Abstract
A novel series of quinazoline compounds (2–14) incorporating biologically active heterocyclic moieties were designed and synthesized. The structure of the newly synthesized compounds was recognized on the basis of elemental analyses, IR, 1H-NMR, 13C-NMR and mass spectral data. All compounds were evaluated for their ability to induce the cytoprotective enzyme NAD(P)H:quinone oxidoreductase 1 (NQO1) using a quantitative bioassay and a docking study was performed in the Kelch domain of Keap1 obtained from the Protein Data Bank (PDB ID: 4IQK) to explore the ability of the synthesized compounds to block the Nrf2-binding site of Keap1. All of the synthesized compounds showed concentration-dependent inducer activity with potencies in the low- or sub-micromolar range. Compound 12 was the most potent inducer in this new series, with a concentration that doubles the specific activity of NQO1 (CD value) of 70 nM. The identification of this compound offers a new chemical scaffold for future development of highly potent inducers.
Keywords:
Introduction
NAD(P)H:quinone oxidoreductase 1 (NQO1) is a cytosolic enzyme that catalyses the obligatory two-electron reduction of many endogenous and environmental quinones using flavin adenine dinucleotide as a cofactorCitation1–3. It is a homodimer and is biochemically distinguished by its prominent ability to use either NADH or NADPH as hydride donors, and by its inhibition by the anticoagulant dicumarolCitation4.
NQO1 is generally considered as a detoxification enzyme due to its ability to metabolize reactive quinones and quinone imines to their less reactive and less toxic hydroquinone forms. NQO1 is a substantially inducible enzyme that is expressed in many tissues, and its expression is regulated by the antioxidant response element (ARE) both in basal and oxidative stress conditionsCitation5. The gene encoding NQO1 contains ARE sequence in the promoter region and is known to be regulated by NF-E2 p45-related factor 2 (Nrf2, gene name Nfe2l2)Citation6. Nrf2 belongs to the basic leucine zipper transcription factor family, a member of the Cap “n” Collar family of transcription factors that binds to the ARE leading to induction of many cytoprotective and antioxidant genes. Under basal conditions, Nrf2 is continuously targeted for ubiquitination and proteasomal degradation, which is primarily mediated by Kelch-like ECH-associated protein 1 (Keap1), a Cullin-3/Rbx1 ubiquitin ligase substrate adaptor proteinCitation7,Citation8. Keap1 has specific highly reactive cysteine residues which serve as sensors for small molecule oxidants and electrophilesCitation9,Citation10, resulting in inactivation of the substrate adaptor activity of Keap1, and consequently to Nrf2 stabilization, which then binds to the ARE (as a heterodimer with a small Maf transcription factor) and regulates expression and induction of its target genes, including NQO1Citation11. Indeed, Nrf2 knockout cells and animals exhibit reduction in the constitutive expression of NQO1 and impaired inductionCitation5. NQO1 gene expression is induced together with other detoxifying enzyme genes in response to antioxidants, xenobiotics, electrophiles, heavy metals and radiationCitation12. Upon entering cells, these inducers can directly scavenge free radicals and can also provoke electrophilic stress signals that trigger proteins linked to diverse cellular signaling pathwaysCitation6–11,12. Evidence for the significance of the antioxidant functions of NQO1 in suppression of oxidative stress is provided by manifestations that induction of NQO1 levels or its reduction are associated with reduced and raised susceptibilities to oxidative stress, respectivelyCitation6–11,12.
Quinazolines are excellent reservoir of bioactive substances. A number of biological activitiesCitation13–17 are associated with quinazolines especially antioxidant activityCitation18.
The stability of the quinazoline nucleus has inspired medicinal chemists to introduce many bioactive moieties to this nucleus to synthesize new potential medicinal agents.
In the present study, and in continuation of our research programme aiming to synthesize biologically active heterocyclesCitation18–20 particularly quinazoline derivativesCitation21,Citation22 several amino nitrogenous heterocyclic moieties have been incorporated at position -4- of the quinazoline to develop novel series 4-amino-substituted-quinazoline derivatives that are likely to have superior cytoprotective activity. In addition, a docking study was performed in the Kelch domain of Keap1 obtained from the Protein Data Bank (PDB ID: 4IQK) to explore the ability of the synthesized compounds to block the Nrf2-binding site of Keap1.
