Abstract
New derivatives of two isomeric types of azaphenothiazines, 1,8- and 2,7-diazaphenothiazine, containing the triple bond substituents and additionally tertiary cyclic and acyclic amine groups, were synthesized and tested for their anticancer activity. The compounds exhibited differential inhibitory activities. Better results were obtained when the acetylenic group was transformed via the Mannich reaction to the dialkylaminobutynyl groups. The most active was 2,7-diazaphenothiazine with the N-methylpiperazine-2-butynyl substituent against the human ductal breast epithelial tumor cell line T47D, more potent than cisplatin. The 2,7-diazaphenothiazine system turned out to be more active than isomeric 1,8-diaza one. For the most active compound, the expression of TP53, CDKN1A, BCL-2 and BAX genes was detected by the RT-QPCR method. The gene expression ratio BACL-2/BAX suggests the mitochondrial apoptosis in T47D cells. The synthesis makes possible to obtain many new bioactive phenothiazines with the dialkylaminoalkynyl substituents inserting various tertiary cyclic and acyclic amine moieties to the substituents.
Introduction
Classical tricyclic phenothiazines, representing the dibenzo-1,4-thiazine ring system, attract considerable attention because of their significant biological activities and interesting chemical features. Those with aminoalkyl substituents at the nitrogen atom are valuable drugs exhibiting neuroleptic, antihistaminic, antitussive and antiemetic activitiesCitation1. The molecular scaffold of phenothiazine was very prolific for the structure modification by introduction of new substituents and substitution of one or two benzene rings with homoaromatic and heteroaromatic rings. The substitution with an azine ring leads to formation of azaphenothiazine. Depending on the aromatic rings, new phenothiazines and azaphenothiazines contain not only the tricyclic ring system, but also tetra- and pentacyclic ones with up to four additional nitrogen atoms in the aromatic ringsCitation2–4. Such structure modifications changed potency and type of biological properties exhibiting very promising anticancer, antibacterial, antifungal, anti-inflammatory activities, reversal of multidrug resistanceCitation4–9 and a potential benefit in treatment of Alzheimer’s, Creutzfeldt-Jakob’s and AIDS-associated diseasesCitation10–12.
Both classical and modified phenothiazines exert anticancer and cancer chemopreventive activity, and potential as an adjuvant to chemo- and radiotherapy. These compounds affect cancer cells mainly through cell death and cell-cycle arrest. Their ability to induce apoptosis is well recognized; however, the mechanism behind this property is not clearly understood. Various studies demonstrated that phenothiazines inhibit calmodulin, tubulin and protein kinase C action, P-glycoprotein transport function and recently induce lysosomal dysfunctionCitation13–15.
Most of the classical bioactive phenothiazines contain flexible pharmacophoric dialkylaminopropyl substituents at the thiazine nitrogen atom. As aromatic compounds with the alkynyl groups exhibit wide variety of activities such as anticancerCitation16–23, anti-inflammatoryCitation24, antimicrobialCitation25,Citation26, antiviralCitation27,Citation28, antioxidantCitation29, it was interesting to synthesize and study phenothiazines with more rigid dialkylamino substituents, containing a triple bond linker. We found only one paper in the phenothiazines studies with the dialkylaminoalkynyl substituents exhibiting promising anticancer and multidrug resistance reverting activityCitation30.
Lipophilicity has been considered for a long time as a vital molecular property in the drug design. This parameter plays a crucial role in the transport of compounds through a biological system and may also influence the formation of a complex with a receptor or a biomacromolecule at the site of action and is modeled by partition of a solute between n-octanol and water as logP. The lipophilicity of bioactive compounds can be correlated with many physicochemical, pharmacokinetic and pharmacodynamic drug properties, including biological activity, solubility, permeability, distribution, metabolism, target protein and plasma protein bindingCitation31–35. It may also influence the formation of a complex between a compound and a receptor or a biomacromolecule at the site of action. It was found for anticancer compounds that lipophilicity can be regarded as one of the main properties correlating with cytotoxicityCitation36–38.
The introduction of two pyridine rings instead of the benzene ones in the phenothiazine scaffold leads to various diazaphenothiazines of the dipyrido[1,4]thiazine structure. We synthesized 1,8- and 2,7-diazaphenothiazines with varied alkyl, aryl, heteroaryl, dialkylaminoalkyl, amidoalkyl, sulfonamidoalkyl and “half-mustard” substituents. Some of those compounds exhibited very promising anticancer, immunosuppressant and antioxidant activities, and low toxicityCitation39–42.
In this article, two series of new azaphenothiazines, 1,8- and 2,7-diazaphenothiazines, with more rigid substituents containing a triple bond were synthesized. For those compounds, the anticancer action on the selected tumor cell lines and lipophilic property were investigated. For the most active compound, the expression of TP53, CDKN1A, BCL-2 and BAX genes was detected using the RT-QPCR method.
Methods
Chemistry
Melting points were determined in open capillary tubes on a Boetius melting point apparatus and are uncorrected. The 1H NMR spectra were recorded on a Bruker Fourier 300 and Bruker DRX spectrometers at 300 and 600 MHz in deuteriochloroform with tetramethylsilane as the internal standard. Fast Atom Bombardment mass spectra (FAB MS, in glycerol) were run on a Finnigan MAT 95 spectrometer at 70 eV. The thin layer chromatography was performed on aluminum oxide 60 F254 neutral (type E) (Merck 1.05581) with CHCl3-EtOH (10:1 v/v) as eluents.
