Abstract
Objective. Preterm labor is associated with excessive maternal thrombin generation, as evidenced by increased circulating thrombin–antithrombin (TAT) III complexes concentration. In addition to its hemostatic functions, thrombin has uterotonic properties that may participate in the mechanism leading to preterm birth in cases of intrauterine bleeding. Thrombin also has a proinflammatory role, and inflammation is associated with increased thrombin generation. The aim of this study was to determine whether intra-amniotic infection/inflammation (IAI) is associated with increased amniotic fluid (AF) thrombin generation in women with preterm and term deliveries.
Study design. This cross-sectional study included the following groups: (1) mid-trimester (n = 74); (2) term not in labor (n = 39); (3) term in labor (n = 25); (4) term in labor with IAI (n = 22); (5) spontaneous preterm labor (PTL) who delivered at term (n = 62); (6) PTL without IAI who delivered preterm (n = 59); (7) PTL with IAI (n = 71). The AF TAT III complexes concentration was measured by enzyme linked immunosorbent assay (ELISA). Non-parametric statistics were used for analysis.
Results. (1) TAT III complexes were identified in all AF samples; (2) patients with PTL who delivered preterm, with and without IAI, had a higher median AF TAT III complexes concentration than those with an episode of PTL who delivered at term (p < 0.001, p = 0.03, respectively); (3) among patients with PTL without IAI, elevated AF TAT III complexes concentration were independently associated with a shorter amniocentesis-to-delivery interval (hazard ratio, 1.5; 95% CI, 1.07–2.1); (4) among patients at term, those with IAI had a higher median AF TAT III complexes concentration than those without IAI, whether in labor or not in labor (p = 0.02); (5) there was no significant difference between the median AF TAT III complexes concentration of patients at term with and without labor; (6) patients who had a mid-trimester amniocentesis had a lower median AF TAT III complexes concentration than that of patients at term not in labor (p < 0.001).
Conclusions. We present herein a distinct difference in the pattern of intra-amniotic thrombin generation between term and preterm parturition. PTL leading to preterm delivery is associated with an increased intra-amniotic thrombin generation regardless of the presence of IAI. In contrast, term delivery is associated with an increased intra-amniotic thrombin generation only in patients with IAI.
Introduction
Thrombin plays a central role in the coagulation cascade and participates in transforming fibrinogen into fibrin and platelet activation [Citation1,Citation2], as well as the activation of the fibrinolytic and anticoagulation systems [Citation3–6]. Other activities of thrombin include the activation of endothelial cells [Citation7–9] and leukocytes (lymphocytes, monocytes and neutrophils) [Citation10–17]. These activities are mediated at least in part by protease-activated receptors (PARs), which are G protein-coupled receptors activated through cleavage by thrombin and other coagulation factors [Citation18–22].
The reaction of thrombin with its major inhibitor, antithrombin III, results in the formation of an inactive stable complex, the thrombin–antithrombin (TAT) III complex. In order to study the activation of the coagulation system, it is necessary to measure either peptides released from coagulation factor zymogens or complexes formed between activated coagulation factors and their natural inhibitors. This is required because activated coagulation factors have a short half-life and direct measurement of these factors during the activation of the coagulation cascade is difficult [Citation23]. The presence and/or concentration of TAT III complexes is widely accepted as an index of thrombin generation in vivoCitation24–27].
During pregnancy, changes in the coagulation system are considered to be adaptive to prevent hemorrhage at the time of delivery [Citation28–32]. Indeed, normal pregnancy has been associated with excessive maternal thrombin generation [Citation31,Citation33] and a tendency for platelets to aggregate in response to agonists [Citation34,Citation35]. In addition, increased thrombin generation in the maternal circulation has been reported in several obstetrical syndromes including preterm labor (PTL) [Citation33,Citation36], preeclampsia [Citation37–45], fetal growth restriction [Citation37,Citation46,Citation47] and preterm prelabor rupture of membranes (PROM) [Citation33,Citation48].
The administration of actively clotting blood, but not blood treated with heparin, into the uterine cavity has been associated with increased uterine contractility, and this has been attributed to a thrombin-specific uterotonic property, even at low concentrations [Citation49]. This phenomenon has been implicated in the initiation of labor in cases of intrauterine bleeding, and perhaps infection [Citation50]. In addition, thrombin may play a role in membrane rupture by activation of matrix metalloproteinase (MMP)-1 or interstitial collagenase [Citation51,Citation52], which can degrade type I and type III collagens, important components of the membranes. MMP-1 concentrations are elevated in the amniotic fluid of women with term as well as preterm PROM [Citation53].
PTL is associated with an increased thrombin generation suggested by a higher median maternal plasma concentrations of TAT III complexes in patients with PTL compared to women with a normal pregnancy [Citation33,Citation36]. Moreover, high plasma concentrations of TAT III complexes are not associated with a history of vaginal bleeding during pregnancy [Citation33,Citation36] or the presence of intra-amniotic infection/inflammation (IAI) [Citation33], suggesting that the activation of the maternal coagulation cascade is associated with an episode of PTL, regardless of the underlying mechanism responsible for the preterm parturition syndrome [Citation54–64].
Amniotic fluid (AF) has procoagulant activity [Citation65–68], which is higher than that of the maternal plasma, this activity has been attributed to tissue factor [Citation69]. Indeed, the concentrations of tissue factor, are higher in AF than in maternal plasma [Citation57]. However, the changes in AF thrombin generation during an episode of PTL have not been systematically studied. The aim of this study was to determine the changes in AF TAT III complexes concentration in women with preterm and term labor and delivery with and without IAI.