Materials and methods
Melting points (uncorrected) were determined in open capillary on a Gallen Kamp melting point apparatus (Sanyo Gallen Kamp, Southborough, UK). Precoated silica gel plates (Kieselgel 0.25 mm, 60 F254, Merck, Darmstadt, Germany) were used for thin layer chromatography. A developing solvent system of chloroform/methanol (8:2) was used and the spots were detected by ultraviolet light. IR spectra (KBr disc) were recorded using an FT-IR spectrometer (Perkin Elmer, Norwalk, CT). 1H-NMR spectra were scanned on a NMR spectrometer (Bruker AXS Inc., Flawil, Switzerland), operating at 500 MHz for 1H- and 125.76 MHz for 13C. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard, using DMSO-d6 as a solvent. Mass spectra were recorded on a 600 GC/MS (Clarus, Middletown, CT) and TQ 320 GC/MS/MS mass spectrometers (Varian, West Sussex, UK). Elemental analyses were done on a model 2400 CHNSO analyser (Perkin Elmer, Norwalk, CT). All values were within ± 0.4% of the theoretical values. All reagents used were of AR grade. The starting material 4-chloro-2-phenylquinazoline 1 was purchased from Sigma (St. Louis, MO) and was directly used for the preparation of target compounds.
Chemistry
General procedure
Synthesis of aminoquinazoline derivatives (2–14). A mixture of 1 (2.41 gm, 0.01 mol) and required amines (0.01 mol) in dry dimethylformamide (20 mL) was heated under reflux for 24 h, then left to cool. The solid product formed was collected by filtration and recrystallized from dioxane to give compounds 2–15, respectively.
2-Phenyl-N-(pyridine-2-yl)quinazolin-4-amine (2). Yield, 86%; m.p. >360 °C. IR (KBr, cm−1): 3411 (NH), 3057 (CH arom.), 1618 (C=N). 1H-NMR (DMSO-d6): 7.5–8.3 [m, 13H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 102.6, 117.0 (2), 126.3, 127.9 (2), 128.2, 129.0 (2), 131.6 (2), 133.1, 135.0, 147.6 (2), 153.7, 160.7 (2). MS m/z (%): 298 (M+) (10.43), 221 (100). Anal. Calcd. For C19H14N4(298.12): C, 76.49; H, 4.73; N, 18.78. Found: C, 76.18; H, 4.45; N, 18.50.
2-Phenyl-N-(pyridine-4-yl) quinazolin-4-amine (3)Citation23. N-(2-chloropyridin-3-yl)-2-phenylquinazolin-4-amine (4). Yield, 86%; m.p. 243.2 °C. IR (KBr, cm−1): 3414 (NH), 3058 (CH arom.), 1601 (C=N), 696 (C-Cl).1H-NMR (DMSO-d6): 7.3–8.2 [m, 12H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 117.2, 121.4 (2), 126.3, 127.0 (2), 127.9, 128.2, 129.0 (2), 131.8 (2), 133.1 (2), 135.0, 152.8 (2), 162.7 (2). MS m/z (%): 333 (M+) (17.35), 295 (100). Anal. Calcd. For C19H13ClN4 (333.08): C, 68.57; H, 3.94; N, 16.84. Found: C, 68.26; H, 3.66; N, 17.09.
N-(5-chloropyridin-2-yl)-2-phenylquinazolin-4-amine (5). Yield, 91%; m.p. 108.9 °C. IR (KBr, cm−1): 3299 (NH), 3058 (CH arom.), 1623 (C=N), 826 (C-Cl).1H-NMR (DMSO-d6): 6.5–8.5 [m, 12H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 109.9, 115.3, 121.4, 125.3, 126.8 (2), 127.0, 127.8, 128.6 (2), 131.2, 133.1, 135.0, 137.3, 145.5, 152.8, 158.8, 162.7, 163.4. MS m/z (%): 333 (M+) (8.48), 205 (100). Anal. Calcd. For C19H13ClN4 (333.08): C, 68.57; H, 3.94; N, 16.84. Found: C, 68.19; H, 3.62; N, 16.49.
