Abstract
β-Lactam antibiotics are a broad class of antibiotics, consisting of all antibiotic agents that contain a β-lactam ring in their molecular structures. Synthesis of β-lactam analogs, which are containing dichloride atoms and N-methyl, N-aromatic rings, was achieved by Schiff bases and dichloroketene compounds. All the synthesized imines and β-lactam analogs were tested against two physiologically relevant carbonic anhydrase isozymes (hCA I and II) and acetylcholinesterase (AChE). They demponstrated effective inhibitory profiles with Ki values in ranging of 3.22-11.18 nM against hCA I, 3.74-10.41 nM against hCA II, and 0.50-1.57 nM against AChE. On the other hand, acetazolamide and dorzolamide clinically used as CA inhibitors, showed Ki value of 170.34 and 129.26 nM against hCA I, and 115.43 and 135.67 nM against hCA II, respectively. Also, tacrine used as standard AChE inhibitor showed Ki value of 5.70 nM against AChE.
Introduction
The effect of a substituent such as halogen on the biological activity of a potential drug structure or bioactive organic compound still has to be planned for experimentally in biological studies designed to detect intended activity. In some position on molecule skeleton, the presence of active atoms such as a chlorine atom is very important for the specific activity of a compound obtained by organic reactions. Sometimes chlorine atom demonstrates to be the optimum substituent of a chemical molecule for developed activity. As it is known that chlorinated organic compounds are not generally hazardous and toxic, the chlorine atom is used as a useful substituent in synthetic organic chemistry by chemists. Nowadays, it remains an important tool for investigation between structure and activity relationships in biochemistry researches and as a substituent in organic molecular structures in commercialized compoundsCitation1. Especially, chloro atom containing β-lactam derivatives are interesting compounds in terms of pharmaceutical and synthetic organic chemistry. Due to bioacvtivity properties, β-lactam molecules have widely large area in drug chemistry; new synthetic methods developed for these molecules are an precious issue in organic chemistry. As known, the β-lactam structure is part of the moiety of several antibiotic molecules such as carbapenems (1), pennicillins (2), and cephalosporins (3), therefore, also called β-lactam antibioticsCitation1. Particularly, all of β-lactam antibiotics inhibit bacteria cell wall biosynthesis. Up to now, more than thousand different β-lactamase enzymes have been reported in several species of bacteria. These enzymes influence widely β-lactam structure and catalytic efficienciesCitation2.
The carbonic anhydrases (CAs, EC 4.2.1.1) are widespread metalloenzymes family and catalyze the reversible hydration of carbondioxide (CO2) and water (H2O) to () and proton (H+)Citation3–7. CAs have six genetically and distinct enzyme families, including the α-, β-, γ-, δ-, ζ-, and n-CACitation8–10. This regulatory reaction supports many physiological processes associated with pH control, fluid secretion, ion transport, and several biosynthetic processesCitation11–15. The inhibition of CAs has been exploited clinically for many decades, as most CA isoforms of the 16 known in humans are therapeutic targets with the potential to be inhibited or activated Citation16–20. The two of them (hCA I and II) are cytosolic ones. The catalytic activity of hCA I, which shows blood enzyme, is much lower compared to that of hCA II, and in addition hCA I is inhibited by the chloride and bicarbonate present in the plasma, raising a lot of questions regarding the physiologic function of this isoformCitation15,21–24. The physiologically dominant isoform is the red blood cell CA II, which present in many other tissues and its inhibitors have widespread use in a variety of applications as diuretics, antiglaucoma, and antiepileptic agentsCitation25–30. The discovery of new classes of CA inhibitors (CAIs), which possessing different inhibition mechanisms when compared with the classical inhibitorsCitation15,31–35. For such reasons, the design of novel classes of potent, possibly isoform-selective inhibitors targeting other human CA (hCA) isoforms, may lead to clinical applications for treating a multitude of diseases such as edema, epilepsy, obesity, neuropathic pain, and other neurological disordersCitation36–40.
Alzheimer’s disease (AD) is a progressive neuro-degenerative disorder and the fourth leading cause of death in people over 65-year-old worldwide. AD is characterized by the atrophy of cholinergic neurons, behavioral abnormalities, deterioration of cognition, memory loss, loss of speech, and, eventually, deathCitation41–44. Although the etiology of this diseae is still elusive, several hallmarks, such as low levels of acetylcholine, oxidative stress, β-amyloid deposits, γ-protein aggregation, and the dyshomeostasis of biometals, are thought to play crucial roles in the development of ADCitation41,Citation45–47. Because of the complex pathogenesis of AD, there is no ideal drug for the prevention or treatment of AD up to now. Acetylcholinesterase (AChE, EC 3.1.1.7), a serine protease, is responsible for acetylcholine (ACh) hydrolysis and plays a fundamental role in impulse transmission by terminating the action of the neurotransmitter ACh at the cholinergic synapses and neuromuscular junctionCitation48,Citation49. One of the best primary pharmacological strategies for the treatment of AD is to improve cholinergic neurotransmission by decreasing the rate of decomposition of ACh at synapses in the brain with the use of acetylcholinesterase inhibitors (AChEIs)Citation50.
The aim of this study is to design and synthesize some imines (10–14) and β-lactam analogs (15–19) and to investigate their potential carbonic anhydrase isoenzymes I and II (CA I and II) and acetylcholinesterase (AChE) inhibition properties.