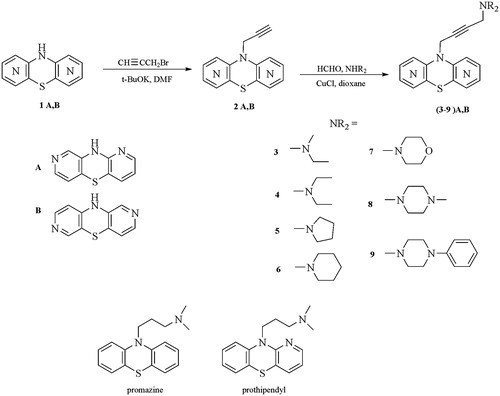
10H-1,8-diazaphenothiazine (1A), 10H-2,7-diazaphenothiazine (1B), 10-propargyl-1,8-diazaphenothiazines (2A) and 10-propargyl-2,7-diazaphenothiazines (2B) were obtained according to the reported procedures in the literatureCitation42–44.
General procedure for synthesis of compounds (3–9)a,B
A mixture of 10-propargyl-diazaphenothiazine 2A or 2B (0.5 mmol), paraformaldehyde (0.5 mmol), amine (0.7 mmol) and cuprous chloride (catalitic amount) in peroxide-free, dry dioxane (10 ml) was heated with continuous stirring at 70–80 °C for 3 h. After cooling 20 ml water was added and mixture was extracted with chloroform, dried with Na2SO4, and evaporated in vacuo. The dry residue was dissolved in CHCl3 and purified by column chromatography (aluminum oxide, CHCl3) to give:
10-[4-(N-ethyl-N-methyl)amino-but-2-ynyl]-1,8-diazaphenothiazine (3A)
(0.116 g, 75%); An oil. 1H NMR (CDCl3) δ: 1.03 (t, J = 7.2 Hz, 3H, CH3), 2.26 (s, 3H, N–CH3), 2.44 (q, J = 7.2 Hz, 2H, N–CH2), 3.35 (s, 2H, CH2), 4.79 (s, 2H, CH2), 6.81 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.95 (d, J = 4.9 Hz, 1H, H6), 7.24 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.07 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.11 (d, J = 4.9 Hz, 1H, H7), 8.39 (s, 1H, H9). FAB MS m/z: 311 (M + 1, 20), 252 (M + 1–C3H5N, 100), 201 (M + 1–C7H12N, 35). Anal. Calcd for: C17H18N4S C 65.78, H 5.84, N 18.05. Found: C 65.58, H 5.79, N 17.98.
10-[4-(N-ethyl-N-methyl)amino-but-2-ynyl)-2,7-diazaphenothiazine (3B)
(0.124 g, 80%); An oil. 1H NMR (CDCl3) δ: 1.08 (t, J = 7.2 Hz, 3H, CH3), 2.31 (s, 3H, N–CH3), 2.49 (q, J = 7.2 Hz, 2H, N–CH2),3.43 (s, 2H, CH2), 4.54 (s, 2H, CH2), 6.99 (d, J = 5.5 Hz, 1H, H-9), 7.04 (d, J = 5.5 Hz, 1H, H-4), 8.14 (s, 1H, H-1), 8.17 (d, J = 5.5 Hz, 1H, H-3), 8.32 (d, J = 5.5 Hz, 1H, H-8), 8.40 (s, 1H, H-6). FAB MS m/z: 311 (M + 1, 100), 252 (M + 1–C3H5N, 18), 201 (M + 1–C7H12N, 55). Anal. Calcd for: C17H18N4S C 65.78, H 5.84, N 18.05. Found: C 65.52, H 5.77, N 17.91.
10-[4-(N,N-diethyl)amino-but-2-ynyl]-1,8-diazaphenothiazine (4A)
(0.134 g, 82%); An oil. 1H NMR (CDCl3) δ: 1.04 (t, J = 7.2 Hz, 6H, 2CH3), 2.50 (q, J = 7.2 Hz, 4H, 2 N–CH2), 3.43 (s, 2H, CH2), 4.78 (s, 2H, CH2), 6.82 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.94 (d, J = 4.9 Hz, 1H, H6), 7.23 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.07 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.11 (d, J = 4.9 Hz, 1H, H7), 8.38 (s, 1H, H9). FAB MS m/z: 325 (M + 1, 15), 252 (M + 1–C4H10N, 100), 201 (M + 1–C8H14N, 20). Anal. Calcd for: C18H20N4S C 66.63, H 6.21, N 17.27. Found: C 66.42, H 6.25, N 17.02.
10-[4-(N,N-diethyl)amino-but-2-ynyl]-2,7-diazaphenothiazine (4B)
(0.126 g, 78%); An oil. 1H NMR (CDCl3) δ: 1.10 (t, J = 6.0 Hz, 6H, 2 CH3), 2.55 (q, 5.32 J = 6.0 Hz, 4H, 2 CH2), 3.52 (s, 2H, CH2), 4.50 (s, 2H, CH2), 6.98 (d, J = 5.5 Hz, 1H, H-9), 7.40 (d, J = 5.5 Hz, 1H, H-4), 8.12 (s, 1H, H-1), 8.17 (d, J = 5.5 Hz, 1H, H-3), 8.32 (d, J = 5.5 Hz, 1H, H-8), 8.39 (s, 1H, H-6). FAB MS m/z: 325 (M + 1, 100), 201 (M + 1–C8H14N, 30). Anal. Calcd for: C18H20N4S C 66.63, H 6.21, N 17.27. Found: C 66.40, H 6.20, N 17.00.
