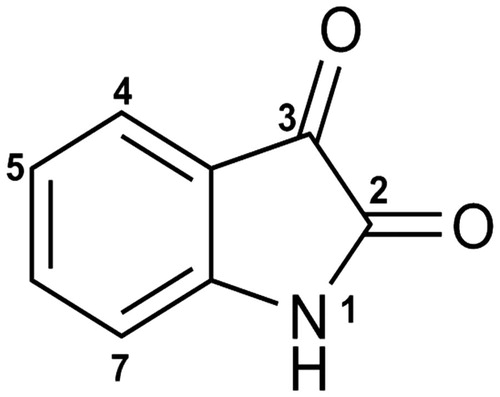
Abstract
Tryptophan 2,3-dioxygenase (TDO) is a cytosolic protein with a proven immunomodulatory function that promotes tumoral immune resistance and proliferation. Despite the interest in TDO as a therapeutic target in cancer treatment, the number of biologically useful inhibitors is limited. Herein, we report isatin derivatives as a new class of TDO inhibitors. Through structure–activity relationships and molecular docking studies, we optimized the inhibition potency of isatin derivatives by >130-fold and elucidated the mechanistic details that control their mode of action. Hydrogen bond interactions between the compound and key active site residues of TDO, freedom upon rotation of the C3 chemical moiety and the presence of chlorines in the benzene ring of the compound comprise the properties that an isatin-based inhibitor requires to effectively inhibit the enzymatic activity of TDO.
Introduction
The kynurenine pathway for tryptophan catabolism has become an attractive target for drug discovery related to various diseases (e.g. cancer, neurological, and autoimmune diseases)Citation1,Citation2. In the case of cancer therapy it is the enzymes involved in the first and rate-limiting step of this pathway that are of particular interest, but despite over 20 years of research an FDA-approved drug is yet to be developed. These targets, tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase-1 (IDO1), the two main enzymes that catalyze oxidation of l-tryptophan to form N-formylkynurenine, have an established role in tumor survival and escape via their ability to modulate immune system cellsCitation3,Citation4. TDO is predominantly expressed in the liver but it has also been found in other tissues after stimuli. On the other hand, IDO1 is expressed throughout the body with the exception of the liver. Due to the early identification of IDO1 as an enzyme with immunomodulatory function and its broad expression in the body, it became the main target for drug discovery. Many years of research have revealed a large number of IDO1 inhibitors with affinities that reach the low nanomolar regionCitation1,5–8. Of these, the most promising candidate, known as INCB024360, is currently under investigation in clinical trialsCitation9. Contrastingly, the number of TDO inhibitors identified is fewer and less diverseCitation10–13. The most potent class of TDO inhibitors belong to the family of 3-(2-pyridylethenyl)-indoles, with potencies in the nanomolar rangeCitation14,Citation15. Recently, a low micromolar affinity TDO inhibitor with a flavonoid scaffold and selectivity over IDO1 was reportedCitation16.
The knowledge gained so far suggests that targeting cancer cells via inhibition of IDO1 alone is not the most effective strategy for two reasons. First, tumor cells can overcome this problem using TDO as a tryptophan catabolizing enzyme; while expression of TDO is normally restricted to liver and brain, in malignancies the enzyme was found in a wide number of cancers including bladder, breast, renal, colon, head and neck carcinomas, hepatocarcinoma, melanoma, mesothelioma, neuroblastoma, sarcoma, and leukemiaCitation17. Second but equally important is intratumor heterogeneity, a phenomenon that is mainly found in advanced cancers. Tumors that predominantly express IDO1 can also contain a small number of TDO-expressing cells that can be activated when the tumor is under attack. A recent study that examined the expression pattern of TDO and IDO1 in various types of cancers supports the idea that intratumor heterogeneity may contribute to tumor escape and proliferationCitation17.
