/kg7.
Unifiram and Sunifiram elicited their nootropic effect without changing animal’s gross behaviour or modifying motor coordination as revealed by the Rotarod test (mouse, 1–10 mg/kg i.p.). The spontaneous motility and the inspection activity were unmodified by administration of the studied compounds (mouse and rat, 1 mg/kg i.p) as revealed by Animex and Hole Board testing in comparison with saline-treated mice.
Unifiram and Sunifiram, tested within the NIMH Psychoactive Drug Screening Program by the lab of Dr Brian Roth (https://pdspdb.unc.edu/pdspWeb/), did not reveal any affinity for the most important CNS receptors, channels, and transporters (see Table 1 in Supplemental information).
The results of experiments with NBQX in the kynurenate test seem to suggest that AMPA receptors are involved in the anti-amnesic activity of Unifiram and Sunifiram. Indeed Unifiram increases AMPA current in rat CA1 slices (EC50 value = 27 ± 6 nM). However, when the compounds were tested on recombinant AMPA (GluR1/GluR2) receptors, no potentiation of the AMPA-R responses was observed (unpublished results).
Rat adipocytes were chosen as model to study in vitro the pharmacological activity of the compounds. Sunifiram increases NO production in rat adipocyte, with a maximal effect (2.75-fold increase over basal) at 17.5 nM, and then it decreased (bell-shaped curve). This effect was completely prevented by 100 μM l-NAME (Nω-nitro-l-arginine methyl ester, a NOS inhibitor), and it was antagonized by mecamylamine and by methyllycaconitine (10−7 M), indicating the involvement of α7 nicotinic receptor activationCitation19. However, no agonist effect or potentiation of ACh stimulation was detected when Sunifiram was tested on rat recombinant α7 nicotinic receptor in Xenopus oocytes (unpublished results). In addition, Sunifiram was not able to revert l-NAME-induced amnesia in the mouse passive avoidance test (unpublished results).
There is an evident pharmacological similarity between our series of compounds and piracetam-like nootropics, the main difference being the potency of Unifiram and Sunifiram: some 30 000 times higher than piracetam and more than 1000 times that of the most potent piracetam-like drugs such as oxiracetam, nefiracetam, etiracetam, and aniracetam. Moreover, our compounds seem to share the lack of toxicity of piracetam-like nootropics. However, as far as chemical structure is concerned, Unifiram has, in common with piracetam-like compounds, the 2-pyrrolidinone ring; in the equipotent Sunifiram, this feature does not seems necessary for nootropic activity.
The problem of the mechanism of action
The different families of cognition enhancers have been reviewedCitation20–26. As far as piracetam-like nootropics are concerned, the lack of a common mechanism of action has been one of the main obstacles to their acceptance as drugs. This is a problem also for Sunifiram and Unifiram. On one hand, the high potency against scopolamine and the release of AChCitation6 would suggest a cholinergic mechanism of action but we were unable to evidence any interaction, orthosteric or allosteric, with muscarinic and nicotinic cholinergic receptors. On the other hand, it seems clear that Unifiram and Sunifiram can modulate the cholinergic system. However, the mechanism remains unknown even if there are strong indications that a specific target is involved. The finding that Unifiram and Sunifiram can reverse the amnesia induced by NBQX and were able to produce a NBQX sensitive reversal of the kynurenate-induced antagonism in the “kynurenate test” would support the involvement of AMPA receptors even if this is not the only mechanism of actionCitation18.
We hoped that the amnestic activity of compounds like 3, 6, and 20Citation8,Citation14 would help to find a solution, but even for them we found no interaction with known central receptors even if the fact that their amnesing activity was contrasted by Unifiram and Sunifram indicates a precise site of interaction. As a matter of fact when in a series of congeneric compounds agonist and antagonists are found, one can be reasonably sure that there is a specific site of interaction: receptor, enzyme, channel, etc. In conclusion, based on the results of our pharmacological research, unifi nootropics seem slightly active on a variety of biological targets with a kind of pleiotropic behavior.
Other research groups have tried to study the mechanism of action of Unifiram and Sunifiram. In 2004, Naftalin, who studied the antagonism of piracetam and like compounds on the inhibition by barbiturates of glucose transport in human erythrocytes, included Sunifiram in the experiment and found that it was a potent antagonist (Ki value = 26.0 ± 3.0 µM) of glucose transport inhibition and that the level of inhibition correlated with nootropic potencyCitation27. It was still another example of the variety of actions of Sunifiram but very likely does not represent the main mechanism of action.