2-Phenyl-N-(pyrimidin-2-yl) quinazolin-4-amine (6). Yield, 81%; m.p. 98.6 °C. IR (KBr, cm−1): 3413 (NH), 3060 (CH arom.), 1615 (C=N). 1H-NMR (DMSO-d6): 7.0–8.4 [m, 12H, Ar-H], 11.1 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 114.7 (2), 114.6, 115.8, 124.1, 125.7 (2), 126.4, 127.8, 128.7 (2), 129.7, 131.2, 131.9, 150.6, 156.4 (2), 161.3, 161.9, 170.6. MS m/z (%): 299 (M+) (11.63), 204 (100). Anal. Calcd. For C18H13N5(299): C, 72.23; H, 4.38; N, 23.40. Found: C, 72.57; H, 4.07; N, 23.18.
N-(4,6-dimethylpyrimidin 2-yl)-2-phenylquinazolin-4-amine (7). Yield, 69%; m.p. 70.1 °C. IR (KBr, cm−1): 3413 (NH), 3063 (CH arom.), 2957, 2876, 2805 (CH aliph.), 1615 (C=N). 1H-NMR (DMSO-d6): 2.5 [s, 6H, 2CH3], 7.3–8.5 [m, 10H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 23.9 (2), 114.8 (2), 124.8, 126.5 (2), 128.2, 128.3, 129.5 (2), 130.5, 132.8, 138.8, 152.7, 158.3 (2), 163.2 (3). MS m/z (%): 327.15 (M+) (3.42), 251 (100). Anal. Calcd. For C20H17N5(327.15): C, 73.37; H, 5.23; N, 21.39. Found: C, 73.61; H, 4.87; N, 21.07.
1, 3-Dimethyl-6-(2-phenylquinazolin-4-ylamino) pyrimidine-2,4(1H,3H)dione (8). Yield, 77%; m.p. 343.8 °C. IR (KBr, cm−1): 3404 (NH), 3100 (CH arom.), 2928, 2836 (CH aliph.), 1711, 1675 (2C=O),1608 (C=N). 1H-NMR (DMSO-d6): 3.2, 33 [2s, 6H, 2N-CH3], 7.0 [s, 1H, CH pyrimidine], 7.4–8.4 [m, 10H, Ar-H], 9.0 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 28.0, 29.9, 86.2, 114.7, 124.2, 126.5 (2), 127.0, 128.1, 128.6 (2), 129.0, 131.8, 132.7, 150.8, 151.3, 160.7, 163.2, 163.8, 165.5. MS m/z (%): 359 (M+) (5.39), 219 (100). Anal. Calcd. For C20H17N5O2 (359.14): C, 66.84; H, 4.77; N, 19.49. Found: C, 66.48; H, 4.36; N, 19.11.
5-(2-Phenylquinazolin-4-ylamino)pyrimidine-2, 4(1H, 3H)-dione (9). Yield, 77%; m.p. >360 °C. IR (KBr, cm−1): 3428, 3400 (NH), 3066 (CH arom.), 1699, 1684 (2C=O),1618 (C=N). 1H-NMR (DMSO-d6): 7.4–8.4 [m, 10H, Ar-H], 9.1 [s, 1H, NH, exchangeable with D2O], 11.0 [s, 1H, NHCO, exchangeable with D2O], 11.7 [s, 1H, CO-NH-CO, exchangeable with D2O]. 13C-NMR (DMSO-d6): 114.1, 123.2, 126.4 (2), 128.3 (2), 128.5 (2), 128.8 (2), 130.7, 133.6, 136.6, 150.6, 150.9, 159.6, 162.1, 162.7. MS m/z (%): 331 (M+) (17.32), 220 (100). Anal. Calcd. For C18H13N5O2 (331.11): C, 65.25; H, 3.95; N, 21.14. Found: C, 65.55; H, 4.26; N, 21.46.
