Abstract
Pyruvate kinase isoenzyme M2 (PKM2) is one of the most important control point enzyme in glycolysis pathway. Hence, its inhibitors and activators are currently considered as the potential anticancer agents. The effect of 28 polyphenolic compounds on the enzyme activity was investigated in vitro. Among these compounds, neoeriocitrin, (−)-catechin gallate, fisetin, (±)-taxifolin and (−)-epicatechin have the highest inhibition effect with IC50 value within 0.65–1.33 µM range. Myricetin and quercetin 3-β-d-glucoside exhibited the highest activation effect with 0.51 and 1.34 µM AC50 values, respectively. Twelve of the compounds showed inhibition effect within 7–38 µM range of IC50 value. Sinapinic acid and p-coumaric acid showed an activation effect with 26.2 and 22.2 µM AC50 values, respectively. The results propose that the polyphenolics may be the potential PKM2 inhibitors/activators, and they may be used as lead compounds for the synthesis of new inhibitors or activators of this enzyme.
Introduction
It is a proven fact for years that cancerous tissues produce more glucose than normal cells, and this difference is widely used in diagnosis and treatment of the diseaseCitation1. Metabolic processes in these cells are revised as over-expression of different isoenyzmes of functioning enzymesCitation2. New approaches targeting these enzymes are developed and such studies yielded positive resultsCitation3. Pyruvate kinase isoenzyme M2 (PKM2) is the most significant regulatory enzyme functioning in intracellular control of glucose consumption specific to cells with overgrowth tendency. Inactive form of the enzyme is used for the synthesis of biomolecules required for cell growth from glycolysis intermediate products, while active form enables synthesis of molecules, such as pyruvate and lactate for ATP and cell metastasisCitation4–6. Since inhibition and activation of this enzyme controls intracellular consumption of glucose, it is an excellent target for cancer treatment. It takes place in the literature that both its inhibitors and activators can be used as chemotherapeutic agentsCitation7.
Moreover, some studies point out that glycolysis products, such as lactate and pyruvate are effective in developing drug resistanceCitation3. Hence, as indicated in various studies, inhibitors of this enzyme can be used as combined chemotherapeutic agents to overcome drug resistance in the cell.
Flavonoids are significant sources for pharmacological researches as they are bioactive in a wide area. Some studies have pointed out the regulatory effect of these compounds on some enzyme activities playing critical roles in pathology of cancerCitation8,Citation9. Moreover, they are significant compounds for design of anticancer drugs as they have huge impact on some molecular structures in cancer metabolism with cardinal roles in drug design. PKM2 is one of the primary glycolytic enzymes selected as the target in cancer treatmentCitation10. However, no study on the effect of flavonoids and phenolic acids on this enzyme activity were found in the literature.
This research aims to perform an in-vitro study of the inhibition and activation effect of some flavonoids on PKM2 enzyme activity.
Material and methods
Materials
Recombinant human PKM2 enzyme and other chemicals were purchased from Sigma (Munich, Germany) at the high purity.
Assay of PKM2 activity
The enzyme activity was determined by monitoring the absorbance at 340 nm according to the described methodCitation11. All reactions were 1 mL in volume at room temperature (25 °C). Each measurement was repeated three times. Reaction mixture includes recombinant 50 nano gram enzyme, 50 mM Tris pH 7.5, 5 mM MgCl2, 100 mM KCl, 0.5 mM PEP (Phosphoenol pyruvic acid), 0.6 mM ADP (Adenosine 5′-diphosphate), 180 μM NADH (β-Nicotinamide adenine dinucleotide), 10 μM FBP (D-Fructose 1,6-bisphosphate) and 8 units of lactate dehydrogenase (LDH). One unit of pyruvate kinase activity is designated as the amount of enzyme involved to produce 1.0 µM of pyruvate from PEP in one minute.