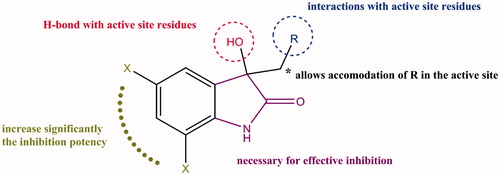
Due to the limited number of drug-like TDO inhibitors, the primary goal of this study was the characterization of a new class of inhibitors that meet the following properties: (i) bioactivity, (ii) anticancer properties, (iii) low cost of synthesis, and (iv) ease of derivatization for optimization. In the search for new TDO inhibitors, 1 (isatin) was an attractive molecule not only because of its tryptophan-like structure but also because of the proven biological and pharmacological role of isatin derivatives as anticancer drugsCitation18,Citation19. In 2006, Sunitinib, an isatin derivative, became the first FDA approved drug for treatment of advanced cancersCitation20. In addition to anticancer activity, isatin derivatives have several other applications including anticonvulsant, antimicrobial, antidepressant, and anxiogenic activitiesCitation18. Isatin was discovered in the nineteenth century as a result of indigo oxidation and is naturally occurring in plants, fruits, animals, and humansCitation19. In humans, it is proposed that isatin is an endogenous product of tryptophan oxidation, a procedure that takes place in the liver after the consumption of tryptophan-rich foodCitation21.
Having isatin (1) as a starting point, we carried out structure–activity relationships (SAR) and molecular docking studies probing the effect of modifications on the inhibition of TDO. These modifications include chlorination of the benzene ring, methylation of N1, substitution at the C3 position and gradual breakdown of the pyrrolidine ring (). The various modifications were chosen carefully in order to obtain information about the importance of the functional groups. Through this process, we optimized the inhibition potency of isatin derivatives by >130-fold and analyzed the role of each functional group. Our findings provide the framework for the development of new therapeutics that target tryptophan oxidation.
Materials and methods
Chemicals
All chemicals used in this study (l-Trp, l-ascorbate, bovine liver catalase, methylene blue, isatin and its derivatives) including those for buffers were of the highest analytical grade (≥95% purity) and were used without further purification. Compounds 1–25 and 27–35 were purchased from Sigma/Aldrich. Compound 26 was purchased from TimTec. Compounds 36–44 were kindly provided by the National Cancer Institute, USA. All the compounds that contain a stereocenter were received and tested as racemic mixtures.
Cloning, expression, and purification
Cloning, expression, and purification of human TDO and IDO1 were carried out as described elsewhereCitation16,Citation22. Briefly, the full nucleotide sequence of TDO and IDO1 were cloned into a pET28a and ToPO plasmids and expressed in Rosetta (DE3) pLysS and BL21 (DE3) E. coli cells respectively. The cells were incubated at 37 °C until the OD600 reached 0.6 and induced with isopropyl-β-d-1-thiogalactopyranoside (IPTG) at 0.6 mM final concentration. The culture medium was supplemented with 5 mM hemin solution to optimize heme incorporation. After induction, the temperature was decreased to 22 °C and the cells were incubated for 12 h. The harvested cells were resuspended in lysis buffer composed of 20 mM Tris HCl pH 8.0, 300 mM NaCl, 10 mM imidazole, 1 mM tris(2-carboxyethyl)phosphine (TCEP). To avoid proteolytic activity of serine and cysteine proteases in E. coli a protease inhibitor tablet was added and the cells were lysed by ultrasonication. Cell debris was then removed via ultracentrifugation for 1 h at 28 000 g, 4 °C, and the hTDO and hIDO1 supernatants collected. The supernatant of hIDO1 was then loaded onto a Ni-NTA column while the hTDO supernatant on batches of 100 ml each to avoid aggregation on the column. hIDO1 and hTDO were washed with 5 column volumes (CV) plus an additional 50 ml of lysis buffer (20 mM Tris HCl pH 8.0, 300 mM NaCl, 20 mM imidazole, 1 mM TCEP) respectively and eluted with the same buffer containing 250 mM imidazole. Imidazole and the small percentage of impurities were removed with size exclusion chromatography (Superdex 200 for hTDO and Superdex 75 for hIDO1) using 20 mM TrisHCl pH 8.0, 1 mM TCEP as running buffer.
