Abstract
Background: Striae Distensae (SD) is a very common dermatologic condition. We evaluated the effectiveness and safety of a novel non-ablative fractional 1565 nm laser (ResurFX) on the appearance of SD. Materials and methods: Twelve Caucasian subjects with various stages of SD received three non-ablative laser treatments. Each treatment consisted of two different laser settings, in order to achieve a demarcated dense impact together with a diffused deep impact. Three months after the last treatment, SD improvement was assessed by blinded and non-blinded reviewers using clinical images and 3D image analyses. Results: Good clinical improvement (between 51% and 75%) was observed in all patients. Most patients showed improvement of > 50% in the volume of depressions and in lesion color (91.7% and 83.3% of patients, respectively). The average pain during treatment was generally defined as tolerable and the average downtime was 4 days. Transient erythema and severe edema were noted immediately after the procedure, but long-lasting or severe adverse effects were not observed. All patients noted a good improvement and were satisfied with the treatment and the results. Conclusions: The treatment with the 1565 nm ResurFX laser resulted in improved pigmentation, volume, and textural appearance of SD.
Key Words::
Introduction
The appearance of Striae Distensae (SD; stretch marks) is an extremely common and well-recognized dermatologic condition that can lead to significant cosmetic concern for patients (Citation1). These atrophic dermal scars with overlying epidermal atrophy are found in all ethnic groups and are usually located on the buttocks, thighs, knees, breasts, calves, and lumbosacral areas (Citation2–4). The exact cause of SD remains unclear; a combination of hormonal factors associated with mechanical stretching and rupture of connective tissue framework, tissue damage by striatoxin, pregnancy, normal growth (especially in adolescents), rapid weight change, and high serum levels of steroids have been suggested (Citation2,Citation3,Citation5). Clinically, immature SD are flattened, or slightly raised pink or red lesions (striae rubrae). Histopathologically, they appear as inflammatory alterations with elastolysis of the mid-dermis (Citation3) and mast cell degranulation (Citation6). Over time, and with atrophic changes, the lesions become white (striae albae; SA). Histopathologic findings of this latter phase demonstrate epidermal atrophy and loss of the rete ridges; densely packed, thin eosinophilic collagen bundles are arranged horizontally, parallel to the surface of the skin, similar to scars (Citation6,Citation7).
SD is not a medical problem, but can frequently pose a significant psychological burden for those affected. While SD may become less conspicuous over time, it rarely resolves without intervention. Various therapeutic approaches [topical tretinoin (Citation9,Citation10), hydrant creams (Citation11,Citation12), different acid peels (Citation13–16), microdermabrasion (Citation17,Citation18), and skin needling (Citation19,Citation20)] have been proposed for the improvement of SD, with disappointing outcomes. Thus, this common skin condition remains a frustrating concern for both physicians and patients.
Inspired by the success of various light and laser-based devices for the treatment of scars and rhytides, fractional lasers have been applied to the treatment of SD, in the hope of achieving similar efficacy. Fractional treatment is achieved through a pattern of microscopic thermal zones produced by the laser beams at specific depths in the dermis. Fractional photothermolysis stimulates the epidermal turnover and dermal collagen remodeling (Citation27).
Other modalities, such as intense pulsed light (Citation21,Citation22), 585 nm flashlamp-pumped pulsed-dye laser (PDL) (Citation23,Citation24), and radiofrequency devices have also been proposed (Citation25,Citation26), with variable results. Recently, non-ablative fractional laser (Citation27–31) and ablative 10,600 nm carbon dioxide fractional laser (Citation32–35) have been suggested as successful approaches for improving the appearance of SD.
The present study was aimed at evaluating the effectiveness and safety of a novel non-ablative fractional 1565 nm device, which uses a “dual impact technique” for targeting stretch marks, to achieve a demarcated dense impact together with a diffused deep impact within the same session. It was hypothesized that the treatment would improve both the aspects of texture and color of SD by elevating the atrophic base of the SD and simultaneously smoothing their ridges.
Materials and methods
Patients
This was a single-center research project conducted at the Istituto Dermatologico Europeo in Milan, Italy. A total of 12 patients with variously-staged SD (classified using the Deprez-Adatto scale, as I, IIa, IIb, IIIa or IIIb, and therefore not larger than 1 cm) were recruited and treated between May and November 2013. Pregnant or breast feeding patients and patients who had been previously treated for SD with mechanical or laser therapy were excluded from the study.