Material and methods
Study groups and inclusion criteria
A cross-sectional study was designed by searching our clinical database and bank of biologic samples, which included samples from pregnant women in the following groups: (1) women in the mid-trimester of pregnancy (n = 74) who underwent amniocentesis for genetic indications and delivered an appropriate-for-gestational age neonate at term; (2) women with a normal pregnancy at term not in labor (n = 39); (3) women with a normal pregnancy at term in labor (n = 25); (4) women with a normal pregnancy at term with IAI (n = 22). Patients with term gestation underwent amniocentesis for the determination of fetal lung maturity or to rule out IAI; (5) women with spontaneous PTL and intact membranes who delivered at term (n = 62); (6) patients with PTL without IAI who delivered preterm (<37 weeks) (n = 59); and (7) PTL with IAI (n = 71).
All women provided written informed consent prior to the collection of AF. The collection of AF and its utilization for research purposes were approved by the Institutional Review Boards of the participating institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH and DHHS. The patients included were recruited from the following centers: Hutzel Hospital, Detroit (women with PTL or mid-trimester), Pennsylvania Hospital, Philadelphia (mid-trimester) and Sotero del Rio Hospital, Santiago, Chile (women at term). Many of these samples have been used previously to study the biology of inflammation, hemostasis, angiogenesis regulation, growth factor concentrations and other processes in normal pregnant women and those with pregnancy complications.
Clinical definitions
PTL was diagnosed by the presence of at least two regular uterine contractions every 10 min associated with cervical changes that required admission to the hospital before 37 weeks gestation. Small-for-gestational age (SGA) neonate was defined as birthweight below the 10th percentile [Citation70]. Placental pathology was classified according to a previously described nomenclature [Citation71]. Intra-amniotic infection was defined by the presence of positive AF cultures for microorganisms and intra-amniotic inflammation by an AF white blood cell (WBC) count ≥100 cells/ml and/or an AF IL-6 concentration >2.6 ng/ml [Citation72–77].
Amniotic fluid collection
AF collection was performed by trans-abdominal amniocentesis as described in other publications by our group [Citation78–85]. AF was transported to the laboratory in a capped plastic sterile syringe, and was cultured for aerobic and anaerobic bacteria, as well as for genital mycoplasmas. WBC count, glucose concentration and Gram stain for microorganisms were performed in AF shortly after collection. The results of the AF analyses were used for clinical management. The remainder of the AF was centrifuged at 4°C for 10 min to remove cellular and particulate debris. Aliquots of the supernatant were stored at (70°C until assay. Samples were not subject to repeat freeze–thaw cycles.
TAT III complex immunoassays
Samples of AF were assayed for TAT III complexes in duplicate using a commercially available immunoassay kit (Enzygnost TAT micro; Behring Diagnostics, Westwood, MA). This assay is a sandwich enzyme linked immunosorbent assay (ELISA). Prior to use on study samples, the assay system was validated for AF using spike and recovery experiments. The sensitivity of the assay was 1.06 μg/l, and the inter- and intra-assay coefficients of variation were 11.6 and 8.3%, respectively.
Statistical analysis
The normality of the data was tested by the Shapiro–Wilk and the Kolmogorov–Smirnov tests. As the AF TAT III complexes concentration were not normally distributed, the Mann–Whitney U and Kruskal–Wallis tests were used to analyze differences between groups. Spearman's rank correlation test was used to determine the relationship between AF TAT III complexes concentration and gestational age in women who had a normal pregnancy. A receiver operating characteristic (ROC) curve was constructed to describe the relationship between the sensitivity (true-positive rate) and the false-positive rate for different values of AF TAT III complexes concentration in the identification of patients at risk for preterm delivery. Survival analyses were performed by a Log rank test. Cox proportional hazard regression analysis was used to determine the association between an elevated TAT III complexes concentration and the amniocentesis-to-delivery interval in patients with PTL without IAI after adjusting for confounding variables. The statistical software package used was SPSS 12.0 (SPSS, Chicago, IL).
Results
Demographic and clinical characteristics
The clinical and demographic characteristics of the term and preterm groups are presented in and . Among the PTL groups, patients with IAI had a lower gestational age at amniocentesis, gestational age at delivery and neonatal birthweight than patients without IAI who delivered preterm and than those who delivered at term ().
Table I. Demographic and clinical characteristics of the study population.
Table II. Demographic and clinical characteristics of the preterm study population.
Changes in amniotic fluid TAT III complexes concentration during normal pregnancy and labor
The AF concentration of TAT III was significantly higher in women at term not in labor than in the mid-trimester of pregnancy (p < 0.001, ). Labor at term was not associated with a significant change in the median AF TAT III complexes concentration (p = 0.7, ).
Figure 1. Amniotic fluid thrombin-antithrombin III complexes concentration in patients in the mid-trimester of pregnancy and women at term not in labor (mid-trimester: median 8.1 μg/L, range 2.1–160.0 vs. term no labor: median 66.9 μg/L, range 10.2–154).
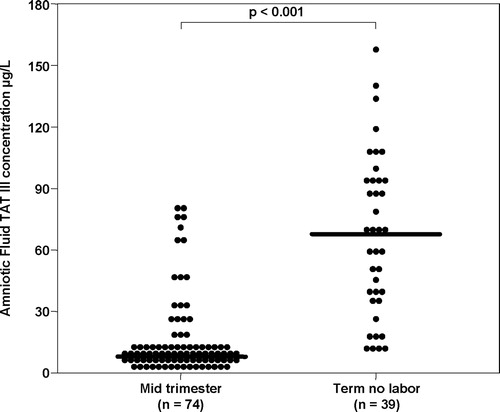
Figure 2. Amniotic fluid thrombin-antithrombin III complexes concentration in patients with term pregnancies not in labor, women at term in labor and patients with term labor and intra-amniotic infection/inflammation (term no labor: median 66.9 μg/L, range 10.2–154; term in labor: median 50.8 mg/L, range 6.8–150.0); term labor with IAI: median 118.5 μg/L, range 26.3–310.0.