Inhibition assay
Both TDO and IDO1 inhibition assays were carried out as described previouslyCitation16,Citation22. The assay was carried out in a 96-well microplate with the components dissolved in 100 mM KPi buffer pH 6.5. Each well contained 200 μl of assay mixture. Briefly, the reaction mixture was composed of sodium ascorbate (40 mM), methylene blue (20 μM), catalase (0.2 mg/ml), l-tryptophan at final concentrations between 0–45 μM for hIDO1 and 0–800 μM for hTDO, 2 μl of the inhibitor dissolved in DMSO (1% final DMSO concentration) and TDO or IDO1 at final concentrations of 20 nM and 10 nM, respectively. The reaction mixture was incubated at room temperature for 20 min (hTDO) and 15 min (hIDO1) and the reaction was terminated by adding 40 μl of trichloroacetic acid (30 w/v) into each well. Subsequently the microplate was transferred into an oven and incubated at 50 °C for 30 min. The microplate was centrifuged for 15 min at 4000 rpm and 125 μl of the supernatant transferred to a new microplate and mixed with equal volume of DMAB in acetic acid (2% w/v). Finally, the absorbance (corresponding to detection of the l-kynurenine-DMAB adduct) was measured at 490 nm. The data were fitted in a Dixon plot, 1/v against [I], using Origin software (Microcal) and the inhibition constants were determined graphically by the intersection point. The kinetic plots of all active compounds along with their highest tested concentrations are provided in Supplementary Figure S1 and Supplementary Table S1.
Molecular modeling
Docking of isatin and its derivatives into the active sites of TDO and IDO1 was carried out using Autodock VinaCitation23. In the case of IDO1, chain A of the human IDO1 crystal structure (PDB ID: 2D0T)Citation24, stripped of the 4-phenylimidazole inhibitor, was used as the protein model. In the case of TDO, a homology model was obtained using SWISS-MODELCitation25 and the structure of the substrate-bound TDO from Xanthomonas campestris (PDB ID: 2NW8)Citation26 [NB. attempts were made to use the Drosophila melanogaster TDO crystal structureCitation27 as a homology model due to its greater sequence similarity with the human enzyme, but these led to models with the active site occluded by the presence of a phenylalanine side chain]. Heme groups were not included in the TDO models provided by SWISS-MODEL, so these were placed manually by superposition of the Xanthomonas TDO structure onto the human TDO homology model and copying the heme coordinates into the TDO model coordinate file. The heme position was optimized manually to avoid steric clashes and to maintain reasonable coordination with the axial histidine ligand. Ligand coordinate files for use by Autodock Vina were prepared using MarvinSketch. Prior to docking of test compounds, the validity of the method was tested by docking l-Trp into the stripped active site of Xanthomonas TDO that previously held bound substrate (data not shown). The preferred conformation was essentially identical to that observed in the original l-Trp-bound structure (PDB ID 2NW8). Subsequent docking of test compounds led to an output of several different poses ranked by calculated binding affinity. For all compounds, the most energetically favorable ligand poses have been used in discussion here. Interactions between 36, 40 and the active sites of TDO and IDO1 were examined using LigPlot plusCitation28. Electrostatic potential maps were generated using the vacuum electrostatics function of PyMOLCitation29.
Results and discussion
Chlorination and N1 methylation of 1
Isatin (1) is a weak inhibitor of TDO and IDO1, with inhibition constants (Ki values) of 132 ± 7 μM and 89 ± 6 μM respectively (). Given the specificity of 1-methyltryptophan for inhibition of IDO1, due to the proposed clash of its N1 methyl group with His 76 of human TDO (based on the observed interaction of l-tryptophan with the equivalent His 55 of Xanthomonas campestris TDO (XcTDO))Citation30, we examined the effect of N1 methylation on 1. Although N1 methylation to produce 2 (1-methylisatin) did not abolish the weak inhibition effect that 1 has on TDO, the inhibition potency reduced further, producing a Ki value of 170 ± 21 μM (). This modest decrease in potency implies that 2 likely does not have the same orientation of the ring system as l-Trp has in the active site of XcTDO (the XcTDO crystal structure is the only one that describes the interactions of l-Trp with the active site residues in the active ferrous form of the enzyme)Citation26. Following this we examined the effect of chlorination at various positions of the benzene ring. The presence of a chlorine atom at the 5-position (3) led to a significant increase in the inhibition potency of the molecule (). Compared to 1, 3 is a 12-fold and 6-fold more potent inhibitor of TDO and IDO1 respectively. Repositioning the chlorine atom to the 7-position of the benzene ring (4) has no significant effect on inhibition of either TDO or IDO1, retaining the inhibition constant at the same level (). Compared to 4, dichlorination at the 4- and 7- (7) or 5- and 7- (8) positions had a noticeable effect on TDO, increasing the potency by 2-fold and 11-fold respectively while the level of IDO1 inhibition remains about the same (). Examination of 5 and 6 suggest that removal of the C2 and/or C3 carbonyl groups of 3 abolishes the effect of the chlorine atoms, turning these compounds into weak inhibitors.