Treatment technique and details
Each patient received a total of three treatments, with a four to five-week interval between each treatment. One hour before each treatment, a very thin layer of an anesthetic cream containing 7% lidocaine and 7% tetracaine (Pliaglis®, GALDERMA) was applied to the treatment area(s). After removal of the anesthetic cream, a careful disinfection of the area was performed with a non-colored benzalkonium chloride disinfectant.
The device used for treating SD is a non-ablative fractional 1565 nm laser (ResurFX, Lumenis Ltd., Yokneam, Israel). At each impact, the laser generates an array of focused microspots with a density adjustable by the operator and varying from 50 to 500 μbeams/cm². The practitioner can choose any of the six different shapes (hexagon, circle, doughnut, square, rectangle, or line), and scan sizes ranging from 5 to 18 mm, with energy of up to 70 mJ/μspot. The device's spot size is derived from the energy level chosen for the treatment. The reusable tip has an integrated contact cooling and a window through which the operator can visualize the aiming beam and therefore target the treatment area with high accuracy. The microspots are emitted in a non-sequential pattern and distributed homogeneously.
We hypothesized that more microcolumns of denaturated dermis at the base of the SD will determine new collagen production, while a less dense deeper treatment of the ridges of the SD can flatten them. Therefore, each treatment comprised two passes over the SD, each with a different setting as follows. Each stria was first treated using a rectangular small spot size inside and in parallel to the long axis of the striae (settings: 300 microspots/cm² with 40–44 mJ/cm²). The full area was then treated using a hexagonal shape large enough to impact inside and laterally to the margins of the striae (settings: 150 microspots/cm² and 50–55 mJ/cm²). These treatment settings were repeated at each of the three treatment sessions.
Immediately after each treatment, a water-based emulsion containing triethanolamine (Biafine, Johnson & Johnson) was applied to the treated area, and the patients were requested to apply a moisturizer multiple times each day, to maintain hydration of the skin. Throughout the treatment course, all patients were required to avoid sun exposure and to use a broad-spectrum sunscreen of no less than 30 SPF on a daily basis, replenishing it as often as needed.
Standard clinical images of the treated SD were acquired using the Canon 5D Mark II camera (Canon Inc., Tokyo, Japan) with an annular flash. To achieve high-quality “before and after” sets, the baseline images were taken under controlled conditions that included the distance, angle, background, and lighting.
The volume of depressions and color (melanin variation) of each stria were analyzed using a 3D imaging system (Antera 3DTM - Miravex, Dublin, Ireland). The system consists of a hand-held imaging device connected to a computer by a USB cable, and uses proprietary software. Images are acquired under varying controlled illumination conditions. Several light emitting diodes (LEDs) illuminate the skin with different colors and different illumination directions. The acquired image data are then used for spatial and spectral analysis for the reconstruction of skin texture, and for analysis of skin constituents. The acquired spectral images are transformed into skin spectral reflectance maps, and the shape of the skins surface is used to compensate for light intensity variation due to the varying directions of incident illumination. The reflectance data are transformed into skin absorption coefficients, and are used to quantify melanin and hemoglobin concentrations using mathematical correlation with known spectral absorption data of these chromophores. Numeric data collected from one image must not be considered as absolute values, but when comparing two or more images, the percentage modification of these data is very useful to demonstrate eventual changes of the analyzed area.
Efficacy and safety evaluations
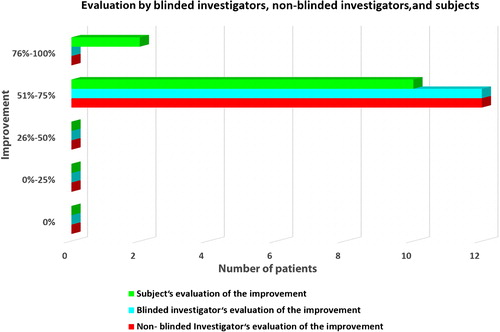
Improvement of SD appearance was assessed by non-blinded and blinded reviewers, 3 months after the last treatment, using a percentage category scale (no improvement – 0%, slight improvement – 1% to 25%, moderate improvement – 26% to 50%, good improvement – 51% to 75%, very good improvement – 76% to 100%). Non-blinded reviews were conducted by the treating physicians. Blinded reviews were performed by two plastic surgeons, who were not involved in any other part of the study, using clinical high-resolution pre- and post-treatment images. The blinded reviewers were asked to blindly select the treated or “better” image, simultaneously evaluating the percentage of improvement. The data were then unblinded, tabulated, and analyzed.