N-(5-Bromopyrimidine -2-yl)-2-phenylquinazolin-4-amine (10). Yield, 89%; m.p. 233.2 °C. IR (KBr, cm−1): 3328 (NH), 3085 (CH arom.), 1618 (C=N). 1H-NMR (DMSO-d6): 6.8–8.6 [m, 11H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 105.6, 114.8, 126.3, 126.5 (2), 127.0, 128.2, 128.7 (2), 130.5, 131.8, 133.2, 152.0, 158.3 (2), 159.5, 162.5, 163.3. MS m/z (%): 378 (M+) (64.21), 296 (100). Anal. Calcd. For C18H12BrN5(378.23): C, 57.16; H, 3.20; N, 18.52. Found: C, 56.84; H, 3.54; N, 18.18.
2-Phenyl-N-(pyrazin-2-yl) quinazolin-4-amine (11). Yield, 90%; m.p. 115.8 °C. IR (KBr, cm−1): 3448 (NH), 3062 (CH arom.), 1612 (C=N). 1H-NMR (DMSO-d6): 7.0–8.4 [m, 12H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 114.8, 114.8, 126.3, 127.9 (2), 128.2, 128.4, 129.0 (2), 130.5, 131.2, 132.8, 133.1, 135.0, 139.8, 149.2, 152.7, 162.7, 163.2. MS m/z (%): 299 (M+) (8.37), 222 (100). Anal. Calcd. For C18H13N5(299.33): C, 72.23; H, 4.38; N, 23.40. Found: C, 72.57; H, 4.07; N, 23.18.
6-Phenyl-8H-benzo[g]quinazolino[4,3-b]quinazolin-8-one (12). Yield, 69%; m.p. 157.8 °C. IR (KBr, cm−1): 3050 (CH arom.), 1675 (C=O), 1593 (C=N). 1H-NMR (DMSO-d6): 7.2–8.8 [m, 15H, Ar-H], 13C-NMR (DMSO-d6): 118.1, 121.2, 125.7, 126.4, 127.0, 127.7 (2), 127.9 (2), 128.1, 128.2, 128.4 (2), 128.5, 129.7, 129.8, 130.0, 130.8, 133.6, 134.3, 150.0, 150.6, 157.6 (2), 170.8. MS m/z (%): 373 (M+) (47.82), 344 (100). Anal. Calcd. For C25H15N3O (373.41): C, 80.41; H, 4.05; N, 11.25. Found: C, 80.76; H, 4.33; N, 10.88.
N-(5,6-Dimethyl-1,2,4-triazin-3-yl)-2-phenylquinazolin-4-amine (13). Yield, 80%; m.p. >360 °C. IR (KBr, cm−1): 3410 (NH), 3100 (CH arom.), 2958, 2847 (CH aliph.), 1617 (C=N). 1H-NMR (DMSO-d6): 2.4, 2.6 [2s, 6H, 2CH3], 6.7–8.6 [m, 9H, Ar-H], 12.5 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 18.5, 23.0, 114.7, 124.8, 126.5 (2), 127.0, 127.9, 128.4 (2), 130.5, 131.8, 133.1, 138.8, 147.3, 149.2, 158.3, 162.7, 163.2. MS m/z (%): 328 (M+) (2.23), 205 (100). Anal. Calcd. For C19H16N6 (328.14): C, 69.50; H, 4.91; N, 25.59. Found: C, 69.23; H, 4.64; N, 25.27.
9-Ethyl-N-(2-phenylquinazolin-4-yl)-9H-carbazol-3-amine (14). Yield, 91%; m.p. 135.7 °C. IR (KBr, cm−1): 3413 (NH), 3052 (CH arom.), 2970, 2882 (CH aliph.), 1618 (C=N). 1H-NMR (DMSO-d6): 1.3 [t, 3H, CH3], 4.4 [q, 2H, CH2], 7.2– 8.8 [m, 16H, Ar-H], 10.0 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 14.2, 37.5, 109.2 (3), 109.7 (2), 114.5, 115.1, 119.2, 120.4, 122.3, 123.5, 126.2, 128.3 (2), 128.7 (2), 130.7, 131.4 (2), 133.5 (2), 137.1, 138.9, 150.6, 158.6, 159.6. MS m/z (%): 415 (M+) (19.53), 372 (100). Anal. Calcd. For C28H22N4(414.50): C, 81.13; H, 5.35; N, 13.52. Found: C, 81.44; H, 5.04; N, 13.26.