Table 1. The effect on strength of inhibition of altering the structure of isatin (1): a structure–activity study.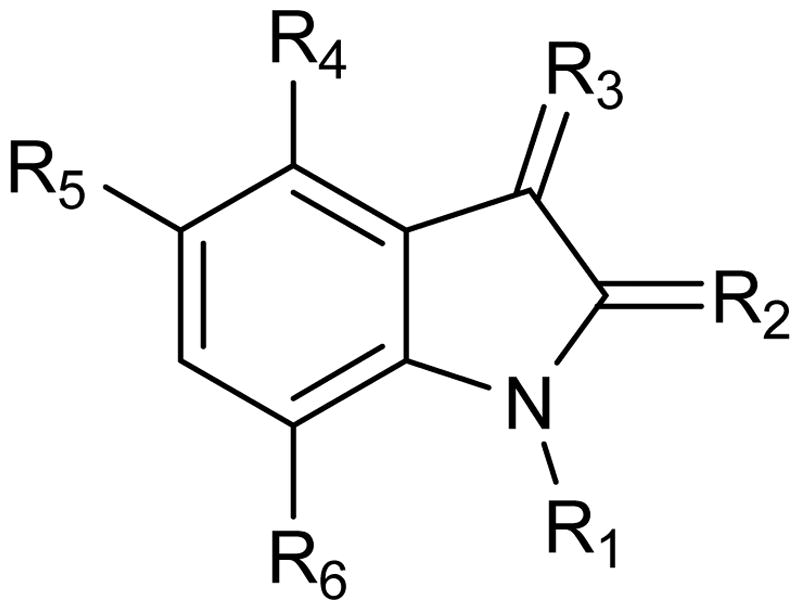
Further derivatization of 8
To better understand the mode of action of isatin derivatives in the active site of TDO, we studied the functional groups of 8 one by one. The molecules used in this trial were divided into two groups. In the first group, we studied the effect of modifications at N1, C2, C3, and the pyrrolidine ring of 8 in the absence of the two chlorines (). In the second group, the two chlorine atoms were retained and alterations were made to the pyrrolidine ring of 8 (). The rationale of this trial was to examine (i) the effect of each group in the absence of the electron-withdrawing chlorines, and (ii) to identify whether chlorines remain important in retaining the inhibition potency of the molecule when the pyrrolidine ring is open. The modifications carried out in the first group (9–15) resulted in weak inhibition of both TDO and IDO1 (). Evaluating the second group (16–20), some interesting results were obtained. Although the two chlorine atoms at the 5- and 7-positions of the benzene ring are important for increasing the inhibition potency of the molecule, this effect is abolished if the functional groups of the pyrrolidine ring are removed or modified (16). Opening of the pyrrolidine ring abolishes compounds 8’s inhibition activity ().
Table 2. The effect of modifications at N1, C2, C3, and the pyrrolidine ring of 8 in the absence of the two chlorines and the resulting Ki values against TDO and IDO1.
Table 3. The importance of the pyrrolidine ring in the inhibition potency of 8.