In vitro effect of polyphenolic compounds
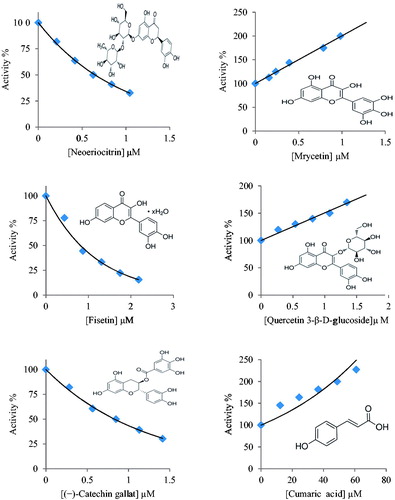
The AC50/IC50 values as the compound concentration that brought about a 50% raise/reduction in enzyme activity under comparable assay and laboratory conditions were calculated from compound dose response curves (for example ). To determine the potential effect of polyphenolic, the initial velocity of the PKM2 reaction was measured at saturating substrate concentrations of phosphoenolpyruvic acid (PEP) and ADP with at least five different concentrations of the compounds. For each PKM2 activity assay the experiment was repeated three times.
Figure 1. Activity (%) versus polyphenols concentrations regression analysis graphs for PKM2 in the presence of some polyphenols.
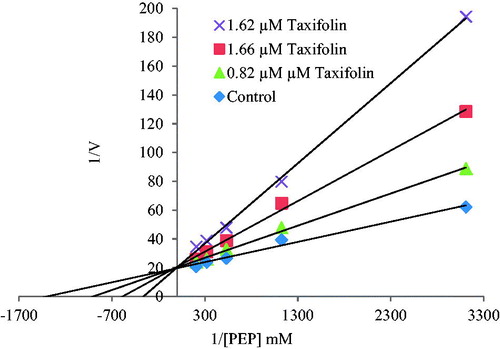
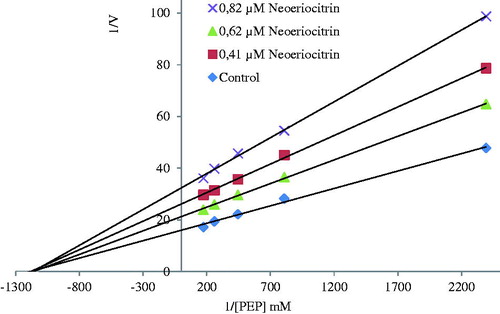
In order to determine Ki constants in the media with inhibitor, substrate (PEP) concentrations were 0.027, 0.048, 0.070, 0.104 and 0.140 mM, respectively. Inhibitor (polyphenolic compounds) solutions were added to the reaction medium, three different inhibitor concentrations were used in 1 mL of total reaction volume. The Lineweaver–Burk graphs were plotted by using 1/V versus 1/[S] values, and Ki constant were calculated from these graphs ( and ). Graphs were plotted using Microsoft Office Excel 2010 (Redmond, WA).
Results
The effect of 28 polyphenol compounds on PKM2 enzyme activity was tested. Concentration activity % graphs were drawn to identify the effects of compounds and IC50 and AC50 values were calculated using the graph. Results are given in . Compounds exhibited different activities on the enzyme. In general terms, flavonols had an inhibition effect of approximately 1 µM IC50 value. Among the flavanones, hesperitin, hesperidin, neohesperidin and naringin showed inhibition effect with IC50 value within 14.20–29.10 µM range, while neoeriocitrin had an inhibition effect at a very low concentration of 0.65 µM. Naringenin is the only flavanone to show activation effect. As seen in the table, phenolic acids showed higher concentrations of inhibition effect compared to flavonoids. IC50 for rutin, galangin, quercetin and kaempferol flavonol compounds is calculated to be approximately 8.5 ± 1.5. Fisetin showed inhibition effect with IC50 value under 1 µM. Myricetin and quercetin 3-β-d-glucoside showed activation effect at very low concentration.
Table 1. PKM2 inhibition or activation data by some polyphenol compounds.
The Lineweaver–Burk graphs were drawn to find out Ki values and inhibition types for compounds with IC50 value under 5 µM (). According to graph results, while (±)-taxifolin perform competitive inhibition on enzyme activity with Ki constant around 0.84 ± 0.25 µM, neoeriocitrin, (−)-catechin gallate, fisetin and (−)-epicatechin showed non-competitive inhibition within 1.99 ± 1.14–4.08 ± 2.27 µM range.