Patients with IAI at term had a higher median AF TAT III complexes concentration (median 118.5 μg/l, range 26.3–310.0) than women at term not in labor (p = 0.006, after Bonferroni correction) and than those in labor (p = 0.02, after Bonferroni correction) (Figure 2).
Changes in amniotic fluid TAT III complexes concentration in preterm labor
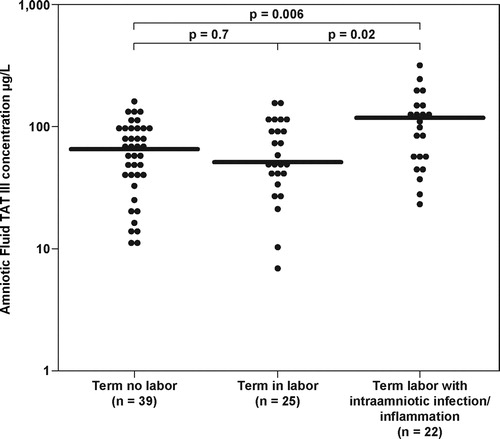
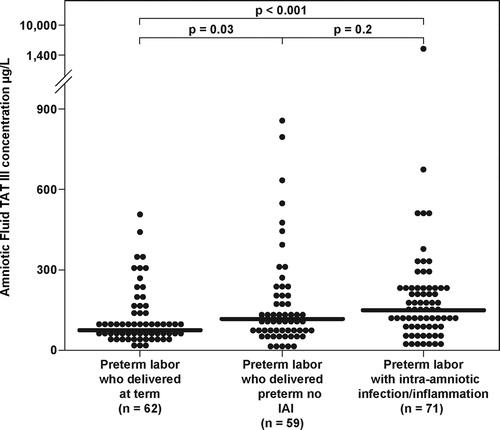
The median AF TAT III complexes concentration differed significantly among patients with PTL and intact membranes (Kruskal–Wallis, p = 0.001). Patients with PTL and IAI had a higher median AF TAT III complexes concentration than that of those with PTL who delivered at term (p < 0.001, ). Patients with PTL who delivered preterm without IAI had a higher median AF TAT III complexes concentration than that of those with PTL who delivered at term (p = 0.03 after Bonferroni correction). Although the median AF TAT III complexes concentration was higher in patients with PTL and IAI than in those with PTL without IAI, the difference did not reach statistical significance (p = 0.2, after Bonferroni correction) ().
Figure 3. Amniotic fluid thrombin-antithrombin III complexes concentration in patients with preterm labor who delivered at term, women with preterm labor who delivered preterm without intra-amniotic infection/inflammation, and women with preterm labor and intra-amniotic infection/inflammation (PTL with IAI: median 147.7 μg/L, range 15.3–1424.8; PTL without IAI: median 116.0 μg/L, range 10.7–2073.9; PTL who delivered at term: median 73.4 μg/L, range 7.6–507.0).
Among patients with PTL, a TAT III complexes concentration of 108.24 μg/l or more was associated with a sensitivity of 63.1% and a specificity of 72.6% for the identification of women who will deliver preterm. According to this cutoff for elevated AF TAT III complexes concentration, patients with PTL with elevated TAT III complexes concentration had an odds-ratio of 4.52 (95% CI, 2.2–9.3) to deliver preterm.
The effect of elevated TAT III complexes concentration on gestational age at delivery and amniocentesis-to-delivery interval among patients with PTL was studied further using survival analyses. An elevated AF TAT III complexes concentration was associated with an earlier gestational age at delivery [Log rank test, p < 0.001, ] and a shorter amniocentesis-to-delivery interval [Log rank test, p < 0.001, ]. When the cases were stratified according to the presence of IAI, patients with PTL without IAI but an elevated TAT III complexes concentration had an earlier gestational age at delivery [Log rank test, p = 0.0002, ] and a shorter amniocentesis-to-delivery interval than patients without an elevated TAT III complexes concentration [Log rank test, p = 0.0085, ]. However, among patients with PTL and IAI who delivered spontaneously, an elevated AF TAT III complexes concentration was not associated with an earlier gestational age at delivery (p = 0.2) or a shorter amniocentesis-to-delivery interval (p = 0.5). To further investigate the effect of an elevated TAT III complexes concentration among patients with PTL without IAI who delivered spontaneously, we conducted a survival analysis using Cox proportional hazard modeling. After correction for confounding factors (including gestational age and cervical dilatation at time of amniocentesis), an elevated AF TAT III complexes concentration was independently associated with a shorter amniocentesis-to-delivery interval (hazard ratio, 1.5; 95% CI 1.07–2.1) ().
Figure 4. (a) The effect of an elevated amniotic fluid thrombin–antithrombin III complexes concentration on gestational age at delivery in patients with preterm labor. (b) The effect of an elevated amniotic fluid thrombin–antithrombin III complexes concentration on the amniocentesis-to-delivery interval in patients with preterm labor.
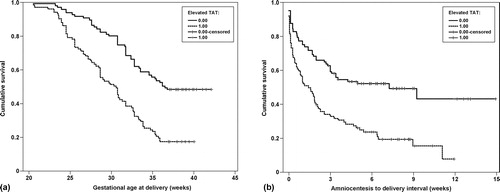
Figure 5. (a) The effect of an elevated amniotic fluid thrombin–antithrombin III complexes concentration on gestational age at delivery, among patients with preterm labor without intra-amniotic infection/inflammation. (b) The effect of an elevated amniotic fluid thrombin–antithrombin III complexes concentration on the amniocentesis-to-delivery interval of patients with preterm labor without intra-amniotic infection/inflammation.