Substitutions at C3
Since removal of the two carbonyl groups has a detrimental effect on the inhibition potency of the chlorinated derivatives (see 5, 6, and 16), we retained the C2 carbonyl group and explored the effect of substitutions at the C3 position alone. As before, the effect of C3 substitutions was assessed in the presence and absence of chlorines. For the non-chlorinated compounds, the C3 carbonyl oxygen was replaced by a variety of aliphatic or aromatic groups (). None of these derivatives (21–35) demonstrated effective inhibition of either TDO or IDO1, confirming that the chlorines are necessary for generating a potent inhibitor. In contrast, C3 substitutions in the presence of chlorines produced reasonable inhibition activities (). Among the nine inhibitors, 36 and 40 were found to be the most promising ones; Ki TDO = 2.0 ± 0.7 μM and Ki TDO = 1.0 ± 0.6 μM for 36 and 40 respectively. The inhibition constants of 36 and 40 are in the same range as that for 8 (0.8 ± 0.5 μM), showing that the C3 carbonyl group is not necessary for creating a potent inhibitor. To understand better our SAR findings, we docked 36 and 40 into the active sites of TDO and IDO1. Our analysis suggests that the 1-(4-hydroxyphenyl)-2-methylpropan-1-one and methylbenzene moieties of 36 and 40 respectively, get into the active site pockets of the two enzymes and stabilized mainly by hydrophobic interactions with active site residues ( and ). The freely rotating bond that is attached to C3 makes the fitting of the 1-(4-hydroxyphenyl)-2-methylpropan-1-one (36) and methylbenzene (40) groups possible. The C3 hydroxyl groups of 36 and 40 play a stabilizing role via hydrogen bond interactions with active site residues. For TDO, the two compounds form hydrogen bond interactions with Ser 148 and Ala 150 while for IDO1 the compounds interact with either Gly 262 and heme (36) or Gly 262 (40) ( and ). Electrostatic potential analyses show that the 1-(4-hydroxyphenyl)-2-methylpropan-1-one and methylbenzene moieties of 36 and 40 respectively are stabilized in the hydrophobic pockets of the TDO and IDO1 active sites ( and ). Replacement of the phenol group of 36, by either aniline (37) or nitrobenzene (38), modestly decreased the inhibition potency of the produced molecule, especially for TDO, something that cannot be said for the chlorobenzene substitution (39) (). Taking into account that 37–39 differ only in one functional group (–NH2, –NO2, or –Cl), the variation in potencies suggest that (i) the C3 chemical moieties of 37, 38, and 39 are involved in interactions with the two active sites and (ii) accommodation of these moieties in the active sites of TDO and IDO1 differ, and is affected by the size of –NH2, –NO2, and –Cl. Examination of the electrostatic potential maps of 36 confirm the above conclusions (). 41 revealed a 4-fold difference in Ki values between TDO and IDO1, indicating that the accommodation of the naphthalene group in the active sites of TDO and IDO1 differs. In the case of 42 and 44, we observed Ki values in the low micromolar range, while 43 was found to be an ineffective inhibitor against both TDO and IDO1. In trying to rationalize the low inhibition potency of 43 we found that this is possibly due to restrictions upon rotation of the 2-methyl-2,3-dihydro-1H-inden-1-one group ().
Figure 2. Stabilization forces between 36 and the active sites of (a) TDO and (b) IDO1 as these defined by LigPlot plus and electrostatic potential analyses. For LigPlot plus analyses (Top), hydrophobic contacts and hydrogen-bond (H-bond) interactions are illustrated as “eyelash” and dashed lines respectively. Compound 36 as well as the H-bond partners are shown as ball-and-stick models. The H-bond distances are in A°. For electrostatic potential analyses (Bottom), 36 and heme are shown as sticks. In both TDO and IDO1 active sites 36 is mainly stabilized by hydrophophic interactions.
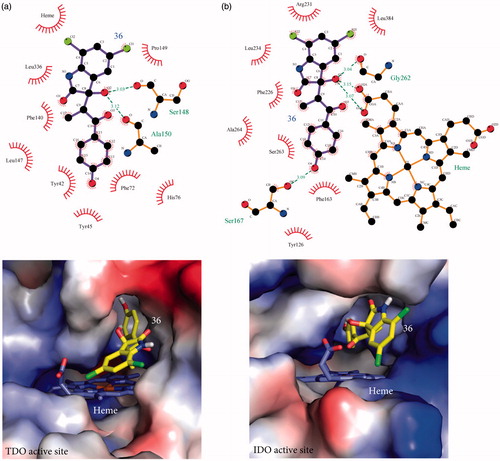
Figure 3. Stabilization forces between 40 and the active sites of (a) TDO and (b) IDO1 as these defined by LigPlot plus and electrostatic potential analyses. For LigPlot plus analyses (Top), hydrophobic contacts and hydrogen-bond (H-bond) interactions are illustrated as “eyelash” and dashed lines respectively. Compound 40 as well as the H-bond partners are shown as ball-and-stick models. The H-bond distances are in A®. For electrostatic potential analyses (Bottom), 40 and heme are shown as sticks. In both TDO and IDO1 active sites 40 is mainly stabilized by hydrophophic interactions.