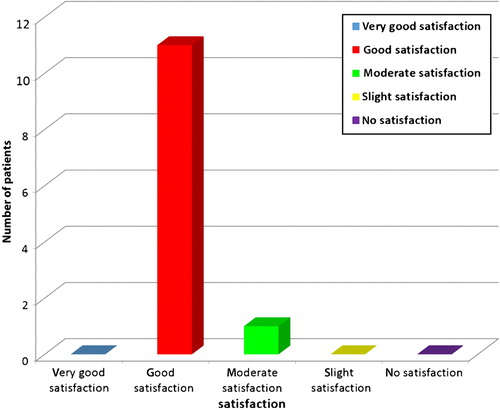
The treated patients were asked to give their own evaluation 3 months after the last treatment, using the percentage scale described above, and giving an overall judgment of satisfaction about the procedure using a 5-point scale (no satisfaction, slight satisfaction, moderate satisfaction, good satisfaction, very good satisfaction).
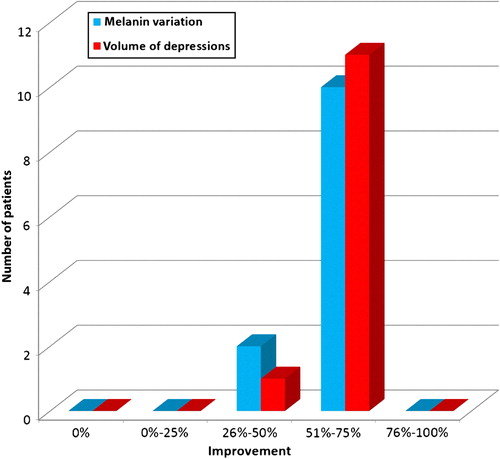
To obtain objective and quantitative data (percentage) on the improvement of the volume of depressions and color (melanin variation) of the SD in each patient, the pre- and post-treatment images were compared using the 3D imaging system software described above. This software automatically matches the images and provides their user modification percentage.
Patient assessment of pain and discomfort was evaluated using a 0–10 Visual Analog Scale (VAS), where 0 represents “no pain” and 10 represents “intolerable pain”. Immediate and short-term responses (erythema, edema, purpura, etc.) were assessed within 30 min post-treatment, according to a 4 level scale: 1 = trace; 2 = moderate; 3 = marked; 4 = severe ( and ). Downtime was defined as the period of time following the procedure, during which the patient had edema and/or erythema and felt uncomfortable.
Statistical analysis
Clinical data were analyzed using descriptive statistics. Differences between results (blinded and non-blinded reviewers) were assessed using the independent t-test and the Pearson correlation test. P-values of < 0.05 were considered statistically significant. A strong correlation was defined as r > 0.7.
Results
Overall, 11 females and one male were treated during the study. All patients were Caucasians with skin type II or III, and their age ranged from 18 to 38 years (mean 27). A total of 17 SD locations were treated among the 12 patients. The anatomic locations treated included the flanks, thighs, buttocks, breasts, abdomen, and shoulders; the majority of treatments were performed at the first four sites. Several patients had SD on multiple areas of the body and were treated accordingly. The time from SD onset ranged between 2 and 12 years (mean, 8 years). The causes of SD were pubertal growth (4 patients, 33.3%), pregnancy (4 patients, 33.3%), weight change (3 patients, 25%), and unknown (1 patient, 8.3%, the male patient).