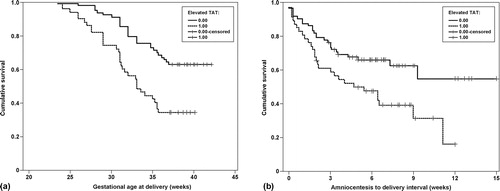
Table III. Cox proportional hazards survival analysis in the prediction of amniocentesis-to-delivery interval.
Comments
Principal findings of the study
(1) TAT III complexes are normally present in AF at all gestational ages from the mid-trimester to term. (2) IAI at term is associated with an increased AF TAT III complexes concentration suggestive of an increase in thrombin generation within the amniotic cavity. (3) Among patients with PTL, those who delivered preterm had a higher median AF TAT III complexes concentration than those who delivered at term, regardless of the presence of IAI. (4) Among patients with PTL without IAI, elevated AF TAT III complexes concentration was associated with a higher risk for preterm delivery and a shorter amniocentesis-to-delivery interval.
Changes in thrombin during normal pregnancy and labor
Normal pregnancy is associated with an increased maternal thrombin generation as determined by the elevated concentrations of fibrinopeptide A, prothrombin fragments 1 and 2, and TAT III complexes [Citation32,Citation85–87]. In addition, the maternal plasma TAT III complexes concentration increases further during labor [Citation88] as well as immediately after delivery [Citation87,Citation88] and decreases during the puerperium.
In contrast to maternal plasma, data about changes in AF TAT III complexes concentration is limited [Citation67,Citation89]. Our finding that the median AF TAT III complexes concentration increases from the mid-trimester to term is consistent with the report of Koh et al. [Citation89] that examined the changes in AF TAT III complexes concentration in the second trimester and during the first stage of labor, and documented an increase in TAT III complexes concentration in the first stage of labor as compared to mid-trimester [Citation89]. Uszynski et al. [Citation67] examined TAT III complexes concentration in AF at term and concluded that they increase during labor [Citation67]. The AF TAT III complexes concentration documented in the latter study [Citation67] during the first stage of labor (54.2 ± 26.4 ng/ml) are in agreement with the concentrations we have found.
The sources and roles of thrombin in AF remain to be elucidated. The AF TAT III complexes concentration of patients with normal pregnancies is 2–4 times higher than that observed in maternal plasma [Citation67]. Similarly, umbilical cord blood TAT III complexes concentration [Citation66] is lower than in AF. These findings, along with the high molecular weight of thrombin (39,000 Da), suggest that thrombin is produced locally. Evidence in support of this view includes the following: (1) Prothrombin [Citation91] and other coagulation factors from both the intrinsic and extrinsic pathways are present in AF [Citation91,Citation92]; (2) Tissue factor is present in AF in high concentrations and can directly convert prothrombin to thrombin [Citation17,Citation65,Citation68,Citation93–95]; and (3) The local production of thrombin is via activated factor X as it has been demonstrated that AF has direct factor X-activating properties [Citation96]. Collectively, these observations provide a mechanism for the local generation of thrombin from prothrombin in the AF.
The association between thrombin generation and intra-amniotic infection/inflammation
The findings of an increased median AF TAT III complexes concentration among patients with PTL who delivered preterm and among patients at term with IAI are novel. The interaction between coagulation and inflammation may be an explanation for the increased thrombin generation in patients with IAI during preterm and term parturition. A solid body of evidence supports the generation of thrombin during the course of inflammation [Citation97–99], mainly through the tissue factor pathway [Citation97,Citation99–102]. Indeed, during inflammation, activated monocytes express tissue factor on their membrane [Citation103–108] and shed micro-particles which contain tissue factor into the plasma [Citation103,Citation109–116]. An important mediator of tissue factor expression during inflammation are interleukin (IL)-6 [Citation23,Citation117], TNF-αCitation118,Citation119], and IL-βCitation119–121]. This is noteworthy, since the AF concentration of these proinflammatory cytokines increases during IAI [Citation122–145] and may be one of the mechanisms by which intra-amniotic inflammation activates the increase AF thrombin generation in these patients.
Activation of the AF complement system can be an additional mechanism by which intra-amniotic thrombin is generated during inflammation. Indeed, patients with PTL and intra-amniotic infection have a higher C5a concentration than patients with PTL who delivered preterm or at term [Citation146]. The association between C5a and tissue factor activation and expression was previously reported [Citation147,Citation148]. C5a induces a 4.9-fold increase in tissue factor activity and a 3.8-fold increase in tissue factor mRNA expression by endothelial cells [Citation147]. Furthermore, the administration of C5a to animals increases the procoagulant activity of alveolar macrophages 5- to 6-fold through tissue factor activation [Citation148]. Thus, the increased concentrations of AF C5a in patients with PTL and intra-amniotic infection may activate AF tissue factor, leading to an increased thrombin generation that is reflected by the elevated AF TAT III complexes concentration in these patients.
Decidual bleeding is an additional mechanism that may be associated with an increased thrombin generation in patients with PTL and intra-amniotic infection/inflammation. Idiopathic vaginal bleeding is associated with IAI [Citation149]. Moreover, patients with vaginal bleeding and microbial invasion of AF had a higher rate of early preterm delivery (before 32 weeks of gestation) than in women without bleeding [Citation149]. Our group has proposed that IAI is associated with decidual bleeding that may be manifested as idiopathic vaginal bleeding. This concept is further supported by the findings that patients with PTL and IAI have a higher median AF total hemoglobin concentration than patients with PTL without IAI and than those who delivered at term [Citation150]. Moreover, among patients with PTL, the fraction of fetal hemoglobin out of the total AF hemoglobin was lower in those with IAI than in those without it, suggesting a higher proportion of maternal hemoglobin in the AF of these patients [Citation151]. Thus, occult decidual bleeding associated with IAI may contribute to activation of the cascade leading to an increased thrombin generation in the AF, as reflected by the elevated TAT III complexes concentration in these patients. Similar mechanisms may contribute to the higher median AF TAT III complexes detected in patients with IAI at term.