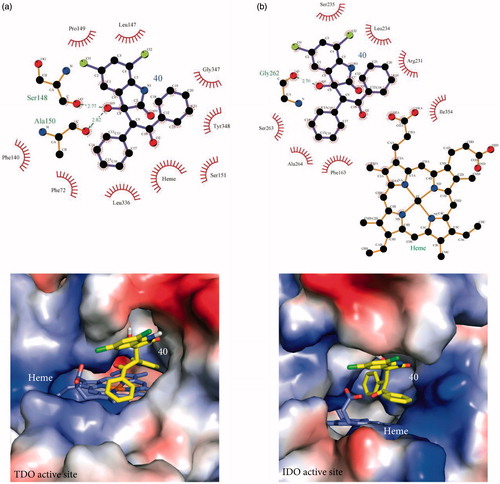
Table 4. Substitutions at C3 and their resulting Ki values against TDO and IDO1.
Table 5. Substitutions at the C3 position in the presence of chlorines and resulting Ki values against TDO and IDO1.
Evaluation of 36 and 40 docking poses
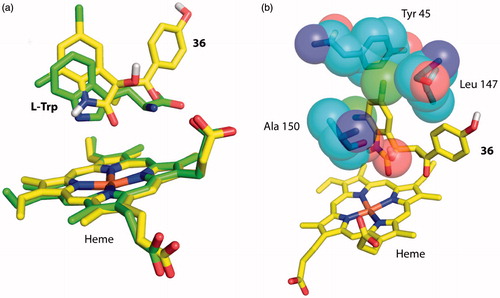
In the trial to evaluate further the accuracy of our docking results, we examined all the possible poses that compounds 36 and 40 may adopt in the two active sites. The three possible scenarios include (i) insertion of the C3 chemical moieties of 36 and 40 into the active sites of TDO and IDO1 and interactions with active sites residues (like the models described above), (ii) insertion of the 5,7-dichloroindolin-2-one moiety of 36 and 40 into the active sites of TDO and IDO1 and interactions with active site residues (like the crystal structure of XcTDO bound to l-Trp)Citation26, or (iii) insertion of the entire molecule and stabilization via interactions with active site residues and/or direct interactions with the heme iron (like the crystal structures of IDO1 with 4PI and Amg-1)Citation24,Citation31. Whereas the first scenario is the most favorable and described in a detail above, we will focus on the other two scenarios. To assess the possibility of having the 5,7-dichloroindolin-2-one group interacting with active site residues, we docked 36 into the active site of human TDO. The most energetically favorable pose, possessing the 5,7-dichloroindolin-2-one group inside the active site, was superposed with the corresponding pose of l-Trp in the crystal structure of XcTDO (). According to our findings, accommodation of the 5,7-dichloroindolin-2-one moiety of 36 and 40 is a possible but energetically unfavorable scenario. In comparison with l-Trp, the 5,7-dichloroindolin-2-one moiety has significant restrictions in movements due to the two chlorines that possess, and it would appear to undergo steric clashes with active site residues (). In addition to these clashes, this scenario has the C3 chemical moiety outside the active site, something that is inconsistent with the SAR findings where functional groups at C3 significantly affect the inhibition potency of the molecule. For the third scenario, we attempted to manually insert 36 and 40 into the active sites of the two protein as none favorable pose was produced by docking. In both cases our efforts failed due to major clashes with active site residues.
Figure 4. Docking of 5,7-dichloroindolin-2-one chemical moiety of 36 into the active site of hTDO. (a) Superposition of 36 with l-Trp in the docking model of hTDO and crystal structure of XcTDO respectively. The pose of 36 is the most favorable one produced by docking and has similar orientation to that of l-Trp in the crystal structure of XcTDO. (b) The most favorable pose of 36 results in clashes with active site residues of hTDO. Clashes were observed between the C5 chlorine atom of 36 and Leu 147 and C3 hydroxyl group of 36 and Ala 150.
Conclusion
In this study, we report the identification of isatin derivatives as a new class of TDO inhibitors. Through SAR and molecular docking studies, we probed optimization of the initially identified weak inhibitor (1) increasing the inhibition potency by over 130-fold for 40 (1.0 ± 0.6 μM). In the absence of drug-like TDO inhibitors, our findings provide insight into the mode of action of isatin derivatives and demonstrate the type of modifications that are necessary for creating a potent TDO inhibitor (summarized in ). The knowledge gained herein will provides the tools for further optimization studies that will enhance the potency and selectivity of isatin derivatives against TDO.
Declaration of interest
The authors declare no competing financial interest.
