Abstract
Objective. An imbalance between angiogenic and anti-angiogenic factors in maternal blood has been observed in several obstetrical syndromes including preeclampsia, pregnancies with fetal growth restriction and fetal death. Vascular lesions have been identified in a subset of patients with spontaneous preterm labor (PTL). It is possible that PTL may be one of the manifestations of an anti-angiogenic state. The aim of this study was to determine if patients prior to the clinical diagnosis of PTL leading to preterm delivery had plasma concentrations of angiogenic and anti-angiogenic factors different from normal pregnant women.
Study Design. This longitudinal nested case–control study included normal pregnant women (n = 208) and patients with PTL leading to preterm delivery (n = 52). Maternal blood samples were collected at 6 gestational age intervals from 6 to36.9 weeks of gestation. The end point (time of diagnosis) of the study, ‘True PTL’, was defined as patients presenting with PTL and delivered within 1 day. Plasma concentrations of sVEGFR-1, sVEGFR-2, sEng and PlGF were determined by ELISA. Analysis was performed with both cross-sectional and longitudinal (mixed effects model) approaches.
Results. (1) Plasma sEng concentration in patients destined to develop PTL was higher than that in normal pregnant women from 15–20 weeks of gestation. The difference became statistical significant at 28 weeks of gestation, or approximately 5–10 weeks prior to the diagnosis of ‘true PTL’. (2) Backward analysis suggests that plasma concentrations of PlGF and sVEGFR-2 were lower, and those of sVEGFR-1 were higher in patients with PTL than in normal pregnant women less than 5 weeks prior to the diagnosis of ‘true PTL’; and (3) Plasma concentrations of sEng and sVEGFR-1 were higher and those of PlGF and sVEGFR-2 were lower in patients diagnosed with PTL and delivery within 1 day than in normal pregnant women who delivered at term.
Conclusion. The changes in sEng are demonstrable several weeks prior to the onset of preterm parturition. In contrast, the changes in the other angiogenic proteins are present close to the onset of PTL and delivery. This observation supports the view that an imbalance of angiogenic factors participates in the pathophysiology of spontaneous preterm parturition.
Introduction
Spontaneous preterm delivery is the leading cause of perinatal mortality and morbidity worldwide [Citation1–3]. Despite considerable effort, the incidence of preterm birth is still rising [Citation4]. We have proposed that spontaneous preterm parturition is a syndrome [Citation1] resulting from multiple pathological processes including intrauterine infection [Citation5–12], uterine overdistension [Citation13–16], allergic-like reaction [Citation17–19], cervical disease [Citation20–22], endocrine disorders [Citation23–26], maternal or fetal stress [Citation27–31]and uterine ischemia. Evidence in support for a role of utero-placental ischemia as a mechanism of disease leading to preterm labor (PTL) includes: (1) an experimental study showed that, after induced uterine ischemia (designed to generate a primate model for preeclampsia), a proportion of animals went into PTL and delivery [Citation32]; (2) patients with PTL and preterm PROM who delivered preterm had a higher percentage of failure of physiologic transformation in myometrial segment of the spiral arteries than women who delivered at term [Citation33,Citation34]; (3) increased impedance to flow in uterine artery in the second trimester increased risk of preterm delivery [Citation35]. Similarly, patients presenting with PTL who had an abnormal uterine artery Doppler velocimetry were more likely to deliver preterm than those with normal Doppler velocimetry [Citation36]; and (4) the presence of vascular lesions in decidual vessels attached to the placenta was more common in patients with PTL and delivery than in women delivered at term gestation [Citation37]. However, the precise mechanisms responsible for the onset of preterm parturition in cases of ischemia have not been determined. Recently, an imbalance between angiogenic and anti-angiogenic factors in maternal blood has been observed in several obstetrical syndromes with perturbation in utero-placental blood supply including preeclampsia [Citation38–56], fetal growth restriction [Citation57–59], placental abruption [Citation60], ‘mirror syndrome’ [Citation61], twin-to-twin transfusion syndrome(TTTS) [Citation62] and unexplained fetal death [Citation63].