The association between thrombin generation and preterm birth in the absence of intra-amniotic infection/inflammation
The association between elevated median AF TAT III complexes concentration and preterm delivery among patients with PTL but without IAI is novel. This observation provides additional evidence to support the distinct underlying mechanisms of term and preterm parturition. Labor at term is a physiologic process that is not associated with a significant increase in AF thrombin generation. However, PTL leading to preterm parturition is a pathologic process that is associated with an increased AF thrombin generation, even in the absence of IAI, emphasizing the syndromic nature of PTL and delivery [Citation54–56,Citation152].
In the current study, among patients with PTL, those with an elevated AF TAT III complexes concentration had a higher risk for preterm delivery. Moreover, an elevated AF TAT III complexes concentration was associated with a shorter amniocentesis-to-delivery interval, only among those with PTL without intra-amniotic infection/inflammation. This observation suggests that the higher AF TAT III complexes concentration observed in cases of IAI (preterm and term) may be part of the inflammatory process in the AF. However, in the absence of infection, increased thrombin generation may be the mechanism actually leading to preterm birth. This assumption is based on the uterotonic properties of thrombin [Citation36,Citation49,Citation50,Citation117] and was proposed in the context of intrauterine bleeding mainly through the association with placental abruption [Citation36,Citation49,Citation50].
The uterotonic effect of thrombin may be exerted by its receptor, the PAR-1, which mediates many of the effects of thrombin on platelet activation [Citation153,Citation154], proinflammatory cytokine secretion [Citation17,Citation155,Citation156], local tissue remodeling after injury [Citation157,Citation158], fetal blood vessel development and stabilization [Citation159–161], as well as uterine contractions [Citation162–164]. Indeed, in a study on stem cells gene expression, PAR-2 and PAR-1 were among the top 25 up-regulated genes in stem cells of AF origin [Citation165], suggesting that thrombin can exert its intracellular and proinflammatory effect via local mechanisms through its receptor. However, it is still not clear which underlying processes lead to an increased AF TAT III complexes concentration in patients with PTL without IAI who delivered preterm.
In summary, the increased AF TAT III complexes concentration in patients with preterm and term IAI is probably mediated by intra-amniotic inflammatory processes. However, the association between elevated AF TAT III complexes concentration and preterm delivery, as well as a shorter amniocentesis-to-delivery interval even without IAI, suggests a role for thrombin in the pathogenesis of preterm parturition even in the absence of infection/inflammation. The precise pathophysiologic role of thrombin in AF remains to be determined because changes occur despite the absence of coagulation, suggesting that thrombin has other functions within the amniotic cavity, which may be unrelated to its role in the coagulation system.
Acknowledgements
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol 1985;101:880–886.
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–594.
- Furie B, Furie BC. Molecular and cellular biology of blood coagulation. N Engl J Med 1992;326:800–806.
- Mann KG, Lorand L. Introduction: blood coagulation. Methods Enzymol 1993;222:1–10.
- Dahlback B. Blood coagulation. Lancet 2000;355:1627–1632.
- Nesheim M. Thrombin and fibrinolysis. Chest 2003;124:33S–39S.
- Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 1989;264:7768–7771.
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol 1994;267:L223–L241.
- Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med 1996;183:821–827.
- Sonne O. The specific binding of thrombin to human polymorphonuclear leucocytes. Scand J Clin Lab Invest 1988;48:831–838.
- Naldini A, Carney DH, Bocci V, Klimpel KD, Asuncion M, Soares LE, Klimpel GR. Thrombin enhances T cell proliferative responses and cytokine production. Cell Immunol 1993;147:367–377.
- Drake WT, Lopes NN, Fenton JW, Issekutz AC. Thrombin enhancement of interleukin-1 and tumor necrosis factor-alpha induced polymorphonuclear leukocyte migration. Lab Invest 1992;67:617–627.
- Bizios R, Lai L, Fenton JW, Malik AB. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol 1986;128:485–490.
- Bar-Shavit R, Kahn A, Wilner GD, Fenton JW. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science 1983;220:728–731.
- Drake WT, Issekutz AC. A role for alpha-thrombin in polymorphonuclear leukocyte recruitment during inflammation. Semin Thromb Hemost 1992;18:333–340.
- Franchini M, Veneri D, Lippi G. Inflammation and hemostasis: a bidirectional interaction. Clin Lab 2007;53:63–67.
- Li T, Wang H, He S. Induction of interleukin-6 release from monocytes by serine proteinases and its potential mechanisms. Scand J Immunol 2006;64:10–16.
- Dery O, Corvera CU. Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 1998;274:C1429–C1452.
- Coughlin SR. Protease-activated receptors and platelet function. Thromb Haemost 1999;82:353–356.
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–264.
- Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost 2001;86:298–307.
- Landis RC. Protease activated receptors: clinical relevance to hemostasis and inflammation. Hematol Oncol Clin North Am 2007;21:103–113.
- Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest 1997;27:3–9.
- Hoek JA, Sturk A, ten Cate JW, Lamping RJ, Berends F, Borm JJ. Laboratory and clinical evaluation of an assay of thrombin-antithrombin III complexes in plasma. Clin Chem 1988;34:2058–2062.
- Pelzer H, Schwarz A, Heimburger N. Determination of human thrombin-antithrombin III complex in plasma with an enzyme-linked immunosorbent assay. Thromb Haemost 1988;59:101–106.
- Bauer KA, Rosenberg RD. Thrombin generation in acute promyelocytic leukemia. Blood 1984;64:791–796.