Evaluation of biological activity
The compounds were evaluated for their ability to induce NQO1 using a quantitative microtiter plate assay in Hepa1c1c7 murine hepatoma cells as described previouslyCitation24. The results are expressed as the ratio of the specific enzyme activity in cell lysates prepared from treated over control wells. The CD value (Concentration of a compound required to Double the specific enzyme activity) represents a measure of inducer potency. Each compound was tested at eight different concentrations in eight replicate wells. Each experiment was performed three times.
Molecular modeling study
The structures of the synthesized 2-phenylquinazoline-4-amine derivatives were built according to the default parameters, using the MOE software version 10.2009 (Montreal, Quebec, Canada). Geometry optimization as well as a systematic conformational search was carried out to an RMS gradient of 0.01 Å employing the ConfSearch module implemented in MOE. All computations were performed with the Merck Force Field (MMFF94s). Molecular docking study was performed using the crystallographic structure of the Kelch domain of Keap1 obtained from the Protein Data Bank (PDB ID: 4IQK) to explore the ability of the synthesized compounds to block the Nrf2-binding site of Keap1. The protein target was prepared for docking by the addition of the missing hydrogens and calculating the partial charges. Internal validation was performed to the native ligand followed by docking of the compounds where the target protein was kept rigid, while ligands were allowed to rotate to accommodate freely inside the protein cavity. Following multiple separate docking simulations using default parameters, the best conformations were chosen based on the combination of S score data, E conformation and appropriate fitting with the relevant amino acids in the binding pocket.
Results and discussion
Chemistry
4-Chloro-2-phenylquinazoline was used as a key starting material for the synthesis of the target compounds. Synthesis of quinazoline derivatives 2–14 has been achieved by substituting 4-chloro group in the starting 1 with different amino nitrogenous heterocyclic moieties namely, 2-amino pyridineroman (all italic comma should change to roman) 4-amino pyridine, 3-amino-2-chloro pyridine, 2-amino-5-chloro pyridine, 2-amino pyrimidine, 2-amino-4,6-dimethyl pyrimidine, 6-amino-1,3-dimethyl uracil, 5-amino uracil, 2-amino-5-bromopyrimidine, 2-amino pyrazine, 3-amino-2-naphthoic acid, 3-amino-5,6-dimethyl-1,2,4-triazine and 3-amino-9-ethyl carbazole as outlined in Scheme 1. In this work, 10 novel compounds, a reported compound 3Citation23 and two commercially available compounds 4 and 6 were synthesized in order to explore their cytoprotective activity.
The synthesized quinazoline derivatives were characterized by IR, 1H-NMR,13 C-NMR and mass spectra. The IR spectra of the compounds perfectly exhibited the absorption bands for NH, CH aromatic and C=N around 3328–3448, 3042–3100, 1601–1625 cm−1, respectively.
In the 1H-NMR spectrum, aromatic protons were observed as complex mulitiplets in the range of δ 6.5–8.8 ppm. In addition, there is one exchangeable secondary amide (–NH) proton in the structures and are observed as broad singlet around δ 9.0–12.5 ppm to prove the introduction of the amino substitution to the structures.
13C-NMR spectra exhibited additional signals for the introduced heterocyclic moieties in the aromatic region around δ 119.2–170.6 ppm which is in conformity with the assigned structures.
The mass spectra of the compounds showed molecular ion peaks at their respective m/e along with the elemental analyses which were found within the limit of 0.4% of theoretical values for all the synthesized compounds.