Materials and methods
The synthesis of Schiff bases from 2-naphthalene aldehyde and amine compounds
Aryl or methyl amines (5–9) (1.0 mmol) were dissolved in 30-mL ethyl alcohol and added for 10 min in droplets to 0.05% 3 mL H2SO4 acid and aldehyde (4) (1.0 mmol) solution dissolved in 20 mL ethyl alcohol. After that, reaction was refluxed at 80–90 °C for 24 h. At the end of the reaction followed by TLC, reaction mixture was taken to a 250 mL beaker glass and added aqueous Na2CO3 solution, so H2SO4 was removed from the reaction medium. Obtained Schiff base mixture was taken to organic phase by extracting and using ethyl acetate (3 × 100 mL). The water in organic phase was removed by drier Na2SO4 after organic phase was washed off in 100 mL saturated NaHCO3. Organic solvent was removed in vacuum (20 °C, 20 mm Hg). Remnant crude product was purified using silica gel through column chromatography in ethylacetate:methanol:n-hexane (7:1:2) solvent mixture.
Typical procedure for dichloroketene cycloadditions
A solution of 0.025 mol of freshly distilled trichloroacetyl chloride in 250 mL of dry ether was added over 4 h to a stirred, refluxing mixture of 0.025 mol of Schiff base (imine) in 250 mL of dry ether and 5 g of activated zinc under a nitrogen atmosphere. The reaction mixture was stirred at reflux for an additional 10 h after the addition was complete. The excess zinc was filtered, washed, and dried. The reaction solution was concentrated to about 50 mL and then stirred with 100 mL of n-hexane. The hexane solution was decanted from the zinc salts and evaporated, and the residue was purificated on silica-jel coloumn chromatography to yield the cycloadduct.
Chemistry
Anhydrous solvents and all reagents were purchased from Sigma-Aldrich and Alfa Aesar Nuclear magnetic resonance (1H-NMR and 13C-NMR); spectra were recorded using a Varian 400 MHz or Bruker Advance 400 DPX MHz spectrometer (Erzurum, Turkey) in CDCl3; Chemical shifts are reported in parts per million (ppm), and the coupling constants (J) are expressed in Hertz (Hz). Splitting patterns are designated as follows: s, singlet; d, doublet; sept, septet; t, triplet; q, quartet; m, multiplet; brs, broad singlet; brm, broad multiplet; dd, double of double; td, triplet of double; tt, triplet of triplet; appd, apparent double; appt, apparent triplet. Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel F-254 plates.
We synthesized the β-lactam analogs containing dichloride atoms and N-methyl, N-aromatic rings as substituents, by Schiff bases and dichloroketene compounds. Synthesis of molecules comprising Schiff base has long been attracting attentions due to their biological activities such as anticancer, antibacterial, antifungal, antidepressant, analgesic, and cytotoxic effects in addition to their regular use in electronic industry, cosmetic, and polymer industry. Schiff bases are frequently used in addition reactions, Staudinger Reactions, Hetero–Diels–Alder reactions, and several ligand complexes, thanks to their structural characteristicsCitation51,Citation52.
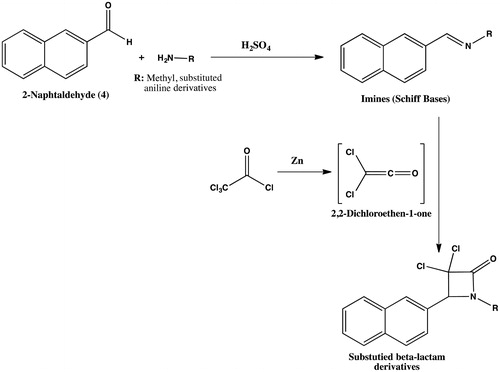
In this study, first, imine groups known as Schiff bases in literature, 1-(naphthalene-2-yl)-N-phenylmethanimine (10)Citation53, N-(4-ethylphenyl)-1-(naphthalene-2-yl)methanimine (11), N-mesityl-1-(naphthalene-2-yl)methanimine (12), 1-(naphthalene-2-yl)-N-(p-tolyl) methan-imine (13)Citation54, N-methyl-1-(naphthalene-2-yl) methanimine (14)Citation55, were synthesized from corresponding aromatic aldehyde (4) and amine derivatives aniline (5), 4-ethylaniline (6), 2,4,6-trimethylaniline (7), p-toluidine (8), methanamine (9). Some Schiff bases (10, 13, 14) as known in literature and other Schif bases were synthesized in this study. After characterization of imine compounds by NMR spectrum, dichloroketene (2,2-dichloroethene-1-one), compounds were obtained with Zn from trichloride acetyl chloride and active zinc metal in basic media in high yields (Scheme 1).
These imines were purified by easy methods such as crystallization method or silica-gel colomn chromatography. The yields and reaction conditions of synthesized imines were shown in .
Table 1. The synthesis of imine derivatives (10–14).