10-(4-Pyrrolidin-1-yl-but-2-ynyl)-1,8-diazaphenothiazine (5A)
(0.129 g, 80%); An oil. 1H NMR (CDCl3) δ: 1.76 (m, 4H, 2CH2), 2.60 (m, 4H, 2CH2),3.41 (s, 2H, CH2), 4.78 (s, 2H, CH2), 6.80 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.94 (d, J = 4.9 Hz, 1H, H6), 7.22 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.05 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.10 (d, J = 4.9 Hz, 1H, H7), 8.37 (s, 1H, H9). FAB MS m/z: 323 (M + 1, 30), 200 (M-C8H12N, 100). Anal. Calcd for: C18H18N4S C, 67.05, H 5.63, N 17.38. Found: C, 66.91, H 5.65, N 17.17.
10-(4-Pyrrolidin-1-yl-but-2-ynyl)-2,7-diazaphenothiazine (5B)
(0.119 g, 74%); An oil. 1H NMR (CDCl3) δ: 1.68 (m, 4H, 2CH2), 2.66 (m, 4H, 2CH2), 3.39 (s, 2H, CH2) 4.57 (s, 2H, CH2), 6.96 (d, J = 5.5 Hz, 1H, H-9), 7.43 (d, J = 5.5 Hz, 1H, H-4), 8.12 (s, 1H, H-1), 8.19 (d, J = 5.5 Hz, 1H, H-3), 8.30 (d, J = 5.5 Hz, 1H, H-8), 8.38 (s, 1H, H-6). FAB MS m/z: 323 (M + 1, 20), 201 (M + 1–C8H12N, 20). Anal. Calcd for: C18H18N4S C, 67.05, H 5.63, N 17.38. Found: C, 66.87, H 5.59, N 17.11.
10-(4-Piperidin-1-yl-but-2-ynyl)- 1,8-diazaphenothiazine (6A)
(0.126 g, 75%); An oil. 1H NMR (CDCl3) δ: 1.34 (m, 2H, CH2), 1.57 (m, 4H, 2CH2), 2.44 (m, 4H, 2CH2), 3.26 (s, 2H, CH2), 4.79 (s, 2H, CH2), 6.79 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.94 (d, J = 4.9 Hz, 1H, H6), 7.22 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.06 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.09 (d, J = 4.9 Hz, 1H, H7), 8.37 (s, 1H, H9). FAB MS m/z: 337 (M + 1, 30), 201 (M + 1–C9H14N, 100). Anal. Calcd for: C19H20N4S C, 67.83, H 5.99, N 16.65. Found: C, 67.59, H 5.92, N 16.40.
10-(4-Piperidin-1-yl-but-2-ynyl)-2,7-diazaphenothiazine (6B)
(0.121 g, 72%); An oil. 1H NMR (CDCl3) δ:1.45 (m, 2H, CH2), 1.70 (m, 4H, 2 CH2), 2.55 (m, 4H, 2CH2),3.40 (s, 2H, CH2), 4.51 (s, 2H, CH2), 6.99 (d, J = 5.5 Hz, 1H, H-9), 7.44 (d, J = 5.5 Hz, 1H, H-4), 8.10 (s, 1H, H-1), 8.15 (d, J = 5.5 Hz, 1H, H-3), 8.31 (d, J = 5.5 Hz, 1H, H-8), 8.33 (s, 1H, H-6). FAB MS m/z: 337 (M + 1, 100), 201 (M + 1–C9H14N, 50). Anal. Calcd for: C19H20N4S C, 67.83, H 5.99, N 16.65. Found: C, 67.55, H 5.90, N 16.45.
10-(4-Morpholin-4-yl-but-2-ynyl)-1,8-diazaphenothiazine (7A)
(0.113 g, 67%); An oil. 1H NMR (CDCl3) δ: 2.54 (m, 4H, 2CH2), 3.29 (s, 2H, CH2), 3.61 (m, 4H, 2CH2), 4.79 (s, 2H, CH2), 6.81(dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.95 (d, J = 4.9 Hz, 1H, H6), 7.23 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.06 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.10 (d, J = 4.9 Hz, 1H, H7), 8.36 (s, 1H, H9). FAB MS m/z: 339 (M + 1, 35), 200 (M-C8H12NO, 100). Anal. Calcd for: C18H18N4SO C 63.88, H 5.36, N 16.56. Found: C 63.72, H 5.41, N 16.39.
10-(4-Morpholin-4-yl-but-2-ynyl)-2,7-diazaphenothiazine (7B)
(0.117 g, 69%); An oil. 1H NMR (CDCl3) δ: 2.52 (m, 4H, 2 CH2), 3.35 (s, 2H, CH2), 3.70 (m, 4H, 2CH2), 4.55 (s, 2H, CH2), 6.90 (d, J = 5.5 Hz, 1H, H-9), 7.41 (d, J = 5.5 Hz, 1H, H-4), 8.12 (s, 1H, H-1), 8.15 (d, J = 5.5 Hz, 1H, H-3), 8.32 (d, J = 5.5 Hz, 1H, H-8), 8.40 (s, 1H, H-6). FAB MS m/z: 339 (M + 1, 40), 201 (M + 1–C8H12NO, 100). Anal. Calcd for: C18H18N4SO C 63.88, H 5.36, N 16.56. Found: C 63.69, H 5.31, N 16.44.