Biological evaluation
All of the new compounds showed a concentration-dependent inducer activity (). The inducer potencies ranged between 5.5 μM (compound 4) and 70 nM (compound 12) (). Thus, compound 12 is ∼80 times more potent than compound 4. Notably, in this series, compound 12 uniquely contains a structurally rigid substituent, strongly suggesting that in addition to chemical reactivity, structural rigidity which perhaps affects its ability to bind to the inducer sensor Keap1, is an important determinant of inducer potency.
Figure 1. Dose-response curves for NQO1 inducer activity of novel 2-phenylquinazolin-4-amine derivatives. Data are expressed as the ratio of treated/control (T/C) values.
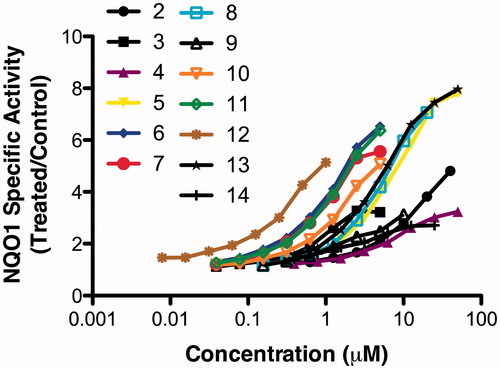
Table 1. NQO1 inducer potency of novel 2-phenylquinazolin-4-amine derivatives.
Molecular modeling study
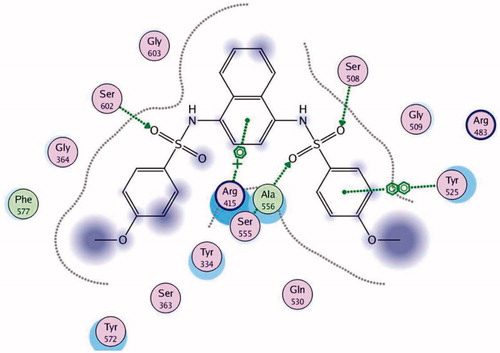
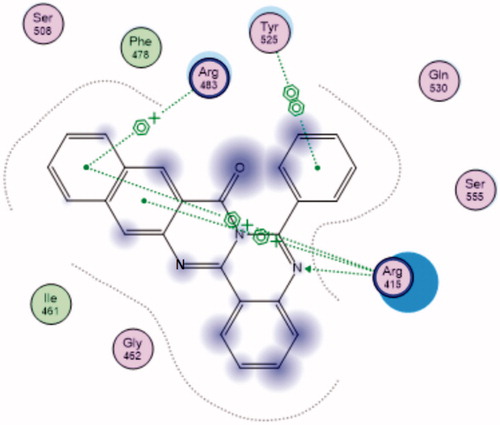
Keap1 binds to Nrf2 promoting its degradation, resulting in low levels of cytoprotective gene product./s. Various compounds were reported to bind to the Keap1 Kelch domain and antagonize its activityCitation6–12. For assessing the ability of the newly synthesized compounds to access and block the Kelch domain of Keap1, a molecular docking study was performed using the Keap1 crystal coordinates obtained from the Protein Data Bank (PDB ID: 4IQK). The main interactions observed between the validated native ligand and the protein target were found to be arene–cation interaction with Arg415, arene–arene interaction with Tyr525 and 3 hydrogen bonds with Ser602, Ser508 and Ser555 with S=−13.306 kcal/mol and rmsd 0.6635 kcal/mol/Å (). Upon docking of the synthesized compounds, they all showed an arene–cation binding interaction with Arg415 via their aromatic rings and one of the nitrogen atoms of quinazoline ring. Whereas Compound 12 (S= −9.571 kcal/mol) has adopted a conformation allowing the presence of additional arene–arene interaction with Tyr525 and arene–cation interactions with Arg483 ().