In second step, the β-lactam derivatives 3,3-dichloro-4-(naphthalene-2-yl)-1-phenylazetidin-2-one (15), 3,3-dichloro-1-(4-ethylphenyl)-4-(naphthalene-2-yl)azetidin-2-one (16), 3,3-dichloro-1-mesityl-4-(naphthalene-2-yl)azetidin-2-one (17), 3,3-dichloro-4-(naphthalene-2-yl)-1-(p-tolyl) azetidin-2-one (18), 3,3-dichloro-1-methyl-4-(naphthalene-2-yl)azetidin-2-one (19) was synthesized with [2 + 2] ketene addition reaction from dichloroketene and corresponding imine derivatives obtained from 10–14 amines and 2-naphtalaldehyde (4) in acidic reaction condition (Scheme 1). All of the β-lactam structures 15–19 were synthesized first time in literature in this study. The dichloroketene (2,2-dichloroethene-1-one) was also obtained from Zn and trichloroacetyl chloride at 40 °C in 10 h (). The yields of addition reaction were changed according to the structure of imine compounds. All of the compounds were purified by coloumn chromatography and characterized by NMR spectroscopy.
Table 2. The addition reactions of imines and dichloroketene compounds.
NMR spectral data of first synthesized imines and β-lactam molecules
N-(4-Ethylphenyl)-1-(naphthalene-2-yl)methanimine (11): 1H-NMR (400 MHz, CDCl3, ppm): δ = 8.46 (s, 1H), 8.19 (m, 2H), 7.92 (m, 3H), 7.56 (m, 2H), 7.28 (m, 4H), 2.71 (q, J = 7.4, 7.6 Hz 2H), 1.3 (t, J = 7.7 Hz, 3H), 13C-NMR (100 MHz, CDCl3, ppm): δ = 159.8, 149.9, 142.6, 135.2, 134.4, 133.4, 131.3, 129.0, 128.9, 128.2, 127.7, 126.8, 124.2, 121.2, 28.7, 15.9.
N-Mesityl-1-(naphthalene-2-yl)methanimine (12): 1H-NMR (400 MHz, CDCl3, ppm): δ = 8.39 (s, 1H), 8.25 (d, J = 1.5 Hz, 8.4 Hz, 1H), 8.18 (s, 1H), 7.95 (m, 3H), 7.57 (m, 2H), 6.9 (s, 2H), 2.3 (s, 3H), 2.2 (s, 6H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 162.9, 149.1, 135.3, 134.1, 133.6, 133.3, 131.1, 129.1, 129.0, 128.2, 127.7, 127.3, 126.9, 123.9, 21.0, 18.5.
3,3-Dichloro-4-(naphthalene-2-yl)-1-phenylazetidin-2-one (15): 1H-NMR (400 MHz, CDCl3, ppm): δ = 7.1–7.9 (m, 12H), 5.6 (s, 1H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 158.6, 133.7, 136.1, 134.1, 133.2, 129.3, 128.7, 128.4, 128.1, 127.9, 127.6, 127.4, 127.1, 125.8, 124.5, 118.3, 74.4.
3,3-Dichloro-1-(4-ethylphenyl)-4-(naphthalene-2-yl)azetidin-2-one (16): 1H-NMR (400 MHz, CDCl3, ppm): δ = 7.3–7.9 (m, 7H), 7.2 (d, J = 8.4 Hz, 2H), 7.1 (d, J = 8.4 Hz, 2H), 5.6 (s, 1H), 2.5 (q, J = 7.3, 7.5 Hz, 2H), 1.1 ppm (t, J = 7.7 Hz, 3H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 165.1, 142.0, 134.1, 133.8, 133.2, 129.9, 129.5, 129.1, 128.9, 128.4, 128.1, 127.8, 127.3, 127.0, 124.6, 118.2, 74.3, 31.8, 15.7.
3,3-Dichloro-1-mesityl-4-(naphthalene-2-yl)azetidin-2-one (17): 1H-NMR (400 MHz, CDCl3, ppm): δ = 7.4–7.8 (m, 7H), 6.8 (bs, 2H), 5.9 (s, 1H), 2.4 (bs, 6H), 2.2 (s, 3H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 160.2, 138.6, 133.9, 133.0, 130.4, 130.2, 129.8, 128.7, 128.4, 128.0, 127.8, 127.3, 126.9, 126.4, 125.2, 124.9, 77.9, 21.0, 19.7.
3,3-Dichloro-4-(naphthalene-2-yl)-1-(p-tolyl)azetidin-2-one (18): 1H-NMR (400 MHz, CDCl3, ppm): δ = 7.0–7.8 (m, 11H), 5.6 (s, 1H), 2.2 (s, 3H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 167.5, 135.7, 130.1, 129.1, 128.8, 128.4, 127.9, 127.7, 127.5, 127.3, 127.0, 126.9, 126.8, 126.7, 126.5, 118.2, 74.3, 21.2.
3,3-Dichloro-1-methyl-4-(naphthalene-2-yl)azetidin-2-one (19): 1H-NMR (400 MHz, CDCl3, ppm): δ = 7.3–7.9 (m, 7H), 5.2 (s, 1H), 3.0 (s, 3H). 13C-NMR (100 MHz, CDCl3, ppm): δ = 162.1, 134.1, 133.2, 129.4, 129.3, 129.0, 128.7, 128.3, 128.1, 127.4, 127.1, 124.7, 75.9, 28.0.
Biochemical studies
For the determination of inhibition effects of imines (10–14) and β-lactam analogs (15–19) on CA isoforms I and II. Both isoenzymes were purified from fresh human erythrocyte using affinity chromatography techniqueCitation56–60. Chromatographic separation technique was widely used for the purification of biomolecules, including protein and enzymes. To this end, sepharose-4B-L-tyrosine-sulfanilamide affinity chromatography was used for the purification of both isoenzymesCitation61–63 as described previouslyCitation64–66.