- Teitel JM, Bauer KA, Lau HK, Rosenberg RD. Studies of the prothrombin activation pathway utilizing radioimmunoassays for the F2/F1 + 2 fragment and thrombin–antithrombin complex. Blood 1982;59:1086–1097.
- Yuen PM, Yin JA, Lao TT. Fibrinopeptide A levels in maternal and newborn plasma. Eur J Obstet Gynecol Reprod Biol 1989;30:239–244.
- Sorensen JD, Secher NJ, Jespersen J. Perturbed (procoagulant) endothelium and deviations within the fibrinolytic system during the third trimester of normal pregnancy. A possible link to placental function. Acta Obstet Gynecol Scand 1995;74:257–261.
- Walker MC, Garner PR, Keely EJ, Rock GA, Reis MD. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol 1997;177:162–169.
- Bellart J, Gilabert R, Miralles RM, Monasterio J, Cabero L. Endothelial cell markers and fibrinopeptide A to D-dimer ratio as a measure of coagulation and fibrinolysis balance in normal pregnancy. Gynecol Obstet Invest 1998;46:17–21.
- de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol 1989;160:95–100.
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:368–373.
- Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Power GG, Araki T. Plasma adenosine levels increase in women with normal pregnancies. Am J Obstet Gynecol 2000;182:1200–1203.
- Sheu JR, Hsiao G, Luk HN, Chen YW, Chen TL, Lee LW, Lin CH, Chou DS. Mechanisms involved in the antiplatelet activity of midazolam in human platelets. Anesthesiology 2002;96:651–658.
- Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol 2001;185:1059–1063.
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002;11:362–367.
- Hayashi M, Numaguchi M, Ohkubo N, Yaoi Y. Blood macrophage colony-stimulating factor and thrombin-antithrombin III complex concentrations in pregnancy and preeclampsia. Am J Med Sci 1998;315:251–257.
- Hayashi M, Inoue T, Hoshimoto K, Negishi H, Ohkura T, Inaba N. Characterization of five marker levels of the hemostatic system and endothelial status in normotensive pregnancy and pre-eclampsia. Eur J Haematol 2002;69:297–302.
- Kobayashi T, Terao T. Preeclampsia as chronic disseminated intravascular coagulation. Study of two parameters: thrombin-antithrombin III complex and D-dimers. Gynecol Obstet Invest 1987;24:170–178.
- Kobayashi T, Tokunaga N, Sugimura M, Suzuki K, Kanayama N, Nishiguchi T, Terao T. Coagulation/fibrinolysis disorder in patients with severe preeclampsia. Semin Thromb Hemost 1999;25:451–454.
- Kobayashi T, Sumimoto K, Tokunaga N, Sugimura M, Nishiguchi T, Kanayama N, Terao T. Coagulation index to distinguish severe preeclampsia from normal pregnancy. Semin Thromb Hemost 2002;28:495–500.
- Schjetlein R, Haugen G, Wisloff F. Markers of intravascular coagulation and fibrinolysis in preeclampsia: association with intrauterine growth retardation. Acta Obstet Gynecol Scand 1997;76:541–546.
- Van Wijk MJ, Boer K, Berckmans RJ, Meijers JC, van der Post JA, Sturk A, Van Bavel E, Nieuwland R. Enhanced coagulation activation in preeclampsia: the role of APC resistance, microparticles and other plasma constituents. Thromb Haemost 2002;88:415–420.
- Schjetlein R, Abdelnoor M, Haugen G, Husby H, Sandset PM, Wisloff F. Hemostatic variables as independent predictors for fetal growth retardation in preeclampsia. Acta Obstet Gynecol Scand 1999;78:191–197.
- Hayashi M, Ohkura T. Elevated levels of serum macrophage colony-stimulating factor in normotensive pregnancies complicated by intrauterine fetal growth restriction. Exp Hematol 2002;30:388–393.
- Ballard HS, Marcus AJ. Primary and secondary platelet aggregation in uraemia. Scand J Haematol 1972;9:198–203.
- Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 2001;10:297–300.
- Elovitz MA, Ascher-Landsberg J, Saunders T, Phillippe M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am J Obstet Gynecol 2000;183:674–681.
- Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol 2000;183:799–804.
- Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol 2004;191:1996–2001.
- Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002;11:11–17.
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–920.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113Suppl 317–42.
- Mazor M, Chaim W, Romero R. [Preterm labor syndrome]. Harefuah 1995;128:111–116.
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–429.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.
- Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–176.
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824.
- Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci 1991;622:355–375.
- Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell GH. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–133.
- Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–183.
- Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15Suppl 241–56.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Lockwood CJ, Bach R, Guha A, Zhou XD, Miller WA, Nemerson Y. Amniotic fluid contains tissue factor, a potent initiator of coagulation. Am J Obstet Gynecol 1991;165:1335–1341.
- Liu EH, Shailaja S, Koh SC, Lee TL. An assessment of the effects on coagulation of midtrimester and final-trimester amniotic fluid on whole blood by Thrombelastograph analysis. Anesth Analg 2000;90:333–336.
- Uszynski M, Kielkowski A, Uszynski W, Zekanowska E. Thrombin-antithrombin III complexes and antithrombin III in amniotic fluid. Gynecol Obstet Invest 1997;43:29–33.
- Uszynski M, Zekanowska E, Uszynski W, Kuczynski J. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in amniotic fluid and blood plasma: implications for the mechanism of amniotic fluid embolism. Eur J Obstet Gynecol Reprod Biol 2001;95:163–166.