Angiogenesis, a process by which new vessels are formed from pre-existing vasculature, is regulated by several growth factors, cytokines and their receptors, including fibroblast growth factors, transforming growth factors, hepatocyte growth factors, angiogenins, angiopoietins, ephrins and vascular endothelial growth factors (VEGF) [Citation64,Citation65]. VEGF-signalling represents a critical step in both physiologic and pathologic angiogenesis [Citation65,Citation66]. VEGF is an endothelial cell-specific growth factor with potent angiogenic properties. While VEGF receptor-1 (VEGFR-1) is considered a ‘decoy’ receptor, VEGFR-2 is the major mediator of the mitogenic, angiogenic, permeability enhancing and endothelial survival effects of VEGF [Citation67]. Placental growth factor (PlGF), another member of the VEGF family, can also bind to VEGFR-1 on endothelial cells or on macrophages, and enhance the angiogenic response of VEGF on endothelial cells or induce migration of macrophages, especially in pathological conditions such as limb ischemia or wound healing [Citation68–70]. The soluble form of VEGFR-1 (sVEGFR-1) has potent anti-angiogenic activity because it can bind to VEGF or PlGF and inhibits their biological functions. The natural form of soluble VEGFR-2 (sVEGFR-2) has recently been detected in human plasma [Citation71]. Under experimental conditions, this protein can bind to VEGF [Citation71] and its recombinant form has anti-angiogenic activity [Citation72,Citation73]. The role of sVEGFR-2 in human health and diseases is still unclear. Endoglin (Eng) is a co-receptor of transforming growth factor (TGF)-β and its soluble form (sEng) has anti-angiogenic activity by modulating the actions of TGF-β1 and TGF-β3 [Citation74].
A balance between angiogenic and anti-angiogenic factors is essential for feto-placental development [Citation75–78]. However, there is a paucity of information regarding the changes of these factors in patients destined to develop spontaneous PTL. The aim of this study was to determine if patients prior to the clinical diagnosis of PTL leading to preterm delivery had plasma concentrations of PlGF, sVEGFR-1, sEng and sVEGFR-2 that were different from normal pregnant women.
Patients and methods
Study design
A retrospective longitudinal nested case–control study was conducted by searching our clinical database and bank of biologic samples from 2002 to 2006. Patients with spontaneous PTL and delivery (n = 52) and normal pregnant women (n = 208) were included. Exclusion criteria were as follows: (1) patients with chronic hypertension, preeclampsia or gestational hypertension; (2) known major fetal or chromosome anomaly; and (3) multiple gestations. All women were enrolled in the prenatal clinic at the Sotero del Rio Hospital, Santiago, Chile and followed until delivery. Prenatal visits were scheduled at 4-week intervals in the first and second trimester, and every 2 weeks in the third trimester until delivery. Blood sampling was performed at enrollment and every visit with the patient's consent.
For this study, subjects were included only if they had plasma samples available at least once before and after 24 weeks of gestation. All patients had a minimum of three samples during pregnancy (3–6 samples). Plasma samples were selected once from each patient of the following six intervals: (1) 6–14 weeks, (2) 15–19 weeks, (3) 20–24 weeks, (4) 25–27 weeks, (5) 28–31 weeks and (6) 32–36 weeks of gestation. The earliest sample for each interval was used.
Clinical definition
Spontaneous PTL and delivery was defined by the presence of regular uterine contractions and cervical changes that led to delivery before 37 completed weeks of gestation. Gestational age (GA) was determined by the last menstrual period or by ultrasound in case the ultrasonographic determination of GA was not consistent with the menstrual dating by >2 weeks. This study included only patients who underwent ultrasound examination for dating before 24 weeks of gestation. An amniocentesis was performed in some patients to assess the microbiologic state of the amniotic cavity at the discretion of the responsible physicians. Some patients may have multiple episodes of preterm contractions or PTL without leading to delivery. The end point (time of diagnosis) of the study was ‘true PTL and delivery’ defined as PTL that leads to preterm delivery within 1 day. Samples that were taken during episodes of ‘false PTL or preterm contraction’ within 1 day were excluded. Pregnant women were considered normal if they had no medical, obstetrical or surgical complications, and delivered a normal term (≥37 weeks) infant whose birth-weight was appropriate for GA (10th–90th percentile) [Citation79]. Acute histologic chorioamnionitis and acute funisitis were diagnosed using previously described criteria [Citation80]. Pathologic findings consistent with maternal hypoperfusion and inflammation were defined according to Redline [Citation81].
The collection and utilisation of the samples was approved by both the Human Investigation Committee of the Sotero del Rio Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago) and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies.
Sample collection and angiogenic factors immunoassays
Venipunctures were performed, and the blood was collected into tubes containing EDTA. Samples were centrifuged and stored at −70°C. Maternal plasma concentrations of PlGF, sVEGFR-2, sEng and sVEGFR-1 were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). All four immunoassays utilised the quantitative sandwich enzyme immunoassay technique and their concentrations in maternal plasma were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation (CV) obtained were as follows: PlGF, 6.02 and 4.8%, respectively, sVEGFR-2, 2 and 4%, respectively; sEng, 2.3 and 4.6%, respectively; and sVEGFR-1, 1.4 and 3.9%, respectively. The sensitivity of the assays were as follows: PlGF, 9.52 pg/ml; sVEGFR-2, 19.01 pg/ml; sEng, 0.08 ng/ml and sVEGFR-1, 16.97 pg/ml.