CA activity determination was realized spectrophotometrically according to Verpoorte et al.Citation67 as described previouslyCitation49. In this method, absorbance change was recorded during 3 min at 25 °C. p-Nitrophenylacetate (NPA) was used as substrate for both isoenzymes and enzymatically converted to p-nitrophenolate ionCitation68. These activity determinations are described in detail in our previous studiesCitation69.
Bradford method was used for the determination of protein quantity during the purification stepsCitation70. This spectrophotometrical protein assay was explained as previouslyCitation71. The bovine serum albumin was used as standard protein which demonstrated maximum activity at 595 nmCitation71–73.
After the purification process of the CA isoenzymes, SDS-PAGE has been carried outCitation74–76. Stacking gel containing (10 and 3%) acrylamide and (0.1%) SDSCitation77,Citation78 was used for running the process using a Minigel system (Mini-PROTEAN Tetra System, China). The method used for visualization of protein has been explained in detail at previous studiesCitation79. Briefly, the gel was fixed and then stained with Coomassie Brilliant Blue R-250 later on the gel destained by using standard methods for detecting protein bands that are belong to purified CA isoenzymesCitation80,Citation81.
The inhibitory effects of imines (10–14) and β-lactam analogs (15–19) on AChE activitiy were measured according to the spectrophotometric method of Ellman et al.Citation82 Acetylthiocholine iodide (AChI) was used as substrate for the enzymatic reaction. 5,5′-Dithio-bis(2-nitro-benzoic) acid (DTNB) was used for the measurement of the AChE activitiy. Briefly, 100 μL of Tris/HCl buffer (1 M, pH 8.0), 10 μL of sample solution dissolved in deionized water at different concentrations, and 50 μL of AChE solution were mixed and incubated for 10 min at 25 °C. Then, 50 μL of DTNB (0.5 mM) was added. Then reaction was initiated by the addition of 50 μL of AChI. The hydrolysis of these substrates was monitored spectrophotometrically by formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by enzymatic hydrolysis of AChI, with an absorption maximum at a wavelength of 412 nmCitation50.
The effect of imines (10–14) and β-lactam analogs (15–19) on both CA isoenzymes was examined using the hydratase activity and recorded in triplicate analysis at the each concentration usedCitation83. For this purpose, different concentrations of imines (10–14) and β-lactam analogs (15–19) were determined in preliminary assays. CA isoenzyme activities were measured in the presence of different quantity of them. The control sample activity in the absence of a imines (10–14) and β-lactam analogs (15–19) was taken as 100%. For each imines (10–14) and β-lactam analogs (15–19), an activity (%)-[imines or β-lactams] was drawn using conventional polynominal regression software. The half maximal inhibitory concentration (IC50) of each imines (10–14) and β-lactam analogs (15–19) was calculated from graphsCitation50. IC50 values are measure of the effectiveness of imines (10–14) and β-lactam analogs (15–19) in inhibiting both CA isoenzymes. For the determination of Ki values, three different imines (10–14) and β-lactam analogs (15–19) concentrations were used. Ki values reflect the binding affinity of imines (10–14) and β-lactam analogs (15–19) to both CA isoenzymes. In this way, The IC50 value is converted to an absolute inhibition constant Ki value. In this experiment, PNA was used as substrate at five different concentrations. Finally, Lineweaver–Burk curves were drawn for each imine (10–14) and β-lactam analog (15–19)Citation50.
Metal-chelating study
Metal-chelating ability of imines (10–14) and β-lactam analogs (15–19) was performed according to Dinis et al.Citation84 with slight modificationCitation85–88. Fe2+-binding capacity of imines (10–14) and β-lactam analogs (15–19) was spectrophotometrically recorded at 562 nmCitation88–91. For this purpose, to a mixture of FeCl2 (0.1 mL, 0.6 mM), three different concentrations (10–30 μg/mL) of imines (10–14) and β-lactam analogs (15–19) in methanol (0.4 mL) were added. The reactions were started by ferrozine solution addition (0.1 mL, 5 mM). After that, the solution was mixed and incubated at room temperature for 10 min. Finally, absorbance value of the mixture of imines (10–14) and β-lactam analogs (15–19) was determined spectrophotometrically at 562 nmCitation92–95.
Result and discussion
Chemistry
Many chlorinated organic compounds are known for their detrimental effects as a toxicological properties and environmentally harmful chemicals. However, chlorinated organic molecules, molecular weight range from 200 to 600, are used as pharmaceutical or biologically active substances in drug chemistry and plant protection agents, which are important and essential chemicals in green chemistry. As a result of the scientific investigation, it has been experimentally found that the introduction of a chlorine atom into several specific positions of a biologically active molecule may be essential to improve the basic biological activity.