- Erez O, Gotsch F, Kusanovic JP, Mazaki-Tovi Shali, Vaisbuch E, Kim CJ, Chaiworapongsa T, Hoppensteadt DA, Fareed J, Than NG, et al. Is Tissue Factor Responsible for the Consumption Coagulopathy Associated with Fetal Death?. Reproductive Sciences 2008;15:259A
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation–a workshop report. Placenta 2005;26Suppl AS114–S117.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–681.
- Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, Shim SS. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–1142.
- Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, Edwin S. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:1143–1148.
- Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, Erez O, Edwin S, Schmidt AM. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008;36:388–398.
- Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee KY, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med 2008;21:697–713.
- Romero R, Scharf K, Mazor M, Emamian M, Hobbins JC, Ryan JL. The clinical value of gas-liquid chromatography in the detection of intra-amniotic microbial invasion. Obstet Gynecol 1988;72:44–50.
- Romero R, Emamian M, Wan M, Yarkoni S, McCormack W, Mazor M, Hobbins JC. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 1988;5:64–69.
- Robert JA, Romero R, Costigan K. Amniotic fluid concentrations of fibronectin and intra-amniotic infection. Am J Perinatol 1988;5:26–28.
- Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–666.
- Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–830.
- Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol 1992;166:618–620.
- Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–816.
- Reber G, Amiral J, de Moerloose P. Modified antithrombin III levels during normal pregnancy and relationship with prothrombin fragment F1 + 2 and thrombin-antithrombin complexes. Thromb Res 1998;91:45–47.
- Reinthaller A, Mursch-Edlmayr G, Tatra G. Thrombin-antithrombin III complex levels in normal pregnancy with hypertensive disorders and after delivery. Br J Obstet Gynaecol 1990;97:506–510.
- Uszynski M. Generation of thrombin in blood plasma of non-pregnant and pregnant women studied through concentration of thrombin-antithrombin III complexes. Eur J Obstet Gynecol Reprod Biol 1997;75:127–131.
- Andersson T, Lorentzen B, Hogdahl H, Clausen T, Mowinckel MC, Abildgaard U. Thrombin-inhibitor complexes in the blood during and after delivery. Thromb Res 1996;82:109–117.
- Koh SC, Anandkumar C, Arulkumaran S, Chua SE, Yuen WK, Ng BL, Ratnam SS. Amniotic fluid plasminogen activators and inhibitors and TAT complex levels during 2nd trimester pregnancy and labour. Fibrinolysis 1995;9:121–126.
- Wilcox GR, Trudinger BJ, Exner T. The coagulation system in placental insufficiency: a study in the fetal circulation. Br J Obstet Gynaecol 1993;100:1101–1106.
- Thompson AR. Factor IX and prothrombin in amniotic fluid and fetal plasma: constraints on prenatal diagnosis of hemophilia B and evidence of proteolysis. Blood 1984;64:867–874.
- Ljung R, Holmberg L, Gustavii B, Philip J, Bang J. Haemophilia A and B–two years experience of genetic counselling and prenatal diagnosis. Clin Genet 1982;22:70–52.
- English CJ, Poller L, Burslem RW. A study of the procoagulant properties of amniotic fluid and their correlation with the lecithin/sphingomyelin ratio. Br J Obstet Gynaecol 1981;88:133–137.
- Heyes H, Leucht W, Musch K. Evaluation of fetal lung maturity by measurement of the procoagulant activity in amniotic fluid. Arch Gynecol 1982;233:7–14.
- Yaffe H, Eldor A, Hornshtein E, Sadovsky E. Thromboplastic activity in amniotic fluid during pregnancy. Obstet Gynecol 1977;50:454–456.
- Phillips LL, Davidson E C Jr.. Procoagulant properties of amniotic fluid. Am J Obstet Gynecol 1972;113:911–919.
- Esmon CT, Fukudome K, Mather T, Bode W, Regan LM, Stearns-Kurosawa DJ, Kurosawa S. Inflammation, sepsis, and coagulation. Haematologica 1999;84:254–259.
- Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005;131:417–430.
- Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost 2006;32:33–39.
- Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004;109:2698–2704.
- Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med 2005;15:254–259.
- Levi M, ten Cate H, Bauer KA, vander PT, Edgington TS, Buller HR, van Deventer SJ, Hack CE, ten Cate JW, Rosenberg RD. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest 1994;93:114–120.
- Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood 2005;105:2764–2770.
- Butenas S, Mann KG. Blood coagulation. Biochemistry (Mosc.) 2002;67:3–12.
- Rivers RP, Hathaway WE, Weston WL. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol 1975;30:311–316.
- Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood 1997;89:3270–3276.
- Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol 2005;25:1493–1498.
- Osterud B. Cellular interactions in tissue factor expression by blood monocytes. Blood Coagul Fibrinolysis 1995;6Suppl 1S20–S25.
- Osterud B. The role of platelets in decrypting monocyte tissue factor. Semin Hematol 2001;38:2–5.
- Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost 2006;32:11–23.
- Satta N, Toti F, Feugeas O, Bohbot A, chary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol 1994;153:3245–3255.
- Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, gnat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 2002;99:3962–3970.
- Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003;102:2678–2683.
- Eilertsen KE, Osterud B. The role of blood cells and their microparticles in blood coagulation. Biochem Soc Trans 2005;33:418–422.
- Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, Caruso P, Bernardi F, Di VF. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J 2007;21:1926–1933.
- Poitevin S, Cochery-Nouvellon E, Dupont A, Nguyen P. Monocyte IL-10 produced in response to lipopolysaccharide modulates thrombin generation by inhibiting tissue factor expression and release of active tissue factor-bound microparticles. Thromb Haemost 2007;97:598–607.
- van der Poll T, Levi M, Hack CE, ten CH, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Buller HR, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med 1994;179:1253–1259.
- Kirchhofer D, Tschopp TB, Hadvary P, Baumgartner HR. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest 1994;93:2073–2083.