IENZ_1170013_Supplementary_material.pdf
Download PDF (656.5 KB)Acknowledgements
We thank National Cancer Institute for proving the compounds 36–44.
References
- Dounay AB, Tuttle JB, Verhoest PR. Challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J Med Chem 2015;58:8762–82.
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 2002;1:609–20.
- Muller AJ, DuHadaway JB, Donover PS, et al. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 2005;11:312–19.
- Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478:197–203.
- Dolusic E, Frederick R. Indoleamine 2,3-dioxygenase inhibitors: a patent review (2008–2012). Exp Opin Therap Pat 2013;23:1367–81.
- Peng YH, Ueng SH, Tseng CT, et al. Important hydrogen bond networks in indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor design revealed by crystal structures of imidazoleisoindole derivatives with IDO1. J Med Chem 2016;59:282–93.
- Malachowski WP, Winters M, DuHadaway JB, et al. O-alkylhydroxylamines as rationally-designed mechanism-based inhibitors of indoleamine 2,3-dioxygenase-1. Eur J Med Chem 2016;108:564–76.
- Lin SY, Yeh TK, Kuo CC, et al. Phenyl benzenesulfonylhydrazides exhibit selective indoleamine 2,3-dioxygenase inhibition with potent in vivo pharmacodynamic activity and antitumor efficacy. J Med Chem 2016;59:419–30.
- Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010;115:3520–30.
- Dolusic E, Larrieu P, Moineaux L, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors: 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem 2011;54:5320–34.
- Banerjee M, Middya S, Shrivastava R, et al. Inhibitors of the kynurenine pathway. Google Patents; 2014.
- Kumar S, Waldo J, Jaipuri F, Mautino M. Tricyclic compounds as inhibitors of immunosuppression mediated by tryptophan metabolization. Google Patents; 2014.
- Cowley P, Wise A. Tryptophan-2,3-dioxygenase (TDO) and/or indolamine-2,3-dioxygenase (IDO) inhibitors and their use. Google Patents; 2015.
- Reinhard JF Jr, Flanagan EM, Madge DJ, et al. Effects of 540C91 [(E)-3-[2-(4′-pyridyl)-vinyl]-1H-indole], an inhibitor of hepatic tryptophan dioxygenase, on brain quinolinic acid in mice. Biochem Pharmacol 1996;51:159–63.
- Salter M, Hazelwood R, Pogson CI, et al. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem Pharmacol 1995;49:1435–42.
- Pantouris G, Mowat CG. Antitumour agents as inhibitors of tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun 2014;443:28–31.
- Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA 2012;109:2497–502.
- Pandeya SN, Smitha S, Jyoti M, Sridhar SK. Biological activities of isatin and its derivatives. Acta Pharm 2005;55:27–46.
- Vine KL, Matesic L, Locke JM, et al. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000–2008. Anticancer Agents Med Chem 2009;9:397–414.
- Samlowski WE, Vogelzang NJ. Emerging drugs for the treatment of metastatic renal cancer. Exp Opin Emerg Drugs 2007;12:605–18.
- Gillam EM, Notley LM, Cai H, et al. Oxidation of indole by cytochrome P450 enzymes. Biochemistry 2000;39:13817–24.
- Pantouris G, Serys M, Yuasa HJ, et al. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids 2014;46:2155–63.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61.
- Sugimoto H, Oda S, Otsuki T, et al. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci USA 2006;103:2611–16.
- Biasini M, Bienert S, Waterhouse A, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 2014;42:W252–8.
- Forouhar F, Anderson JL, Mowat CG, et al. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA 2007;104:473–8.
- Huang W, Gong Z, Li J, Ding J. Crystal structure of Drosophila melanogaster tryptophan 2,3-dioxygenase reveals insights into substrate recognition and catalytic mechanism. J Struct Biol 2013;181:291–9.
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 2011;51:2778–86.
- DeLano WL. The PyMOL molecular graphics system. Palo Alto (CA): DeLano Scientific; 2002.
- Chauhan N, Thackray SJ, Rafice SA, et al. Reassessment of the reaction mechanism in the heme dioxygenases. J Am Chem Soc 2009;131:4186–7.
- Tojo S, Kohno T, Tanaka T, et al. Crystal structures and structure-activity relationships of imidazothiazole derivatives as IDO1 inhibitors. ACS Med Chem Lett 2014;5:1119–23.