Biochemistry
The discovery of new classes of CAIs, possessing different inhibition mechanisms compared with the classical inhibitors of the sulfonamide and anion type, has also seen important developments ultimatelyCitation96. So far, five different CA inhibition mechanisms were reported: (i) the zinc ion (Zn2+) binders are the inhibitors which coordinate to the catalytically crucial Zn2+ from the enzyme active site. In this inhibition type, Zn2+ may be in a tetrahedral or trigonal bipyramidal geometries, with the sulfonamides and sulfamides, sulfamates, most anions, dithiocarbamates, carboxylates, and hydroxamates binding in this wayCitation97; (ii) the inhibitors that anchor to the Zn2+-coordinated water molecule (H2O)/hydroxide ion (–OH), represented by the phenols, some carboxylates, the polyamines, 2-thioxocoumarins, and sulfocoumarinsCitation96,Citation98; (iii) the inhibitors-like coumarins and their isosteres which occlude the entrance to the active site cavity, this binding site coinciding with that where CA activators bindCitation99; (iv) the compounds which bind out of the active site cavity, and (v) compounds for which the inhibition mechanism is not known, among which the secondary or tertiary sulfonamides are the most investigated examplesCitation100.
The hCA I and II isoenzyme inhibitory activity of the imines (10–14) and β-lactam analogs (15–19) was shown in . CA II isoform was chosen because of its antiglaucoma drug targets, however CA I, due to its diffuse distribution in the blood and gastrointestinal tract is one of the main off-targets for such pharmacologic agentsCitation101. On the other hand, AChE was chosen for its significant contribution in drug discovery and development for AD. All the synthesized imines (10–14) and β-lactam analogs (15–19) were tested for the evaluation of their inhibitory activity toward the slower cytosolic hCA I isoform, the more rapid cytosolic hCA II isoenzyme and AChE enzyme. Also, these novel derivatives showed effective ferrous ions (Fe2+)-chelating effects. The following results can be observed from the inhibition data of :
Cytosolic hCA I isoenzyme was potently inhibited by imines (10–14) and β-lactam analogs (15–19). Ki values were found in ranging between 3.22 ± 0.22 and 11.18 ± 1.98 nM (). The best inhibition for this isoform was observed by novel β-lactam analog 16 (3,3-dichloro-1-(4-ethylphenyl)-4-(naphthalene-2-yl)azetidin-2-one), which possess biological active groups of –C=O, –CH3, and Cl with Ki value of 3.22 ± 0.22 nM. It is well-known that CA isoenzymes easily inhibited by the compounds, which had these biologically active groups. On the other hand, as can seen in acetazolamide (AZA, 5-acetamido-1,3,4-thiadiazole-2-sulfonamide) and dorzolamide (DZA, N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide) used as CAI for the medical treatment of some diseases including idiopathic intracranial hypertension, epileptic seizure, glaucoma, altitude sickness, periodic paralysis, cystinuria, central sleep apnea, and dural ectasia, showed Ki values of 170.34 ± 2.48 and 129.26 ± 0.21 nM, respectively. The hCA I is highly abundant in red blood cells and is found in many tissues but its precise physiological function is unknown. CA I is associated with cerebral and retinal edema, and the inhibition of CA I may be a valuable tool for fighting these conditionsCitation6.
The ubiquitous and physiologically predominant cytosolic isoform hCA II is associated with several diseases. For hCA II, β-lactam analogs (15–19) had Ki values ranging in 3.74 ± 0.39–10.29 ± 3.55 nm. On the other hand, acetazolamide (AZA, 5-aceta-mido-1,3,4-thiadiazole-2-sulfonamide), a clinically used compound, was a medium potency CA II inhibition for this isoform, with an inhibition constant of 115.43 ± 1.63 nM. Also, dorzolamide (DZA), a carbonic anhydrase inhibitor, demonstrated Ki value of 135.67 ± 0.32 nM. DZA was used as antiglaucoma agent and acts by decreasing the production of aqueous humourCitation102. All of the derivatives showed highly effective hCA II inhibitory activity, comparable to that of AZA and DZA. As can be seen in CA I, the most inhibition effect of CA II was observed by β-lactam analog 16 (3,3-dichloro-1-(4-ethylphenyl)-4-(naphthalene-2-yl)azetidin-2-one) with Ki values of 3.74 ± 0.39 nM.
Effective AChE inhibitors can be used for AD treatment. Most of the currently available drugs on the market including tacrine, rivastigmine, donepezil, and galantamine intended to treat AD are AChE inhibitorsCitation103. The inhibition effects of some imines (10–14) and β-lactam analogs (15–19) against AChE activities were measured according to spectrophotometric Ellman methodCitation82. AChI was used as substrates of the reaction. This method is based on the amount of thiocholine released when the enzyme AChE hydrolyzes the AChI to thiocholine and acetate. The product thiocholine reacts with DTNB to produce a yellow compound (5-thio-2-nitrobenzoate) anion, which can be detected at a wavelength of 412 nm. AChE was very effectively inhibited by imines (10–14) and β-lactam analogs (15–19). It was found that Ki values were ranging between 0.50 ± 0.20 and 1.57 ± 0.55 nM (). All imines (10–14) and β-lactam analogs (15–19) demonstrated similar inhibiton profile against AChE. On the other hand, tacrine, a first centrally acting cholinesterase inhibitor approved for the treatment of AD, was used as a standard AChE with Ki values of 5.70 ± 1.09 nM. Imines 10 (1-(naphthalene-2-yl)-N-phenylmethanimine), which shown the weakest AChE inhibiton, had 3.63-times AChE inhibiton effects than that of Tacrine. The Ki values of imines (10–14) and β-lactam analogs (15–19) for AChE were calculated from Lineweaver–Burk plotsCitation83.