10-[4-(4-Methylpiperazin-1-yl)but-2-ynyl]-1,8-diazaphenothiazine (8A)
(0.121 g, 69%); An oil. 1H NMR (CDCl3) δ: 1.25 (s, 3H, N-CH3), 2.55 (m, 8H, 4CH2), 3.42 (s, 2H, CH2), 4.79 (s, 2H, CH2), 6.81(dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.95 (d, J = 4.9 Hz, 1H, H6), 7.22 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 8.05 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.09 (d, J = 4.9 Hz, 1H, H7), 8.35 (s, 1H, H9). FAB MS m/z: 351 (M + 1, 100), 201 (M + 1–C9H15N2, 35). Anal. Calcd for: C19H21N5S C 64.93, H 6.02, N 19.93. Found: C 64.75, H 5.91 N 19.69.
10-[4-(4-Methylpiperazin-1-yl)but-2-ynyl]-2,7-diazaphenothiazine (8B)
(0.125 g, 71%); An oil. 1H NMR (CDCl3) δ:1.25 (s, 3H, N-CH3), 2.55 (m, 8H, 4 CH2), 3.40 (s, 2H, CH2), 4.52 (s, 2H, CH2), 6.99 (d, J = 5.5 Hz, 1H, H-9), 7.44 (d, J = 5.5 Hz, 1H, H-4), 8.10 (s, 1H, H-1), 8.15 (d, J = 5.5 Hz, 1H, H-3), 8.31 (d, J = 5.5 Hz, 1H, H-8), 8.33 (s, 1H, H-6). FAB MS m/z: 351 (M + 1, 100), 201 (M + 1–C9H15N2, 40). Anal. Calcd for: C19H21N5S C 64.93, H 6.02, N 19.93. Found: C 64.72, H 5.90 N 19.73.
10-[4-(4-Phenylpiperazin-1-yl)but-2-ynyl]-1,8-diazaphenothiazine (9A)
(0.171 g, 83%); An oil. 1H NMR (CDCl3) δ: 2.73 (m, 4H, 2CH2), 3.22 (m, 4H, 2 CH2), 3.41 (s, 2H, CH2), 4.79 (s, 2H, CH2), 6.82 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H, H3), 6.95 (d, J = 4.9 Hz, 1H, H6), 7.23 (m, 6H, HAr + H4), 8.10 (dd, J = 5.1 Hz, J = 1.5 Hz, 1H, H2), 8.11 (d, J = 4.9 Hz, 1H, H7), 8.39 (s, 1H, H9). FAB MS m/z: 414 (M + 1, 100), 201 (M + 1–C13H17N2, 20). Anal. Calcd for: C24H23N5S C 69.71, H 5.61, N 16.94. Found: C 69.55, H 5.54, N 16.79.
10-[4-(4-Phenylpiperazin-1-yl)but-2-ynyl]-2,7-diazaphenothiazine (9B)
(0.167 g, 81%); An oil. 1H NMR (CDCl3) δ: 2.73 (m, 4H, 2 CH2), 3.25 (m, 4H, 2 CH2), 3.42 (s, 2H, CH2), 4.55 (s, 2H, CH2), 6.95 (d, J = 5.5 Hz, 1H, H-9), 7.10 (d, J = 5.5 Hz, 1H,H-4), 7.23–7.32 (m, 5H, HAr), 8.10 (s, 1H, H-1), 8.13 (d, J = 5.5 Hz, 1H, H-3), 8.31 (d, J = 5.5 Hz, 1H, H-8), 8.33 (s, 1H, H-6). FAB MS m/z: 414 (M + 1, 100), 201 (M + 1–C13H17N2, 30). Anal. Calcd for: C24H23N5S C 69.71, H 5.61, N 16.94. Found: C 69.59, H 5.52, N 16.81.
Anticancer effects in vitro
Cell culture
Compounds were evaluated for their anticancer activity using three cultured cell lines: SNB-19 (human glioblastoma, DSMZ – German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), C32 (human amelanotic melanoma, ATCC—American Type Culture Collection, Manassas, VA, USA) and T47D (human ductal breast epithelial tumor cell line, ATCC, Manassas, VA, USA). The cultured cells were kept at 37 °C and 5% CO2. The cells were seeded (1 × 104 cells/well/100 μl DMEM supplemented with 10% FCS and streptomycin and penicillin) using 96-well plates (Corning).
Cell proliferation and viability
In recent years tetrazolium salts have been described, which can be used for the measurement of cell proliferation and viability. The tetrazolium salts are cleaved to formazan by cellular enzymes. An expansion in the number of viable cells results in an increase in the overall activity of mitochondrial dehydrogenases in the sample. This augmentation in enzyme activity leads to an increase in the amount of formazan dye formed, which directly correlates to the number of metabolically active cells in the culture. The formazan dye produced by metabolically active cells is quantified by a scanning ELISA reader by measuring the absorbance of the dye solution at appropriate wavelengths (λ = 420–480 nm with a reference wavelength λ = 600 nm).