All 12 patients completed the study (and 7 of them asked to be treated in additional anatomic regions of their body). Good clinical improvement (between 51% and 75%) was observed in all patients by the blinded as well as by the non-blinded reviewers ( and ). The 3D analysis showed similar results. The volume of SD depressions improved by more than 50% (mean improvement 58%) in the majority of patients (11/12, 91.66%), and the color of the lesions improved by more than 50% (mean improvement 54%) in 83.33% of patients (10/12) (, and ). The average pain during treatment was generally defined as tolerable (mean 2–3 out of a 10-point scale), and the average downtime was 4 days. Marked erythema and severe edema were noted immediately after the procedure; however, 30 min after the procedure, the edema decreased to trace. All patients noted a good improvement and were satisfied with the treatment and with the results during the follow-up period as well. One patient was only moderately satisfied, more due to the length of the procedure, rather than due to the results themselves (). No long-lasting or severe adverse effects were observed; a single patient presented a prolonged (7 days) micro-crusting effect on the treated area () that shed off with no sequela within a week. The percentage improvements provided by the blinded and non-blinded reviewers were not statistically significantly different between these two groups of reviewers (P = 0.06, by the T-test) and were strongly correlated (r = 0.763). A strong correlation was also observed between the results of the blinded reviewers and those of the 3D analysis (r = 0.709), as well as between the results of the non-blinded reviewers and those of the 3D analysis (r = 0.731), indicating low inter-observer variations.
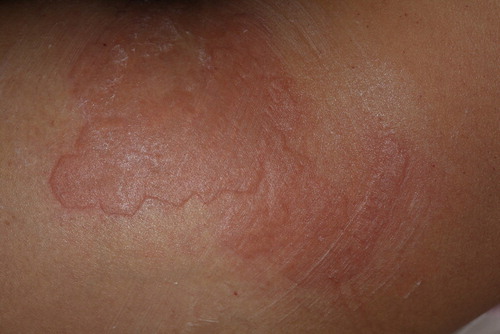
Figure 3. A 28 year-old, ST III female who presented widespread striae alba from pregnancy (an onset of 3 years). The top image was taken before treatment, and the bottom image was taken after 3 treatment sessions, as described in the section, Materials and Methods. Marked improvements in the texture as well as the color of the striae (from IIIb to IIa, according to the Deprez-Adatto scale) can be seen following the treatment, as compared to the baseline.
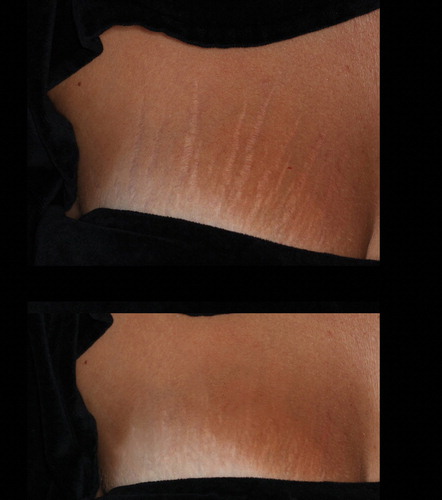
Figure 4. The 3D skin surface analysis of the 28 year-old, ST III female depicted in , before the treatment (left), and 3 months after the end of treatment (right).
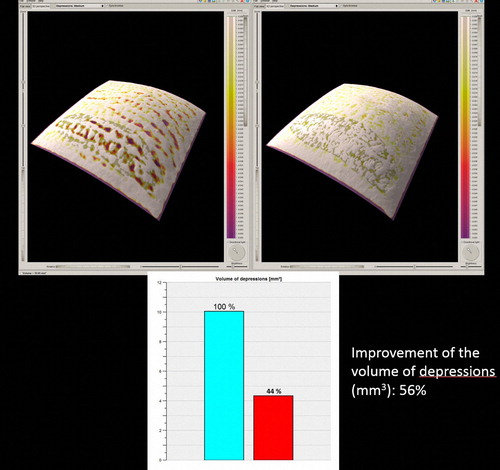
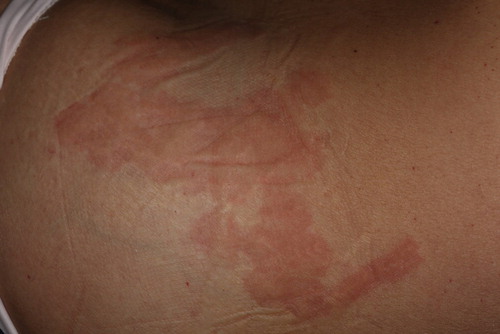
Figure 5. A 22 year-old ST IIIb female who presented with widespread striae alba after weight loss. The image on the left was taken before treatment and the image on the right was taken after 3 treatment sessions, as described in the section, Materials and Methods. Marked improvements in the texture as well as the color of the striae (from IIIb to IIa, according to the Deprez-Adatto scale) can be seen following the treatment, as compared to the baseline.