Metal-chelating measurement is an antioxidant method, which based on the absorbance measurement of ferrous ion (Fe2+)-ferrozine complex after prior treatment of a Fe2+ solution with test materialCitation104–106. Ferrozine forms a complex with free Fe2+, but not with Fe2+ bound to other chelators; thus, a decrease in the amount of Fe2+-ferrozine complex formed after treatment indicates the presence of antioxidant chelatorsCitation107,Citation108. The ferrozine–Fe2+ complex produced a red chromophore with absorbance that can be measured at 562 nmCitation109,Citation110. A significant drawback of this complexation reaction in measuring the presence of antioxidant chelator is that the reaction is affected by both the antioxidant-Fe2+ and ferrozine–Fe2+ complex formation constants, and the competition between the two chelators for binding to iron. Thus, a weak antioxidant iron chelator would be seriously underestimated in quantitative determinationCitation111,Citation112. The metal-chelating capacity was significant since it reduced the concentration of the catalyzing transition metal in lipid peroxidation. It was reported that chelating agents are effective as secondary antioxidants because they reduce the redox potential, and, thereby, stabilize the oxidized form of the metal ionCitation113,Citation114. EDTA is a strong metal chelator; hence, it was used as standard metal chelator agent in this study. The data obtained from reveal that imines (10–14) and β-lactam analogs (15–19) possess marked iron-binding capacity, suggesting that their main action as a peroxidation inhibitor may be related to their iron-binding capacity. The distinction between different concentrations (10–30 μg/mL) of imines (10–14) and β-lactam analogs (15–19) and the control value was fixed to be statistically important (p < 0.01). Furthermore, it is found that IC50 values for imines (10–14) and β-lactam analogs (15–19) were found in the range of 49.50–230.01 μg/mL (). Whereas, IC50 values belonging to Fe2+ ion-chelating capacity of positive controls like α-tocopherol, trolox, BHT, BHA, and EDTA were found to be in range of 12.83–57.75 μg/mL. A lower IC50 value reflects a higher Fe2+ ions-binding activity. These results clearly show that Fe2+ ion-chelating effect of imines (10–14) and β-lactam analogs (15–19) had effective Fe2+ ions chelating; however, these values were lower than that of standard metal chelators. Fe2+ ions are the most efficient pro-oxidants in pharmacology systems and foodCitation115,Citation116. Ferrozine can create complexes with Fe2+. In the presence of Fe2+- chelating compounds, ferrozine-Fe2+ complex formation is a broken down, resulting in a decrease in the red color formation of Ferrozine-Fe2+ complexCitation117,Citation118.
Figure 1. Standard compounds used for carbonic anhydrase I and II isoenzymes (acetazolamide and dorzolamide) and acetylcholinesterase (tacrine) inhibitors.
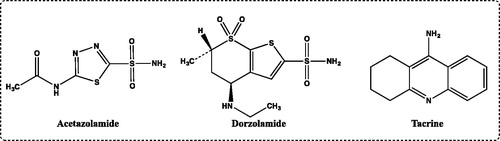
Table 3. The enzyme inhibition values of some imines (10–14) and β-lactam analogs (15–19) against human carbonic anhydrase isoenzymes I and II (hCA I and II) and acetylcholinesterase (AChE) enzyme.
Table 4. Determination of half maximal concentrations (IC50, μg/mL) of Fe2+ chelating of some imines (10–14) and β-lactam analogs (15–19) and standard compounds.
Conclusion
In the present study, practical and efficient method has been developed for N-aryl β-lactams the first time in literature from Schiff bases and simple dichloroketene compound. Schiff base reactions are widely used in chemistry due to their mild reaction conditions and high reaction rates. The synthesis of β-lactams derived 15–19 from these Schif bases and the dichloroketene 4 by using [2 + 2] addition reaction. It is evident from the results of the investigation that a effective method has been obtained, by EtOH solvent under acidic conditions, for the synthesis of Schiff bases from corresponding amines 10–14 and 2-naphtalaldehyde (4) in high yields in our study. The imines (10–14) and β-lactam analogs (15–19) used in the present study showed effective inhibition profiles against AChE enzyme and both hCA isoenzymes. Also, these compounds demonstrated effective ferrous ion (Fe2+)-chelating activity. In this study, nanomolar level of Ki values was observed for each novel imines (10–14) and β-lactam analogs (15–19), and these compounds can be selective inhibitor of both cytosolic CA isoenzymes and AChE enzyme. Also, they can be used as metal chelators in related applications.
Declaration of interest
The authors declare no conflict of interest.