Table 2. Ki values obtained from the Lineweaver–Burk graphs for PKM2 in the presence of three fixed inhibitors and five substrate concentrations for different polyphenol compounds.
Discussion
Cancer is one of the leading diseases threatening the humanity globallyCitation12. Due to the toxic effects of existing treatment methods, various studies are carried to develop new treatment methods with less toxic effects. Cancer cells consume extreme amounts of glucose compared to normal cells. Isoenzymes over-synthesized in cancer cells and functioning in glycolysis pathway, a significant metabolic pathway of glucose, are the new targets in cancer treatment. PKM2 is the most important control point enzyme where glucose consumption is controlled. Thus, it is stated in the literature that inhibitors and activators of the enzyme can be used as drugsCitation13.
Natural compounds, especially the phenolic compounds, are significant sources for pharmacological researches in the discovery of active drug substancesCitation14–16. Due to their structural properties, phenolic compounds can have non-covalent interactions with proteins. The polyphenols were reported as the inhibitors of many enzymes, such as glucosidases, P-glycoprotein and xanthine oxidase in low micromolar rangesCitation17–19. Similarly, the recent studies showed that the carbonic anhydrase isoenzymes were significantly inhibited by natural phenolic compounds and their derivativesCitation20–24. In this study, the effects of some phenolic compounds on PKM2 activity were investigated, and their impact order was determined as flavanonol > flavonol > flavanon > phenolic acids. Flavonoids were generally found to be more active compounds compared to the phenolic acids.
The highest inhibition effect was measured with neoeriocitrin, fisetin and (−)-catechin gallate under 1 µM IC50 value. Moreover, (−)-epicatechin and (±)-taxifolin showed inhibition effect at the value of approximately 1 µM. Flavonoids exhibited high inhibitory effect on enzyme activity having hydroxyl group substitution at position 3′,4′. These groups are considered to be effective in the interaction between compounds and proteins. Given the enzyme kinetics of these compounds, (±)-taxifolin and neoeriocitrin inhibit the enzyme by binding to the active site where the enzyme catalyzes PEP, while other compounds bind to the different amino acid residues outside the active site for inhibition.
Flavonols, such as quercetin, galangin and kaempferol that include 3, 5, 7 OH groups showed a similar inhibition effect. These compounds can interact with the enzyme in these OH groupsCitation25,Citation26. In contrast to the inhibition effect of quercetin, its analogues, myricetinin and quercetin 3-β-d-glucoside showed activation effect with 0.5 and 1.34 µM AC50 values, respectively. However, quercetin 3-d-galactoside, with d-galactose at its three positions, did not show any effect on PKM2 enzyme activity within 27–107 µM range.
It is considered that flavonoids can be potential inhibitors or activators in low concentrations. The changes in substituted groups result in significant differences in activity; therefore, flavonoids can be used as lead compounds in synthesis and design of the inhibitors and activators of this enzyme.
Phenolic acids exhibited different effects within a wide range. Substituents over the benzene ring and acrylic functional groups play an important role in activity differences. Inhibition effect of phenolic acids containing acryl group, such as shikimic acid and 3,4-dihydroxybenzoic acid, is higher than those containing carboxylic acid, such as gallic acid and caffeic acid. Comparison of the effects of syringic-ferulic acid and shikimic-caffeic acid reveals that methoxy groups improve the inhibition effect of the compounds. The change over benzene ring of p-coumaric acid and sinapinic acids did not significantly change the activation effect of the compound.
Drug resistance is an important problem in cancer treatmentCitation27. The PKM2 enzyme inhibition causes a reduction in the amount of metabolites, such as pyruvate and lactate, which involves in drug resistance. Therefore, the compounds inhibiting the enzyme can be used as combined chemotherapeutic agentsCitation28. Moreover, the lactate participates in the formation of micro-acidic environment around the cell, and contributes to metabolic symbiosis as a significant factor in permanence of the other cancer cellsCitation29. Reduction of the lactate amount is a new therapeutic target for cancer treatment. These metabolic processes can be prevented by PKM2 enzyme inhibitors.