WST-1 assay
In this study, the WST-1 assay (Roche Diagnostics, Mannheim, Germany) was used to evaluate the antiproliferative effect of the compounds on the number of cancer cells in cultures. After exposure to tested compounds (at concentrations between 0 and 100 μg/ml) for 72 h, cells were incubated with WST-1 (10 μl) for 1 h. The absorbance of the samples against a background control was read at 450 nm using with a reference wavelength λ = 600 nm a microplate reader. Results are expressed as means of at least two independent experiments performed in triplicate.
The RT-QPCR method
Genes trancriptional activity (TP53, CDKN1A, BCL-2, BAX) was evaluated using real-time RT-QPCR method with OPTICON TM DNA Engine (MJ Research, Watertown, MA) and QuantTect® SYBR® Green RT-PCR Kit (Qiagen, Valencia, CA). Cells were exposed to compound 8B in 0.5 μg/ml concentration for 24 h. The RNA extraction was made by using Quick-RNA™ Kit MiniPrep (ZYMO RESEARCH). Total RNA integrity was analyzed in 1.2% agarose electrophoresis with added ethidium bromide compound. The quantity and purity of the extracted total RNA were determined using spectrophotometric analysis with HP845 (Hewlett Packard, Waldbronn, Germany) spectrophotometer. The statistical analysis was performed using the Statistica 8.0 software (StatSoft, Tulsa, OK). All values were expressed as means ± SE.
Lipophilicity determination
Thin-layer chromatography was performed on 10 cm × 10 cm reversed-phase thin-layer chromatography (RP TLC) plates precoated with silica gel RP-18F254S (Merck, Darmstadt, Germany) with the mobile phase of acetone and aqueous TRIS (tris(hydroxymethyl)aminomethane) buffer pH 7.4 (ionic strength 0.2 M) to meet physiological conditions. The concentration of acetone in the mobile phase ranged from 50% to 85% (v/v) in 5% increments. Diazaphenothiazines (3–9)A and (3–9)B, and the standards I–V (benzamide logP = 0.64Citation45, acetanilide logP = 1.21Citation46, acetophenone logP = 1.58Citation46, 4-bromoacetophenone logP = 2.43Citation45, benzophenone logP = 3.18Citation46) were dissolved in ethanol (2.0 mg/ml) and 2 μl of these solutions were spotted on the plates 10 mm from the bottom edges. Before development of the plates, chromatographic chambers were saturated with the mobile phase for 0.5 h. After development of the plates and drying in a stream of air the chromatograms were observed under UV light at λ = 254 nm. At least three chromatograms were developed for each solute–solvent combination and RF values were averaged. The RM values calculated from experimental RF values using the equation RM = log(1/RF − 1) were linearly dependent on the concentration of acetone.
The RM0 values were obtained by extrapolation to zero acetone concentration by using the equation RM = RM0 + bC, where C is the concentration (%, v/v) of acetone in the mobile phase.
The correlation between the known logP values and the experimental RM0 values for standards I–V gave the calibration equation:
This equation was used for transformation of the RM0 values into the logP values for diazaphenothiazines.
Results and discussion
Chemistry
The parent substrates 10H-1,8-diazaphenothiazine 1A and 10H-2,7-diazaphenothiazine 1B were transformed with propargyl bromide into the prop-2-ynyl derivatives 2A and 2B, and further using the Mannich reaction (with formaldehyde and selected secondary acyclic and cyclic amines) into the 4-dialkylaminobut-2-ynyl derivatives of 1,8- and 2,7-diazaphenothiazines (3–9)A and (3–9)B in good yields (67–83%, Scheme 1). The structures of the new compounds were characterized with the use of 1H NMR and FAB MS spectra and elemental analyses. Two compounds (2A,B) possess the prop-2-ynyl group, 14 compounds possess the dialkylaminobut-2-ynyl groups with the acyclic ((3,4)A,B) and 5-membered and 6-membered cyclic ((5–9)A,B) amine moieties.
Anticancer activity
The activity of two series of compounds (2–9)A and (2–9)B was investigated in vitro using cultured glioblastoma SNB-19, melanoma C-32 and ductal carcinoma T47D cell lines and cisplatin as a reference. To compare the influence of the nitrogen atoms on the anticancer activity classical monoazaphenothiazine drug, prothipendyl, (10-dimethylaminopropyl-1-azaphenothiazine) was also tested.
The tested compounds exhibited different activities against the cell lines (). The T47D cell line was the most resistant for the tested compounds. The derivatives 2A and 2B with the alkynyl group, prop-2-ynyl, (very active in some compoundsCitation17,Citation20,Citation21) were found to be very weak active or inactive (IC50 >50 μg/ml). The transformation of the prop-2-ynyl group into the aminobut-2-ynyl groups led to the compounds with different activities. Most of the dialkylaminobutynyl compounds were more active than the prop-2-ynyl derivatives (). The location of the nitrogen atoms in the tricyclic ring system also influenced the anticancer activity as the 2,7-diazaphenothiazine series was more active than the 1,8-diaza one. The most active was compound 8B with the N-methylpiperazine-2-butynyl group (containing tertiary cyclic amine moiety with two nitrogen atoms) against the T47D cell line. In this case, the activity was even stronger than for cisplatin. The isomeric compound 8A, containing the same substituent, was found unexpectedly inactive. It seems that the kind of the amine group does not influence significantly the tested activity, but rather combination of the effects of the diazaphenothiazine system (the place of the nitrogen atoms) and the substituent. Prothipendyl, containing only one pyridine ring, was less active than the most active diazaphenothiazines against all examined cell lines.