Conclusion
This work was aimed to synthesize novel 4-amino-substituted-quinazoline derivatives as NAD(P)H: quinone oxidoreductase 1 inducers (NQO1). NQO1 is a versatile cytoprotective enzyme which has prominent antioxidant functions due to of its ability to metabolize reactive endogenous and exogenous (dietary or environmental) quinones to their less reactive and less toxic hydroquinone forms by obligatory 2-electron reduction, thereby diverting them from redox cycling reactions and depletion of intracellular antioxidants, such as glutathione. Thus, NQO1 inducers could be of potential therapeutic benefit in oxidative stress-mediated pathologies, such as neurodegenerative diseases. The new compounds showed a concentration-dependent inducer activity with potencies in the low- to sub-micromolar range. Compound 12, which uniquely contains a structurally rigid substituent, is the most potent inducer in this series with activity in the nanomolar concentration range. Molecular docking of the synthesized compounds as Keap1-Nrf2 protein–protein interaction inhibitors was performed. Compound 12 was found to bind to key amino acids in the binding site. These compounds represent promising quinazoline scaffold-based leads for further optimization as cytoprotective agents.
Declaration of interest
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project no. RGP-VPP-302. Maureen Higgins and Albena T. Dinkova-Kostova are grateful to Cancer Research UK (C20953/A18644) for financial support. The authors declare that they have no conflict of interest.
References
- Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med 2000;29:231–40
- Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol 1995;49:127–40
- Ross D, Kepa JK, Winski SL, et al. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000;129:77–97
- Nebert DW, Roe AL, Dieter MZ, et al. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 2000;59:65–85
- Nioi P, Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res 2004;555:149–71
- Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem 2010;285:153–62
- Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999;13:76–86
- Baird L, Lleres D, Swift S, Dinkova-Kostova AT. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc Natl Acad Sci USA 2013;110:15259–64
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 2002;99:11908–13
- McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci USA 2010;107:18838–43
- Motohashi H, O’Connor T, Katsuoka F, et al. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 2002;294:1–12
- Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 1998;17:3145–56
- Cao SL, Feng YP, Jiang YY, et al. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett 2005;15:1915–17
- Govindaraj Y, Sathyamoorthy Karthikeyan V, et al. Synthesis and in-vivo anticancer screening of 2-{[bis-(2-chloroethyl) amino] methyl} -6, 8-dinitro-1-(4-substituted ethyl)-1h-quinazolin-4-one derivatives. Acad J Cancer Res 2009;2:73–7
- Raffa D, Elder MC, Daidone G, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem 2004;39:299–304
- Ho N, Harapanhalli RS, Dahman BA, et al. Synthesis and biologic evaluation of a radioiodinated quinazolinone derivative for enzyme-mediated insolubilization therapy. Bioconjug Chem 2002;13:357–64
- Rajveer C, Kumaraswamy D, Sudharani S, Stephen-Rathinaraj B. Synthesis of some 6-bromo quinazolinone derivatives for their pharmacological activities. Int J Pharm Bio Sci 2010;1:1–10
- AlSaid MS, Ghorab MM, Higgins M, Dinkova-Kostova AT. NAD(P)H: quinone oxireductase 1 inducer activity of some enaminone derivatives. Biomed Res 2015;26:7–12
- Ghorab MM, Higgins M, Alsaid MS, et al. Synthesis, molecular modeling and NAD(P)H:quinone oxidoreductase 1 inducer activity of novel cyanoenone and enone benzenesulfonamides. J Enzyme Inhib Med Chem 2014;29:840–5
- Ghorab MM, Ragab FA, Heiba HI, et al. Synthesis, anticancer and radiosensitizing evaluation of some novel sulfonamide derivatives. Eur J Med Chem 2015;92:682–692
- Ghorab MM, Alsaid MS, El-Gazzar MG, et al. NAD(P)H: quinone oxireductase 1 inducer activity of novel 4-aminoquinazoline derivatives. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2015.1135913
- Ghorab MM, Alsaid MS, Al-Dosari MS, et al. Design, synthesis and anticancer evaluation of novel quinazoline-sulfonamide hybrids. Molecules 2016;21:189–201
- Gellibert F, Fouchet MH, Nguyen VL, et al. Design of novel quinazoline derivatives and related analogues as potent and selective ALK5 inhibitors. Bioorg Med Chem Lett 2009;19:2277–81
- Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Meth Enzymol 2004;382:243–58