Biological, pharmacological and drug properties of polyphenols are widely studied in previous studiesCitation9. It is reported that these compounds affect metabolic pathways and show anticancer activity with action mechanisms, such as inhibition of protein, DNA and RNA synthesis, reduction in cAMP level, and protein kinase and topoisomerase enzyme inhibition in cell metabolism. Likewise, PKM2 enzyme is considered as a potential target in cancer treatment since its inhibition or activation significantly affects the glucose consumption. Therefore, we propose that the inhibition and activation effect of these compounds on PKM2 may have a contribution to their anticancer property.
In conclusion, the present in vitro study reports the effects of some flavonoids and phenolic acids on PKM2 enzyme. A substantial part of these compounds showed inhibition effect, while some compounds exhibited powerful activation effect. These compounds can be considered as potential inhibitors or activators due to their significant effects on the enzyme activity. The results contribute to the understanding of anticancer action mechanisms of these compounds. Further studies might be performed on the effect of new phenolic compounds on the enzyme activity, and additional active compounds may be synthesized using these compounds.
Declaration of interest
This research was financed by TUBITAK (Project No: 112T781).
References
- Goel A, Mathupala SP, Pedersen PL. Glucose metabolism in cancer: evidence that demethylation events play a role in activating type II hexokinase gene expression. J Biol Chem 2003;278:15333–40
- Locasale JW, Cantley LC. Altered metabolism in cancer. BMC Biol 2010;8:88
- Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 2013;4:e532
- Harris I, McCracken S, Mak TW. PKM2: a gatekeeper between growth and survival. Cell Res 2012;22:447–9
- Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 2011;43:969–80
- Spoden GA, Rostek U, Lechner S, et al. Pyruvate kinase isoenzyme M2 is a glycolytic sensor differentially regulating cell proliferation, cell size and apoptotic cell death dependent on glucose supply. Exp Cell Res 2009;315:2765–74
- Mazurek S, Hugo F, Zwerschke W. PKM2 (pyruvate kinase isoenzyme type M2). Atlas Genet Cytogenet Oncol Haematol 2009;13:276–81
- Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 2011;5:1–12
- Ravishankar D, Rajora AK, Greco F, Osborn HMI. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol 2013;45:2821–31
- Ganapathy-Kanniappan S, Geschwind JFH. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 2013;12:152
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008;452:230–3
- Munoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: current perspectives and future directions. Cell Death Dis 2012;3:e248
- Wu S, Le H. Dual roles of PKM2 in cancer metabolism. Acta Biochim Biophys Sinica 2013;45:27–35
- Orlikova B, Legrand N, Panning J, et al. Anti-inflammatory and anticancer drugs from nature. Cancer Treat Res 2014;159:123–43
- Khazir J, Mir BA, Pilcher L, Riley DL. Role of plants in anticancer drug discovery. Phytochem Lett 2014;7:173–81
- Mushtaq M, Wani SM. Polyphenols and human health – a review. Int J Pharma BioSci 2013;4:B338–60
- Kumar S, Narwal S, Kumar V, Prakash O. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev 2011;5:19–29
- Bansal T, Jaggi M, Khar R, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. Int J Pharm Pharm Sci 2009;12:46–78
- Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem 1999;63:1787–90
- Ekinci D, Karagoz L, Ekinci D, et al. Carbonic anhydrase inhibitors: in vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J Enzyme Inhib Med Chem 2014;28:283–8
- Balaydin HT, Durdaği S, Ekinci D, et al. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012;27:467–75
- Koz Ö, Ekinci D, Perrone A, et al. Analysis of saponins and phenolic compounds as inhibitors of α-carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2014;28:412–17
- Ekinci D, Cavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73
- Durdagi S, Entürk M, Ekinci D, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–9
- Sun F, Zheng XY, Ye J, et al. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer 2012;64:599–606
- Adhami VM, Syed DN, Khan N, Mukhtar H. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol 2012;84:1277–81
- Damia G, Garattini S. The pharmacological point of view of resistance to therapy in tumors. Cancer Treat Rev 2014;40:909–16
- Sullivan EJ, Kurtoglu M, Brenneman R, et al. Targeting cisplatin-resistant human tumor cells with metabolic inhibitors. Cancer Chemother Pharmacol 2014;73:417–27
- Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013;12:2723–32