Table 1. Anticancer activities IC50 (μg/ml) of 10-substituted 1,8-diazaphenothiazines (2–9)a and 2,7-diazaphenothiazines (2–9)B.
Apoptosis assay
The cellular stress can change the expression of the TP53 gene encoding the P53 protein, which is known as the “guardian of the genome”. This protein influences cell cycle arrest by changing the expression of CDKN1A gene encoding the P21 protein. The P53 protein also can stimulate the cell to changes in gene expression BCL-2 and BAX involved in mitochondrial pathway apoptosisCitation47–50. Efforts were made to understand the mechanism of anticancer activity for the most active compound 8B with the N-methylpiperazine-2-butynyl group. The compound effect on the transcriptional activity of the TP53 and CDKN1A genes was evaluated after cells incubation by 24 h. The results of analysis of TP53, CDKN1A, BCL-2, BAX genes in T-47D, SNB-19 and C-32 cells after 24 h of treatment are collected in . mRNA copy number of TP53 gene did not shown any significant changes between T47D samples. In SNB-19 cells, compound 8B generated a significant increase in the expression of TP53 but in C-32 cells a reduction of the number of copies mRNA was observed. An increase in the number of CDKN1A copies in T47D and SNB-19 suggests possibility of participating in cell cycle arrest. Analysis of the gene expression ratio BCL-2/BAX in T47 cells showed activation of the mitochondrial apoptosis. Transcriptional activity of these genes in the SNB-19 and C-32 cells suggests a different way of cell death and protective activation.
Table 2. The influence of compound 8B on expression of genes encoding TP53, CDKN1A, BCL-2, BAX in glioblastoma SNB-19, melanoma C-32 and ductal carcinoma T47D cells.
Lipophilicity determination
For lipophilicity determination of diazaphenothiazines (2–9)A,B, a very convenient RP TLC method was usedCitation51–54. The obtained parameter RM0, representing relative lipophilicity (), was transformed in the parameter logP with the use of the calibration curve based on five standards of known logP values. The tested compounds turned out to be low lipophilic with the logP values of 0.92–2.644 at pH = 7.4. Phenothiazines are known as one of the most lipophilic drugs withCitation55,Citation56 the logP values up to 5.9. In comparison with classical phenothiazines, the investigated azaphenothiazines are much less lipophilic mainly due to the presence of additional nitrogen atoms in the ring system. Such a lipophilic property is considered as optimal for achieving appropriate physicochemical characteristics. The latest reports suggested that the compounds with the lipophilic parameter logP < 4 stand a much higher chance of success in achievement of biological target. The optimum region of the lipophilicity lies within a narrow rangeCitation34,Citation35 between 1 and 3. It is worth noting that anticancer and immunosuppressive 1,8-diazaphenothiazines with the alkyl, dialkylaminoalkyl, acetylaminopropyl, methanesulfonylaminopropyl and chloroethylureidopropyl substituents exhibited the logP valuesCitation57 in the range of 0.78–2.60.
Table 3. The RM0, logPTLC values and b (slope) and r (correlation coefficient) of the equation RM = RM0 + bC for diazaphenothiazines (2–9)A,B.
The correlation of the anticancer activities (the IC50 values) of the aminobutynyl derivatives (3–9)A and (3–9)B with lipophilic properties expressed as logP was very weak to moderate with the coefficient r = 0.141–0.625 (). It means that lipophilicity is only one of the parameters influencing the activity.
Table 4. The correlation between the anticancer activities IC50 and the logP values for diazaphenothiazines (2–9)A,B.
Conclusion
New derivatives of two isomeric types of azaphenothiazines, 1,8-diaza- and 2,7-diazaphenothiazine, containing triple bond substituents and additionally tertiary cyclic and acyclic amine groups, were synthesized and tested for their anticancer activity. Our investigations show that the introduction of the slightly acidic acetylenic group into the diazaphenothiazine system did not lead to potent anticancer compounds. Better results were obtained when the acetylenic group was transformed via the Mannich reaction to the dialkylaminobutynyl groups. The most active was the N-methylpiperazine-2-butynyl derivative 8B against the ductal carcinoma T47D cell line, more potent than cisplatin. The 2,7-diazaphenothiazine system turned out to be more active than the 1,8-diaza one. The replacement of the well-known flexible dialkylaminoalkyl substituents in the phenothiazine system with more rigid substituents containing a triple bond and a tertiary acyclic or cyclic amine groups also led to anticancer compounds. Analysis of the gene expression ratio BCL-2/BAX in T47 cells showed activation of the mitochondrial apoptosis. The correlation between anticancer activities and lipophilicity was moderate. As the Mannich reaction enables to insert various tertiary cyclic and acyclic amine moieties to the aminoalkynyl substituents, we think there is a way to obtain many new bioactive phenothiazines with the aminoalkynyl substituents.
Acknowledgements
The work was supported by the Medical University of Silesia (grants KNW-1–073/P/1/0 and KNW-1–023/K/3/0).
Declaration of interest
The authors have declared no conflict of interest.
Part CXLIII in the series of Azinyl Sulfides.