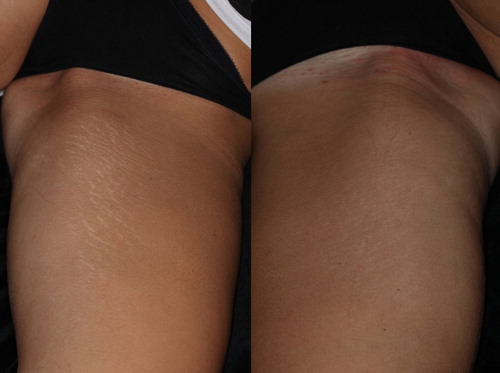
Figure 6. The 3D skin surface analysis of the 22 year-old, ST III female depicted in , before the treatment (left) and 3 months after the end of treatment (right).
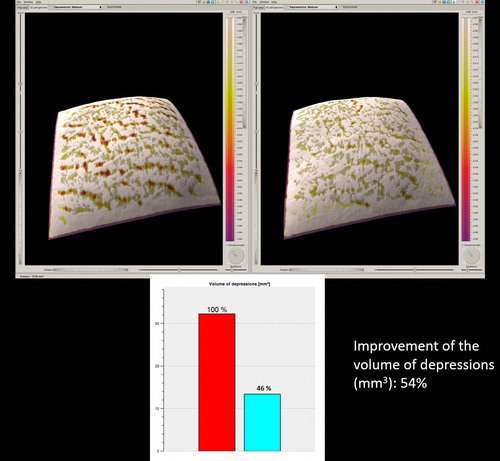
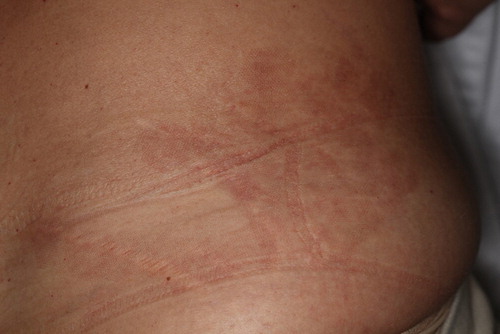
Figure 7. Micro-crusting of the skin, observed 6 days after the procedure in a 31 year-old female patient. This resolved without sequela within a week.
Discussion
Numerous therapeutic strategies for SD have been suggested to date, but no consistent treatment modality has been established yet (Citation2,Citation3,Citation9–20). From the numerous modalities used to improve SD, lasers have recently become a popular therapeutic alternative. Specifically, fractional photothermolysis looks promising in dermal remodeling and subsequent improvement of white and pigmented SD, as well as in effectively treating different kinds of scars (Citation27–35,Citation38–39). Preliminary investigations of fractional non-ablative photothermolysis demonstrated the capability of this technology to induce dermal remodeling (Citation40–42), and these histologic changes (neocollagenesis, increased epidermal thickness) have recently been confirmed in striae studies with the 1550-nm laser (Citation27,Citation29). To the best of our knowledge, the present study was the first to evaluate the effectiveness of a non-ablative fractional 1565 nm device on mature SD. Two laser settings were used for treating each stria (dual impact technique); the first setting was intended to achieve a demarcated dense impact because the base of an atrophic scar needs greater collagen production to elevate it, and the second setting was intended to achieve a diffused deep impact in order to smooth the ridges of the scar, in order to reduce the shadow produced by incident light. Indeed, the volume of depressions and the color of the lesions were both improved by more than 50% in over 80% of the patients following treatment. Pain experienced during the treatment was tolerable. Erythema and edema were expected in this type of treatment, but subsided shortly after treatment.
Both ablative and non-ablative fractional treatments have shown promising results for treating SD, but controlled studies are scarce. In a study comparing the effect of ablative CO2 fractional laser with that of non-ablative 1,550 nm Erbium (ER): Glass fractional laser on SD in Asian patients, both modalities showed similar improvements of SD; however treatment with the ablative CO2 fractional laser was considered more painful than the treatment with the non-ablative fractional laser, and resulted in more post-inflammatory hyperpigmentation and longer post-treatment erythema (Citation34).