Very recently, some interesting data were published in two papers by Moriguchi and coworkers. In the first paperCitation28, using olfactory bulbectomized (OBX), mice that show in hippocampus-dependent memory impairment, it was shown that Sunifiram (0.01–1.0 mg/kg p.o.) improves cognitive deficit observed in OBX mice via CaM kinase II and proteine kinase C activation. Sunifiram also restored hippocampal LTP (long-term potentiation) impaired in OBX mice. From these and other experiments, the authors concluded that these data indicate that Sunifiram ameliorates OBX-induced deficits of memory-related behaviors and impaired LTP in the hippocampal CA1 region via stimulation of glycine-binding site of N-methyl-d-aspartate receptor (NMDA-R). In the second paperCitation29, the authors, to address the question whether Sunifiram affects (NMDAR)-dependent synaptic function in the hippocampal CA1 region, studied the effects of Sunifiram on NMDAR-dependent LTP confirming that Sunifiram enhances hippocampal synaptic efficacy via glycine-binding site of NMDA receptor. At the moment, the relation of this result with our finding on AMPA receptors involvement is unclear.
The direct involvement of the NMDA-R, obtained by Moriguchi on animal tissues, needs to be confirmed in an in vitro assay that unequivocally identify the biological target, such as a functional assay on recombinant systems expressing only the NMDA receptor, or binding studies. If it works, the results of Moriguchi et al. could open the way to a better understanding of the pharmacology of nootropics, taking advantage also of the other chemical tools in our hands, i.e., compounds 3, 6, and 20 that act in an opposite way with respect to nootropic Unifiram and Sunifiram, and the rigid analog 18; this would also help further design.
The patents
We rushed to patent our new series of nootropics first in Italy and then in EuropeCitation30 and at the same time, with the assistance of the Notarbartolo & Gervasi studio and a small funding from the University of Florence, we proposed our patent to several pharmaceutical companies, since we did not have an opportunity to extend the preclinical tests, not to speak of moving the compounds to clinical trials. The responses from all the companies contacted were negative with a variety of reasons, the most frequent being that our proposal did not fit their programs or that the company already had a research line in that field. There was also the request of pharmacokinetic, metabolic, and toxicological data that we were not able to produce in our labs. So, since our budget, due to the incumbent financial crisis, was shrinking, we left the patent applications to expire and turned to other research subjects. Ironically, later on, when we had already published our results (under the publish or perish rule) and let to go the patents, we were contacted by a couple of small companies interested in the results of this research; we were invited to contact them again, if and when we identified the biological target and did find new good and patentable compounds. Unfortunately, I retired in 2009 and our last paper on the topic was in 2012.
Is this the end of the story? Not really.
The website
“Clinical trials” online
I confess that for the first time I did not pay due attention to the message of John Doe and I simply answered that we did not perform any pharmacokinetic or toxicity studies on Sunifiram, so I was unable to help him. I also asked my former collaborator Prof. Maria Novella Romanelli who was responsible of the research, to add more details if any. I overlooked two things in the message: (i) he wanted to buy Sunifiram to consume it himself, (ii) he had already found six suppliers of the drug, a thing that was difficult to believe since we were not aware of such an interest for our compound. When I realized that he wanted to consume the drug, I explicitly warned him about the possible consequences of that. Then he informed me that he was going to delay the purchase of Sunifiram to gather more information. However, a few days later, in a message to request a reprint, he wrote that he was going to order 1 g sample of Sunifiram to try it on himself. The day after, I again warned him about the risks that he would face since neither the pharmacokinetic nor toxicology of such experimental compound was known.
A year later, when we had nearly forgotten about this episode, I received a message where John Doe announced that the drug was now available in the US at the online market. Moreover, Sunifiram was also available at low prices and in large quantities from a Chinese manufacturer. We were kind of shocked and a window suddenly opened on a new world when we run at the web to verify. We discovered that Sunifiram was sold by dozens of companies that provided also the documents of its purity (NMR, GC, and HPLC). At that time, Unifiram was neglected since Sunifiram is much simpler to synthesize, but was gaining credit. We realized that Unifiram and Sunifiram were quite popular in the web (in a search by the browser safari in a day of august 2014, they had 13 800 and 39 800 entries, respectively). Moreover, they were well cited in Wikipedia and Sunifiram is actually available for sale on Amazon. We also learned that our molecules are sold side-by-side online with other drugs (including other nootropics) that have passed clinical trials while ours have never been tested on humans. In addition to the usual issues on the efficacy of nootropics and the ethical concerns that arise from their useCitation31,Citation32, non-approved compounds may present toxicity problems, in particular in the long termCitation33.
For us, however, what was most interesting was that people consuming them were discussing their experience in several forum and blogs, which sound like clinical trial reports. Of course, John Doe did not refrain to send its own report to us; it is too long to be inserted here. The following is a tentative synopsis of his message:
He was already used to take nootropics among which piracetam, oxiracetam alone, or together in grams doses.