References
- Gupta RR, Kumar M. Synthesis, properties and reactions of phenothiazines. In: Gupta RR, ed. Phenothiazine and 1,4-benzothiazines – chemical and biological aspect. Amsterdam: Elsevier; 1988:1–161
- Silberg IA, Cormos G, Oniciu DC. Retrosynthetic approach to the synthesis of phenothiazines. In: Katritzky AR, ed. Advances in heterocyclic chemistry. New York: Elsevier; 2006:205
- Pluta K, Morak-Młodawska B, Jeleń M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J Heterocycl Chem 2009;46:355–91
- Pluta K, Morak-Młodawska B, Jeleń M. Recent progress in biological activities of synthesized phenothiazines. Eur J Med Chem 2011;46:3179–89
- Motohashi N, Kawase M, Saito S, Sakagami H. Antitumor potential and possible targets of phenothiazine-related compounds. Curr Drug Targets 2000;1:237–45
- Motohashi N, Kawase M, Satoh K, Sakagami H. Cytotoxic potential of phenothiazines. Curr Drug Targets 2006;7:1055–66
- Aaron JJ, Gaye Seye MD, Trajkovska S, Motohashi N. Bioactive phenothiazines and benzo[a]phenothiazines: spectroscopic studies and biological and biomedical properties and applications. Top Heterocycl Chem 2009;16:153–231
- Dasgupta A, Dastridara SG, Shirataki Y, Motohashi N. Antibacterial activity of artificial phenothiazines and isoflavones from plants. Top Heterocycl Chem 2008;15:67–132
- Sadandam YS, Shetty MM, Bhaskar Rao A. 10H-Phenothiazines: a new class of enzyme inhibitors for inflammatory diseases. Eur J Med Chem 2009;44:197–202
- Amaral L, Kristiansen JE. Phenothiazines: potential management of Creutzfeldt-Jacob disease and its variants. Int J Antimicrob Agents 2001;18:411–17
- Viveiros M, Martins M, Couto I, et al. The in vitro activity of phenothiazines against Mycobacterium avium: potential of thioridazine for therapy of the co-infected AIDS patient. In Vivo 2005;19:733–6
- González-Muñoz GC, Arce MP, López B, et al. Acylamino-phenothiazines: neuroprotective agents displaying multifunctional activities for a potential treatment of Alzheimer’s disease. Eur J Med Chem 2011;46:2224–35
- Sudeshna G, Parimal K. Multiple non-psychiatric effects of phenothiazines: a review. Eur J Pharmacol 2010;648:6–14
- Jaszczyszy A, Gąsiorowski K, Świątek P, et al. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep 2012;64:16–23
- Zong Z, Zielinska-Chomej K, Juntti T, et al. Harnessing the lysosome-dependent antitumor activity of phenothiazines in human small cell lung cancer. Cell Death Dis 2014;5:e1111
- Schimler SD, Hall DJ, Debbert SL. Anticancer (hexacarbonyldocobalt)propargyl aryl ethers: synthesis, antiproliferative activity, apoptosis induction, and effct on cellular oxidative stress. J Inorg Biochem 2013;119:28–37
- Kode NG, Phadtare S. Synthesis and cytotoxic activity of some new 2,6-substituted purines. Molecules 2011;16:5840–60
- Boryczka S, Bębenek E, Wietrzyk J, et al. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013;18:4526–43
- Piras S, Carta A, Briguglio I, et al. 2[N-Alkyl(R-phenyl)-aminomethyl]-3-phenyl-7-trifluoromethylquinoxalines as anticancer agents inhibitors of folateenzymes. Eur J Med Chem 2014;75:169–83
- Zilla MK, Nayak D, Vishwakarma RA, et al. A convergent synthesis of alkyneeazide cycloaddition derivatives of 4-a,b-2-propyne podophyllotoxin depicting potent cytotoxic activity. Eur J Med Chem 2014;77:47–55
- Ma K, Liu Y, Zhu Q, et al. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One 2011;6:e20525
- Romagnoli R, Baraldi PG, Cruz-Lopez O, et al. Synthesis of novel antimitotic agents based on 2-amino-3-aroyl-5-(hetero)arylethynyl thiophene derivatives. Bioorg Med Chem Lett 2011;21:2746–51
- Xiao F, Xue Y, Luo Y, et al. Synthesis and cytotoxic activity of 7-alkynyl camptothecin derivatives. Chinese Chem Lett 2009;20:566–8
- Vasilevsky SF, Govdi AI, Shults EE, et al. Efficient synthesis of the first betulonic acid-acetylene hybrids and their hepatoprotective and anti-inflammatory activity. Bioorg Med Chem 2009;17:5164–9
- Al-Kaissi EN, Al-Ghrary NF, Al-Kaisi NK, et al. Synthesis and antimicrobial evaluation of 4,5-diaryl-2-[4-(tamino)-2-butynyl]-2,4-dihydro-3H-1,2,4-triazol-3-ones. Bioorg Med Chem Lett 2012;21:3390–5
- Wube A, Guzman JD, Hüfner A, et al. Synthesis and antibacterial evaluation of a new series of N-alkyl-2-alkynyl/(E)-alkenyl-4-(1H)-quinolones. Molecules 2012;17:8217–40
- Aly YL, Pedersen EB, La Colla P, Lodda R. Synthesis and anti-HIV-1 activity of new MKC-442 analogues with an alkynyl-substituted 6-benzyl group. Arch Pharm 2007;340:225–35