- Hoffman M, Cooper ST. Thrombin enhances monocyte secretion of tumor necrosis factor and interleukin-1 beta by two distinct mechanisms. Blood Cells Mol Dis 1995;21:156–167.
- Hahn CL, Best AM, Tew JG. Rapid tissue factor induction by oral streptococci and monocyte-IL-1beta. J Dent Res 2007;86:255–259.
- Herbert JM, Savi P, Lale A, Laplace MC, Baudry N, Pereillo JM, Emonds-Alt X. Malformin-A1 inhibits the binding of interleukin-1 beta (IL1 beta) and suppresses the expression of tissue factor in human endothelial cells and monocytes. Biochem Pharmacol 1994;48:1211–1217.
- Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–550.
- Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–193.
- Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, Dehan M, Frydman R, Ville Y. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–77.
- Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–779.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–681.
- Jun JK, Yoon BH, Romero R, Kim M, Moon JB, Ki SH, Park JS. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:868–873.
- Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–1129.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, Jun JK. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–1167.
- Fukuda H, Masuzaki H, Ishimaru T. Interleukin-6 and interleukin-1 receptor antagonist in amniotic fluid and cord blood in patients with pre-term, premature rupture of the membranes. Int J Gynaecol Obstet 2002;77:123–129.
- Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003;82:120–128.
- Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90.
- Lee KY, Jun HA, Kim HB, Kang SW. Interleukin-6, but not relaxin, predicts outcome of rescue cerclage in women with cervical incompetence. Am J Obstet Gynecol 2004;191:784–789.
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–1587.
- Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol 1993;169:825–829.
- Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol 1993;30:49–57.
- Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol 1991;41:123–127.
- Mitchell MD, Branch DW, Lundin-Schiller S, Romero RJ, Daynes RA, Dudley DJ. Immunologic aspects of preterm labor. Semin Perinatol 1991;15:210–224.
- Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids 1990;41:35–38.
- Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids 1989;37:183–186.
- Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–341.
- Harris AN, Perlman M, Schiller SL, Romero R, Mitchell MD. Characterization of prostaglandin production in amnion-derived WISH cells. Am J Obstet Gynecol 1988;159:1385–1389.
- Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999;181:1142–1148.
- Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–830.
- Soto E, Romero R, Richani K, Chaiworapongsa T, Yoon BH, Nien JK, Edwin S, Kusanovic JP, Espinoza J, Espinoza J. Evidence for complement activation in premature labor associated with intra-amniotic infection. Am J Obstet Gynecol 2006;195:S74
- Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost 1997;77:394–398.
- Sitrin RG, Kaltreider HB, Ansfield MJ, Webster RO. Procoagulant activity of rabbit alveolar macrophages. Am Rev Respir Dis 1983;128:282–287.
- Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–37.
- Vaisbuch E, Romero R, Erez O, Kusanovic JP, Gotsch F, Than NG, Mazaki-Tovi S, Mittal P, Edwin S, Hassan SS. Total hemoglobin concentration in amniotic fluid is increased in intraamniotic infection/inflammation. Am J Obstet Gynecol 2008;199:426–427.
- Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Mazaki-Tovi S, Kim CJ, Kim JS, Chaiworapongsa T, Edwin SS, Than NG, et al. Fetal Hemoglobin Concentration In Amniotic Fluid Is Elevated In Preterm Labor Or Prelabor Rupture Of Membranes. Reproductive Sciences 2008;15:254A
- Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome 2004;First:28–60.
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 1999;103:879–887.
- Hung DT, Vu TK, Wheaton VI, Ishii K, Coughlin SR. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J Clin Invest 1992;89:1350–1353.
- Li T, He S. Induction of IL-6 release from human T cells by PAR-1 and PAR-2 agonists. Immunol Cell Biol 2006;84:461–466.
- Naldini A, Carney DH, Pucci A, Pasquali A, Carraro F. Thrombin regulates the expression of proangiogenic cytokines via proteolytic activation of protease-activated receptor-1. Gen Pharmacol 2000;35:255–259.
- Hirano K, Kanaide H. Role of protease-activated receptors in the vascular system. J Atheroscler Thromb 2003;10:211–225.
- Duarte M, Kolev V, Soldi R, Kirov A, Graziani I, Oliveira SM, Kacer D, Friesel R, Maciag T, Prudovsky I. Thrombin induces rapid PAR1-mediated non-classical FGF1 release. Biochem Biophys Res Commun 2006;350:604–609.
- Connolly AJ, Ishihara H, Kahn ML, Farese R V Jr, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature 1996;381:516–519.
- Darrow AL, Fung-Leung WP, Ye RD, Santulli RJ, Cheung WM, Derian CK, Burns CL, Damiano BP, Zhou L, Keenan CM, et al. Biological consequences of thrombin receptor deficiency in mice. Thromb Haemost 1996;76:860–866.
- Major CD, Santulli RJ, Derian CK, Andrade-Gordon P. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler Thromb Vasc Biol 2003;23:931–939.
- O'Brien M, Morrison JJ, Smith TJ. Expression of prothrombin and protease activated receptors in human myometrium during pregnancy and labor. Biol Reprod 2008;78:20–26.
- O'Sullivan CJ, Allen NM, O'Loughlin AJ, Friel AM, Morrison JJ. Thrombin and PAR1-activating peptide: effects on human uterine contractility in vitro. Am J Obstet Gynecol 2004;190:1098–1105.
- Shintani Y, Hirano K, Nishimura J, Nakano H, Kanaide H. Enhanced contractile response to thrombin in the pregnant rat myometrium. Br J Pharmacol 2000;131:1619–1628.
- Tsai MS, Hwang SM, Chen KD, Lee YS, Hsu LW, Chang YJ, Wang CN, Peng HH, Chang YL, Chao AS, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells 2007;25:2511–2523.