He had taken 25 mg six times a day for a total of 150 mg of Sunifiram.
From the day he started assumption of Sunifiram “I have not spent one second tired” he says.
The sleep duration was reduced from about 8.5 to about 5 h per night without any “sleep deficit” and no tiredness.
In his mind “Sunifiram = modafinil 2.0.
He reports that among the members of the blogs, there has been a problem but only when the person used Sunifiram plus 5 g of caffeine.
I never heard from him again since this message. The only things I know about him is what he shared in the first e-mail. It is possible that he used a pseudonym as his signature. I did note naïveté in his questions about chemical compound structures and pharmacology. In one of his messages, John Doe says: “your dreams have now come true”, but I disagree. In fact, our dream was to discover a medicine for sick persons and then, if possible, collect funding to support our research.
Traditionally one examines the morale of a story. My thinking is that, if our government had adequately funded our research, if our university paid more attention to the potential of in-house patents (as it is doing now), if at least one company had the foresight to put money on it, and if the pharmaceutical industry were more open to outside research, today we would have, perhaps, more of a medicine and less of a street drug. And, last but not least, we would have likely collected financial gain for our efforts.
Supplementary material available online.
Supplemental Information
GENZ_1021252_Supplementary_Material.pdf
Download PDF (204.7 KB)Acknowledgements
I wish to dedicate this work to my former staff with particular acknowledgement to Serena Scapecchi who departed us too early, and to all the scientists that have contributed to its success.
Medicinal chemists
Staff: Cristina Bellucci, Silvia Dei, Dina Manetti, Maria Novella Romanelli, Serena Scapecchi, and Elisabetta Teodori.
PhD students and post doc: Luca Guandalini, Cecilia Martelli, Elisabetta Martini, Michele Melchiorre, and Simona Pagella.
Chemists: Gianluca Bartolucci and Carlo Bertucci.
Pharmacologists: Alessandro Bartolini, Elisabetta Baldi, Corrado Bucherelli, Nicoletta Galeotti, Carla Ghelardini, Monica Norcini, Anna Pittaluga, Annamaria Pugliese, and Alberto Salvicchi.
Many thanks to my daughter-in-law Montine Blank who revised the paper, fixing my broken English.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
References
- Jarvik ME, Kopp R. An improved one trial passive avoidance learning situation. Psychol Rep 1967;21:221–4
- Mondadori C, Preiswerg G, Jaekel J. Treatment with a GABAB receptor blocker improves the cognitive performance of mice rats and rhesus monkeys. Pharmacol Commun 1992;2:93–7
- Morris RGM. Development of a water–maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47–60
- Manetti D, Borea PA, Ghelardini C, et al. Reduced flexibility hybrids of the nicotinic agonist 1,1-dimethyl-4-acetylpiperazinium iodide and 2-(dimethylamino)methyl-5-methyl cyclopentanone methyiodide. Med Chem Res 1997;7:301–12
- Manetti D, Ghelardini C, Bartolini A, et al. Design, synthesis and preliminary pharmacological evaluation of 1,4-diazabicyclo[4.3.0.]nonan-9-ones as a new class of highly potent nootropic drugs. J Med Chem 2000;43:1969–74
- Manetti D, Ghelardini C, Bartolini A, et al. Molecular simplification of 1,4-diazabicyclo[4.3.0]nonan-9-ones gives piperazine derivatives that maintain high nootropic activity. J Med Chem 2000;43:4499–507
- Martini E, Ghelardini C, Bertucci C, et al. Enantioselective synthesis and preliminary pharmacological evaluation of the enantiomers of unifiram (DM232), a potent cognition-enhancing agent. Med Chem 2005;1:473–80
- Scapecchi S, Martini E, Manetti D, et al. Structure–activity relationship studies on unifiram (DM232) and sunifiram (DM235), two novel and potent cognition enhancing drugs. Bioorg Med Chem 2004;12:71–85
- Zarrinmayen H, Bleakman D, Gates MR, et al. [3H]N-2-(4-(N-benzamido)phenyl)propyl-2-propanesulfonamide. A novel AMPA receptor potentiator and radioligand. J Med Chem 2001;44:302–4
- Martini E, Di Cesare Mannelli L, Bartolucci L, et al. Synthesis and biological evaluation of 3,7-diazabicyclo[4.3.0]nonan-8-one as potential nootropic and analgesic drugs. J Med Chem 2011;54:2512–16