Supplementary material available online
IENZ1170014_Supplementary_Material.pdf
Download PDF (1 MB)References
- Naumann K. How chlorine in molecules affects biological activity. Brussels: Science Dossier; 2003:1–37
- Holten KB, Onusko EM. Appropriate prescribing of oral beta-lactam antibiotics. Am Fam Phys 2000;62:611–20
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–16
- Beydemir Ş, Gülçin İ. Effects of melatonin on carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2004;19:193–7
- ArasHisar Ş, Hisar O, Beydemir Ş, et al. Effect of vitamin E on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Acta Vet Hung 2004;52:413–22
- Taslimi P, Gulcin İ, Ozgeris B, et al. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J Enzyme Inhib Med Chem 2016;31:152–7
- Scozzafava A, Kalin P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810
- Hisar O, Beydemir Ş, Gülçin İ, et al. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhyncytes mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–9
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Hisar O, Beydemir Ş, Gülçin İ, et al. The effect of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk J Vet Anim Sci 2005;29:841–5
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Scozzafava A, Passaponti M, Supuran CT, Gülçin İ. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91
- Supuran CT. Carbonic anhydrase inhibitors: an editorial. Expert Opin Ther Pat 2013;23:677–9
- Arabaci B, Gülçin İ, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2015;19:10103–14
- Supuran CT. Carbonic anhydrases. Bioorg Med Chem 2013;21:1377–8
- Göçer H, Akıncıoğlu A, Göksu S, et al. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Akbaba Y, Bastem E, Topal F, et al. Synthesis and carbonic anhydrase inhibitory effects of novel sulfamides derived from 1-aminoindanes and anilines. Arch Pharm 2014;347:950–7
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg Med Chem 2014;22:3537–43
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902
- Pinard MA, Boone CD, Rife BD, et al. Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases. Bioorg Med Chem 2013;21:7210–15
- Çetinkaya Y, Göçer H, Gülçin İ, Menzek A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm 2014;347:354–9
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32
- Aksu K, Nar M, Tanç M, et al. Synthesis and carbonic anhydrase inhibitory properties of sulfamides structurally related to dopamine. Bioorg Med Chem 2013;21:2925–31
- Akıncıoğlu A, Akbaba Y, Göçer H, et al. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg Med Chem 2013;21:1379–85
- Gülçin İ, Beydemir S. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6
- Öztürk Sarıkaya SB, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62
- Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors: inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
- Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9
- Innocenti A, Gülçin İ, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3
- Innocenti A, Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64
- Mao F, Li J, Wei H, et al. Tacrine-propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem 2015;30:995–1001
- Öztaşkin N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57
- Polat Köse L, Gülçin İ, Gören AC, et al. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crops Prod 2015;74:712–21
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266–75
- Iqbal K, del C, Alonso A, Chen S, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 2005;1739:198–210
- Pratico D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci 2008;29:609–15
- Aksu K, Topal F, Gülçin I, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm 2015;348:446–55
- Göçer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch Pharm 2013;346:783–92
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2016;31:441–7
- Gülçin İ, Scozzafava A, Supuran CT, et al. The effect of caffeic acid phenethyl ester (CAPE) metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione s-transferase, lactoperoxidase and carbonic anhydrase ısoenzymes I, II, IX and XII. J Enzyme Inhib Med Chem 2015;9:1–7
- Tidwell TT. Hugo (Ugo) Schiff, Schiff Bases, and a century of β-lactam synthesis. Angewandte Chemie 2008;47:1016–20
- Jia Y, Li J. Molecular assembly of schiff base interactions: construction and application. Chem Rev 2015;115:1597–621
- Naka H, Koseki D, Kondo Y. Catalytic deprotonative functionalization of propargyl silyl ethers with imines. Adv Synth Catal 2008;350:1901–6
- Orita A, Uehara G, Miwa K, Otera J. Rate acceleration of organic reaction by immediate solvent evaporation. Chem Commun 2006;7:4729–31
- Artunç T, Çetinkaya Y, Göçer H, et al. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Des 2016;87:594–607
- Atasever A, Özdemir H, Gülçin İ, Küfrevioğlu Öİ. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70
- Beydemir Ş, Gülçin İ, Küfrevioğlu Öİ, Çiftçi M. Glucose 6-phosphate dehydrogenase: in vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 2003;55:787–92
- Gülçin İ, Küfrevioğlu Öİ, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302
- Gülçin İ, Yıldırım A. Purification and characterization of peroxidase from Brassica oleracea var. Acephala. Asian J Chem 2005;17:2175–83
- Beydemir Ş, Gülçin İ, Hisar O, et al. Effect of melatonin on glucose-6-phospate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. J Appl Anim Res 2005;28:65–8
- Gülçin İ, Beydemir Ş, Hisar O. The effect of α-tocopherol on the antioxidant enzymes activities and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Acta Vet Hung 2005;53:425–33
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–70
- Şentürk M, Gülçin İ, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Göcer H, Akıncıoğlu A, Göksu S, İ Gülçin I. Carbonic anhydrase inhibitory properties of phenolic sulfonamides derived from dopamine related compounds. Arab J Chem 2016. [Epub ahead of print]. http://dx.doi:10.1016/j.arabjc.2014.08.005
- Taslimi P, Gülçin İ, Öztaşkin N, et al. The effects of some bromophenol derivatives on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:603–7