Within non-ablative devices, the results are variable. Bak et al. (Citation27) reported a mean clinical improvement of 25% to 50% with a 1550 nm laser, the majority graded the improvement no greater than 25%. Of the eight patients randomly evaluated in a study with the 1550 nm laser by Stotland et al. (Citation35), 63% of the patients demonstrated improvements of 26% to 50%. On the other hand, de Angelis et al. (Citation31) demonstrated an improvement between 50% and 75% for all patients. These studies also demonstrated a thickening of the epidermis and dermis, as well as an increased number of collagen fibers at 30 days after the last treatment. Additionally, they observed an increased number and more uniform distribution of elastic fibers in the papillary dermis.
The limitation of the present study includes the lack of histologic assessment, a limited number of patients, the absence of dark-skinned patients, and the lack of a longer follow-up period; however the study presents, for the first time, an objective evaluation of the improvement of SD after treatment with this modality.
In conclusion, the current study demonstrated a significant improvement in texture, volume, and color of white SD, with minimal adverse effects. These results were supported by the correlation between the blinded and the non-blinded evaluation of improvement after treatment, as well as by the correlation between the reviewers and the 3D analysis. The procedures were extremely well-tolerated by the patients, and the results were so convincing that 58% of them asked to be treated in another anatomic region of their body.
The risk of eventual associated hyperpigmentation should never be underestimated, and therefore, additional studies are required. In general, SD remain a challenge; they are difficult to treat, and patients’ expectations are always very high. For these reasons, we are still persuaded that any therapy and associated side effects should be critically discussed with the patient before therapy.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yamaguchi K, Suganuma N, Ohashi K. Quality of life evaluation in Japanese pregnant women with striae gravidarum: a cross-sectional study. BMC Res Notes. 2012;21:450.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: An update. Dermatol Surg. 2009;35:563–573.
- Al-Himdani S, Ud-Din S, Gilmore S, Bayat A. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. 2014;170: 527–547.
- Gauglitz GG, Reinholz M, Kaudewitz P, Schauber J, Ruzicka T. Treatment of striae distensae using an ablative Erbium: YAG fractional laser versus a 585-nm pulsed-dye laser. J Cosmet Laser Ther. 2014;16:117–119.
- Burrows NP, Lovell CR. Disorders of connective tissue. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: WileyBlackwell; 2010. p. 45–45.
- McDaniel DH. Laser therapy of stretch marks. Dermatol Clin. 2002;20:67–76.
- Watson RE, Parry EJ, Humphries JD, Jones CJP, Polson DW, Kielty CM, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138: 931–937.
- Hermanns JF, Pierard GE. High-resolution epiluminescence colorimetry of striae distensae. J Eur Acad Dermatol Venereol. 2006;20:282–287.
- Elson ML. Treatment of striae distensae with topical tretinoin. J Dermatol Surg Oncol. 1990;16:267–270.
- Kang S, Kim KJ, Griffith CE, Wong TY, Talwar HS, Fisher GJ, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519–526.
- Young GL, Jewell D. Creams for preventing stretch marks in pregnancy. Cochrane Library. 2008;1:1–7.
- Mallol J, Belda MA, Costa D, Noval A, Sola M.Prophylaxis of striae gravidarum with a topical formulation. A double blind trial. Int J Cosm Sci. 1991;13:51–57.
- Ash K, Lord J, Zukowski M, McDaniel DH.Comparison of topical therapy for striae alba (20% glycolic acid/0.05% tretinoin versus 20% glycolic acid/10% L-ascorbic acid). Dermatol Surg. 1998;24:849–856.
- Adatto MA, Deprez P. Striae treated by a novel combination treatment – sand abrasion and a patent mixture containing 15% trichloracetic acid followed by 6–24 hrs of a patent cream under plastic occlusion. J Cosmet Dermatol. 2003; 2:61–67.
- Deprez P. Easy peel for the treatment of stretch marks. Int J Cosmet Surg Aesthet Dermatol. 2000;2:201–204.
- Mazzarello V, Farace F, Ena P, Fenu G, Mulas P, Piu L, et al. A superficial texture analysis of 70% glycolic acid topical therapy and striae distensae. Plast Reconstr Surg. 2012; 129:589–590.
- Karimipour DJ, Kang S, Johnson TM, Orringer JS, Hamilton T, Hammerberg C, et al. Microdermabrasion: a molecular analysis following a single treatment. J Am Acad Dermatol. 2005;52:215–223.
- Abdel-Latif AM, Albendary AS. Treatment of striae distensae with microdermabrasion: a clinical and molecular study. J Egyptian Women Dermatol Soc. 2008;5:24–30.