- Yamada KA. Therapeutic potential of positive AMPA receptors modulators in the treatment of neurological disease. Exp Opin Inv Drug 2000;9:765–78
- Martini E, Ghelardini C, Dei S, et al. Design, synthesis and preliminary pharmacological evaluation of new piperidine and piperazine derivatives as cognition enhancers. Bioorg Med Chem 2008;16:1431–43
- Manetti D, Martini E, Ghelardini C, et al. 4-Aminopiperidine derivatives as a new class of potent cognition enhancing drugs. Bioorg Med Chem Lett 2003;13:2303–6
- Martini E, Norcini M, Ghelardini C, et al. Design synthesis and preliminare pharmacological evaluation of new analogues of DM232 (unifiram) and DM235 (sunifiram) as cognition modulator. Bioorg Med Chem 2008;16:10034–47
- Martini E, Salvicchi A, Ghelardini C, et al. Design, synthesis and nootropic activity of new analogues of sunifiram and sapunifiram, two potent cognition enhancers. Bioorg Med Chem 2009;17:7606–814
- Ghelardini C, Galeotti N, Gualtieri F, et al. The novel nootropic compound DM232 (UNIFIRAM) ameliorates memory impairment in mice and rats. Drug Dev Res 2002;56:23–32
- Ghelardini C, Galeotti N, Gualtieri F, et al. DM235 (Sunifiram): a novel nootropic with potential as cognitive enhancer. Naunyn-Schmiedeberg’s Arch Pharmacol 2002;365:419–26
- Galeotti N, Ghelardini C, Bartolini A, et al. AMPA-receptor activation is involved in the antiamnesic effect of DM232 (unifiram) and DM235 (sunifiram). Naunyn-Schmiedeberg’s Arch Pharmacol 2003;368:538–45
- Raimondi L, Ghelardini C, Gualtieri F, et al. DM232 and DM235, two potent cognition-enhancers, increase NO levels in rat adipocytes. Proceedings of Frontiers in CNS and Oncology Medicinal Chemistry, Siena, Italy; 2007 Oct 7–9, p 47
- Goulaiev AH, Senning A. Piracetam and other structurally related nootropics. Brain Res Rev 1994;19:180–222
- Gualtieri F, Manetti D, Romanelli MN, Ghelardini C. Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs. Curr Pharm Des 2002;8:125–38
- Gualtieri F, Guandalini L, Manetti D, et al. Cognition-enhancing drugs in mild cognitive impairment (MCI) and Alzheimer’s disease (AD): an update[1]. Med Chem Rev-Online 2005;2:471–87
- Romanelli MN, Galeotti N, Ghelardini C, et al. Pharmacological characterization of DM232 (unifiram) and DM235 (sunifiram) new potent cognition enhancers. CNS Drug Rev 2006;12:39–53
- Froestl W, Muhs A, Pfeiffer A. Cognitive enhancers (nootropics). Part 1 Drugs interacting with receptors. Update 2014. J Alzheimer’s Dis 2014;41:961–1019
- Froestl W, Muhs A, Pfeiffer A. Cognitive enhancers (nootropics). Part 2 Drugs interacting with enzymes. Update.2014. J Alzheimer’s Dis 2014;42:1–68
- Froestl W, Muhs A, Pfeiffer A. Cognitive enhancers (nootropics). Part 3 Drugs interacting with targets other than receptors and enzymes. Disease modifying drugs. J Alzheimer’s Dis 2014;42:1079–149
- Naftalin RJ, Cunningham P, Afzal-Ahmed I. Piracetam and TRH analogues antagonize inhibition by barbiturates, diazepam, melatonin and galanin of human erythrocyte D-glucose transport. Br J Pharmacol 2004;142:594–608
- Moriguchi S, Tanaka T, Tagashira H, et al. Novel nootropic drug sunifiram improves cognitive deficits via CaM kinase II and protein kinase C activation in olfactory bulbectomized mice. Behav Brain Res 2013;242:150–7
- Moriguchi S, Tanaka T, Narahashi T, Fukunaga K. Novel nootropic drug sunifiram enhances hippocampal synaptic efficacy via glycine-binding site of N-methyl-d-aspartate receptor. Hippocampus 2013;23:942–51
- Bartolini A, Bellucci C, Galeotti N, et al. (University of Florence). Derivatives possessing nootropic activity, preparation and use. EP1118612 (A1), July 25, 2001. Application number: EP20000101165 20000121
- Lanni C, Lenzken SC, Pascale A, et al. Cognition enhancers between treating and doping the mind. Pharmacol Res 2008;57:196–213
- Bostrom N, Sandberg A. Cognitive enhancement: methods, ethics, regulatory challenges. Sci Eng Ethics 2009;15:311–41
- Kluger J. Safety concerns raised over popular wakefulness drug. Time 2009, March 17. Available from: http://content.time.com/time/health/article/0,8599,1885825,00.html [last accessed 27 Feb 2015]