- Cristofoli WA, Wiebe LI, De Clercq E, et al. 5Alkynyl analogs of arabinouridine and 2‘-deoxyuridine: cytostatic activity against herpes simplex virus and varicella-zoster thymidine kinase gene-transfected cells. J Med Chem 2007;50:2851–7
- Bräthe A, Andresen G, Gundersen LL, et al. Antioxidant activity of synthetic cytokinin analogues: 6-alkynyl- and 6-alkenylpurines as novel 15-lipoxygenase inhibitors. Bioorg Med Chem 2002;10:1581–6
- Bisi A, Meli M, Gobbi S, et al. Multidrug resistance reverting activity and antitumor profile of new phenothiazine derivatives. Bioorg Med Chem 2008;16:6474–82
- Rutkowska E, Pająk K, Jóźwiak K. Lipophilicity-methods of determination and its role in medicinal chemistry. Acta Pol Pharm 2013;70:13–18
- Dąbrowska M, Komsta Ł, Krzek J, Kokoszka K. Lipophilic study of eight cephalosporins by reversed-phase thin-layer chromatographic method. Biomed Chromatogr 2015. [Epub ahead of print]. DOI: 10.1002/bmc.3490
- Liu X, Testa B, Fahr A. Lipophilicity and its relationship with passive drug permeation. Pharm Res 2011;28:962–77
- Arnot JA, Planey SL. The influence of lipophilicity in drug discovery and design. Expert Opin Drug Discov 2012;7:863–75
- Waring MJ. Lipophilicity in drug discovery. Expert Opin Drug Discov 2010;5:235–48
- Baguley BC, Finlay GJ. Derivatives of amsacrine: determinants required for high activity against Lewis lung carcinoma. J Natl Cancer Inst 1988;80:195–9
- Facchetti I, Grandi M, Cucchi P, et al. Influence of lipophilicity on cytotoxicity of anthracyclines in LoVo and LoVo/Dx human cell lines. Anticancer Drug Des 1991;6:385–97
- Maliepaard M, de Mol NJ, Janssen LHM, et al. Role of lipophilicity in the in vitro antitumour activity of a series of new mitosene compounds. Anticancer Drug Des 1992;7:415–25
- Zimecki M, Artym J, Kocięba M, et al. The immunosuppressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell Mol Biol Lett 2009;14:622–35
- Pluta K, Jeleń M, Morak-Młodawska B, et al. Anticancer activity of newly synthesized azaphenothiazines from NCI's anticancer screening bank. Pharmacol Rep 2010;62:319–32
- Morak-Młodawska B, Pluta K, Matralis AN, Kourounakis AP. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Arch. Pharm. (Weinheim) 2010;343:268–73
- Morak-Młodawska B, Pluta K, Zimecki M, et al. Synthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazines. Med Chem Res 2015;24:1408–18
- Morak-Młodawska B, Pluta K. Acyl and sulfonyl derivatives of 10-aminoalkyl-2,7-diazaphenothiazines. Heterocycles 2009;78:1289–98
- Morak-Młodawska B, Suwińska K, Pluta K, Jeleń M. 10-(Prop-2-yn-1-yl)-2,7-diazaphenothiazine. Acta Crystallogr Sect E Struct Rep Online 2012;68:o1590–1
- Mannhold R, Cruciani G, Dross K, Rekker R. Multivariate analysis of experimental and computational descriptors of molecular lipophilicity. J Comput Aided Mol Des 1998;12:573–81
- Bodor N, Gabanyi Z, Wong CK. A new method for the estimation of partition coefficient. J Am Chem Soc 1989;111:3783–6
- Hemann MT, Lowe SW. The p53-Bcl-2 connection. Cell Death Differ 2006;13:1256–9
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 2001;276:11615–23
- Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 2003;304:437–44
- Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res 2000;256:50–7
- Völgyi G, Deák K, Válmos J, et al. RPTLC determination of log P of structurally diverse neutral compounds. J Planar Chromatogr 2008;21:143–9
- Vaštag D, Perišić-Janjić N, Tomić J, Petrović S. Evaluation of the lipophilicity and prediction of biological activity of some N-cyclohexyl-N-substituted-2-phenylacetamide derivatives using RP-TLC. J Planar Chromatog 2011;24:435–40
- Casoni D, Sârbu C. Comprehensive evaluation of lipophilicity of biogenic amines and related compounds using different chemically bonded phases and various descriptors. J Sep Sci 2012;35:915–21
- Paszkowska J, Kania B, Wandzik I. Evaluation of the lipophilicity of selected uridine derivatives by use of RP-TLC, shake flask, and computational methods. J Liq Chromatogr Rel Technol 2012;35:1202–12
- Mannhold R, Dross K. Calculation procedures for molecular lipophilicity: a comparative study. Quant Struct-Act Relat 1996;15:403–9
- Franke U, Munk A, Wiese M. Ionisation constants and distribution coefficients of phenothiazines and calcium channel antagonists determined by a pH-metric method and correlation with calculated partition coefficients. J Pharm Sci 1999;88:89–95
- Morak-Młodawska B, Pluta K, Jeleń M. Estimation of the lipophilicity of new anticancer and immunosuppressive 1,8-diazaphenothiazine derivatives. J Chromatogr Sci 2015;53:462–6