- Park KY, Kim HK, Kim SE, Kim BJ, Kim MN. Treatment of striae distensae using needling therapy: a pilot study. Dermatol Surg. 2012;38:1823–1828.
- Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421–1429.
- Hernandez-Perez E, Colombo-Charrier E, Valencia-Ibiett E. Intense pulsed light in the treatment of striae distensae. Dermatol Surg. 2002;28:1124–1130.
- Al-Dhalimi MA, Abo Nasyria AA. A comparative study of the effectiveness of intense pulsed light wavelengths (650 nm vs 590 nm) in the treatment of striae distensae. J Cosmet Laser Ther. 2013;15:120–125.
- Jimenez GP, Flores F, Berman B, Gunja-Smith Z. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362–365.
- McDaniel DH, Ash K, Zukowoski M. Treatment of stretch marks with the 585 nm flashlamp pumped pulsed dye laser. Dermatol Surg. 1996;22:332–337.
- Manuskiatti W, Boonthaweeyuwat E, Varothai S. Treatment of striae distensae with a TriPollar radiofrequency device: a pilot study. J Dermatolog Treat. 2009;20:359–364.
- Issa MC, de Britto Pereira Kassuga LE, Chevrand NS, do Nascimento Barbosa L, Luiz RR, Pantaleão L, et al. Transepidermal retinoic acid delivery using ablative fractional radiofrequency associated with acoustic pressure ultrasound for stretch marks treatment. Lasers Surg Med. 2013;45: 81–88.
- Bak H, Kim BJ, Lee WJ, Bang JS, Lee SY, Choi JH, et al.Treatment of striae distensae with fractional photothermolysis. Dermatol Surg. 2009;35:1215–1220.
- Stotland M, Chapas AM, Brightman L, Sukal S, Hale E, Karen J, et al. The safety and efficacy of fractional photothermolysis for the correction of striae distensae. J Drugs Dermatol. 2008;7:857–861.
- Kim BJ, Lee DH, Kim MN, Song KY, Cho WI, Lee CK, et al. Fractional photothermolysis for the treatment of striae distensae in Asian skin. Am J Clin Dermatol. 2008;9:33–37.
- Katz TM, Goldberg LH, Friedman PM. Non ablative fractional photothermolysis for the treatment of striae rubra. Dermatol Surg. 2009;35:1430–1433.
- de Angelis F, Kolesnikova L, Renato F, Liguori G. Fractional nonablative 1540-nm laser treatment of striae distensae in Fitzpatrick skin types II to IV: clinical and histological results. Aesthet Surg J. 2011;31:411–419.
- Lee SE, Kim JH, Lee SJ, Lee JE, Kang JM, Kim YK, et al.Treatment of striae distensae using an ablative 10,600-nm carbon dioxide fractional laser: a retrospective review of 27 participants. Dermatol Surg. 2010;36:1683–1690.
- Bin Cho S, Lee SJ, Lee JE, Kang JM, Kim YK, Oh SH. Treatment of striae alba using the 10.600-nm carbon dioxide fractional laser. J Cosmet Laser Ther. 2010;12:118–119.
- Yang YJ, Lee GY. Treatment of Striae Distensae with nonablative fractional laser versus ablative CO(2)fractional laser: A randomized controlled trial. Ann Dermatol. 2011;23: 481–489.
- Alexiades-Armenakas M, Sarnoff D, Gotkin R, Sadick N. Multi-center clinical study and review of fractional ablative CO2 laser resurfacing for the treatment of rhytides, photoaging, scars and striae. J Drugs Dermatol. 2011;10: 352–362.
- Cho S, Park ES, Lee DH, Li K, Chung JH. Clinical features and risk factors for striae distensae in Korean adolescents. J Eur Acad Dermatol Venereol. 2006;20:1108–1113.
- Sisson WR. Colored striae in adolescent children. J Pediatr. 1954;45:520–530.
- Haedersdal M, Moreau KE, Beyer DM, Nymann P, Alsbjorn B. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189–195.
- Vasily DB, Cerino ME, Ziselman EM, Zeina ST. Nonablative fractional resurfacing of surgical and posttraumatic scars. J Drugs Dermatol. 2009;8:998–1005.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169–176.
- Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426–438.
- Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg Med. 2006;38:142–149.