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Gül Hİ, Kucukoglu K, Yamali C, et al. Synthesis of 4-(2-substitutedhydrazinyl)benzenesulfonamides and their carbonic anhydrase inhibitory effects. J Enzyme Inhib Med Chem 2016;31:568–73
- Gocer H, Aslan A, Gülçin İ, Supuran CT. Spirobisnaphthalenes effectively inhibit carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:503–7
- Bradford MM. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein dye binding. Anal Biochem 1976;72:248–54
- Ayık O, Hisar O, Gülçin İ, et al. Effects of light on trace elements and thriiodothyronine levels in plasma of mirror carp. Biol Trace Elem Res 2005;108:147–54
- Birdane FM, Cemek M, Birdane YO, et al. Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J Gastroenterol 2007;13:607–11
- Cankaya M, Hernandez AM, Ciftci M, et al. An analysis of expression patterns of genes encoding proteins with catalytic activities. BMC Genomics 2007;8:232
- Şişecioğlu M, Çankaya M, Gülçin İ, Özdemir M. Interactions of melatonin and serotonin to lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2010;25:779–83
- Şişecioğlu M, Gülçin İ, Çankaya M, et al. Purification and characterization of peroxidase from Turkish black radish (Raphanus sativus L.). J Med Plants Res 2010;4:1187–96
- Koksal Z, Kalın R, Gülçin İ, et al. The impact of some avermectins on lactoperoxidase from bovine milk. Int J Food Propert 2016;19:1207–16
- Şişecioğlu M, Kireçci E, Çankaya M, et al. The prohibitive effect of lactoperoxidase system (LPS) on some pathogen fungi and bacteria. Afr J Pharm Pharmacol 2010;4:671–7
- Köksal E, Ağgül AG, Bursal E, Gülçin İ. Purification and characterization of peroxidase from sweet gourd (Cucurbita Moschata Lam. Poiret). Int J Food Propert 2012;15:1110–19
- Şişecioğlu M, Gülçin İ, Çankaya M, Özdemir H. The inhibitory effects of L-adrenaline on lactoperoxidase enzyme (LPO) purified from buffalo milk. Int J Food Propert 2012;15:1182–9
- Ozturk Sarikaya SB, Sisecioglu M, Cankaya M, et al. Inhibition profile of a series of phenolic acids on bovine lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2015;30:479–83
- Aydin B, Gülcin I, Alwasel SH. Purification and characterization of polyphenol oxidase from Hemşin apple (Malus communis L.). Int J Food Propert 2015;18:2735–45
- Ellman GL, Courtney KD, Andres V, Featherston RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212
- Talaz O, Gülçin İ, Göksu S, Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–9
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25:44–53
- Kalın P, Gülçin İ, Gören AC. Antioxidant activity and polyphenol content of Vaccinium macrocarpon. Rec Nat Prod 2015;9:496–502
- Sehitoglu MH, Han H, Kalin P, et al. Pistachio (Pistacia vera L.) Gum: a potent inhibitor of reactive oxygen species. J Enzyme Inhib Med Chem 2015;30:264–9
- Bursal E, Köksal E, Gülçin İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res Int 2013;51:66–74
- Gülçin İ, Elmastaş M, Aboul-Enein HY. Antioxidant activity of clove oil – a powerful antioxidant source. Arab J Chem 2012;5:489–99
- Çetinkaya Y, Göçer H, Menzek A, Gülçin İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Pharm 2012;345:323–34
- Gülçin İ, Beydemir S, Topal F, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587–94
- Bursal E, Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–9
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Ferraroni M, Carta F, Scozzafava A, Supuran CT. Thioxocoumarins show an alternative carbonic anhydrase inhibition mechanism compared to coumarins. J Med Chem 2016;59:462–73
- Isik S, Vullo D, Bozdag M, et al. 7-Amino-3,4-dihydro-1H-quinolin-2-one, a compound similar to the substituted coumarins, inhibits α-carbonic anhydrases without hydrolysis of the lactam ring. J Enzyme Inhib Med Chem 2015;30:773–7
- Küçükbay FZ, Küçükbay H, Tanc M, Supuran CT. Synthesis and carbonic anhydrase I, II, IV and XII inhibitory properties of N-protected amino acid-sulfonamide conjugates. J Enzyme Inhib Med Chem. [Epub ahead of print]. http://dx.doi.org/10.3109/14756366.2016.1147438
- Durdagi S, Vullo D, Pan P, et al. Protein-protein interactions: inhibition of mammalian carbonic anhydrases I-XV by the murine inhibitor of carbonic anhydrase and other members of the transferrin family. J Med Chem 2012;55:5529–35
- Grover S, Apushkin M, Fishman G. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol 2006;141:850–8
- Stepankova S, Komers K. Cholinesterases and cholinesterase inhibitors. Curr Enzyme Inhib 2008;4:160–71
- Gülçin İ, Topal F, Çakmakçı R, et al. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:C585–93
- Gülçin İ, Topal F, Oztürk Sarikaya SB, et al. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–75
- Gülçin İ. Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food 2011;14:975–85
- Köksal E, Bursal E, Dikici E, et al. Antioxidant activity of Melissa officinalis leaves. J Med Plants Res 2011;5:217–22
- Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43–53
- Gülçin İ, Bursal E, Şehitoğlu HM, et al. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–38
- Şerbetçi Tohma H, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71
- Gülçin İ, Kirecci E, Akkemik E, et al. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.). Turk J Biol 2010;34:175–88
- Balaydın HT, Gülçin İ, Menzek A, et al. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–95
- Gülçin İ. Antioxidant properties of resveratrol: a structure-activity insight. Innov Food Sci Emerg 2010;11:210–18
- Gülçin İ. Antioxidant activity of L-adrenaline: a structure-activity insight. Chem Biol Interact 2009;179:71–80
- Köksal E, Gülçin İ, Öztürk Sarıkaya SB, Bursal E. On the in vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395–405
- Gülçin İ. In vitro prooxidant effect of caffeine. J Enzyme Inhib Med Chem 2008;23:149–52
- Köksal E, Gülçin İ. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk J Agric For 2008;32:65–78
- Gülçin İ, Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95