Statistical analysis
Cross-sectional analysis
Shapiro–Wilk and Kolmogorov–Smirnov tests were used to test for normal distribution of the data. Kruskal–Wallis and post-hoc Mann–Whitney U tests were utilised to determine the differences of the median among and between groups. Chi-square and Fischer's Exact tests were employed for comparisons of proportions. Multivariate logistic regression was applied to examine the association between high plasma concentrations of sEng or sVEGFR-1 (defined as plasma sEng or sVEGFR-1 concentrations above the third quartile of normal pregnancy), low plasma concentrations of PlGF or sVEGFR-2 (defined as plasma PlGF or sVEGFR-2 concentrations below the first quartile of normal pregnancy) and the development of ‘true spontaneous preterm parturition’ in samples obtained prior to the clinical diagnosis of the disease in various GA intervals after adjusting for potential confounders. Cox-regression analysis was applied to determine the association of high or low plasma angiogenic factor concentrations and duration to delivery while adjusting for potential confounders. The statistics package used was SPSS V.15 (SPSS, Chicago, IL). A p value of <0.05 was considered significant.
Longitudinal analysis
Changes in the plasma concentrations of the four angiogenic factors over time and between groups were tested using a linear mixed effects model (fixed effects + random effects). The fixed effects were the diagnosis (a factor with two levels: normal pregnancy and PTL), the linear and quadratic effects of GA, the interaction term between the diagnosis and GA and other covariates including: maternal age, body mass index (BMI), smoking, nulliparity, previous preeclampsia and sample storage time. The random effects were the patient identification numbers, therefore allowing examination of the deviation of each individual from the average profile of each diagnostic group and accounting for the unknown variability among patients. The model was fitted to the transformed plasma concentration [log10 concentration +1)] of the analytes. This logarithmic transformation was employed to achieve normality of the data and stabilise variance across the entire range of GA. Statistical significance of fixed effects was assessed using t-scores, and a p value <0.05 was considered significant. A false discovery rate algorithm was applied to adjust for multiple analytes and multiple covariates. The analysis was performed using the nlme (Nonlinear Mixed Effects Model) package of the R statistical environment (www. r-project.org).
To identify the GA at which the difference in the median concentration between groups became significant, a moving window approach was used. Unlike in the cross-sectional study, where the limits of the intervals were predetermined in the moving window approach, the number of data points was fixed to 250 for each window. All observations were sorted as a function of the GA in ascending order. A set of 250 data points was chosen starting with the smallest gestation age and moving up the list. A Wilcoxon test was used to determine if there was significant difference between the two groups in each window of GA. The procedure was repeated until the point when the difference between groups remained significant (p < 0.05) in the current window and all consecutive ones. The median GA in the current window recorded.
Results
Clinical characteristics of the study population are displayed in . As expected, patients with spontaneous PTL and delivery had a history of preterm delivery more frequently than those of normal pregnant women who delivered at term. There were 7 (13.5%) and 13 (25%) patients who delivered before 32 and 34 weeks of gestation, respectively. Eleven patients underwent amniocentesis and two (18%) had a positive amniotic fluid culture result for Candida albicans.
Table I. Clinical characteristics of the study population.
The GA at which patients presented with ‘true spontaneous PTL and delivery’ varied. One patient was diagnosed with PTL and delivered at 26 weeks of gestation, five at 28–31 weeks and 46 at 32–36 weeks. There was no significant difference in the median GA at which venipuncture was performed by the interval window between the group of patients who delivered preterm and the control group (all p > 0.05, ).
Table II. Plasma concentrations of angiogenic factors in normal pregnancy and preterm labor who delivered preterm.
Plasma sEng concentrations are elevated prior to the clinical manifestation of ‘true spontaneous PTL and delivery’: forward cross-sectional approach
The median plasma sEng concentrations in women who subsequently experienced PTL and delivery were higher than in normal pregnant women from 15 to 19 weeks of gestation until delivery (p < 0.05, except at 32–37 weeks p = 0.06; ). In contrast, no significant difference in the median plasma concentration of sVEGFR-1, PlGF and sVEGFR-2 between patients with PTL before the clinical diagnosis and normal pregnant women was observed in any of the six GA intervals, except one. The median plasma concentration of PlGF in patients who subsequently delivered preterm was significantly lower than that of those with a normal pregnancy at 28–31 weeks of gestation (p = 0.03; ).
To examine the association between high plasma concentrations of sEng (above the 3rd quartile of normal pregnancy) and the development of spontaneous PTL and delivery, multivariate logistic regression was applied to adjust for potential confounders. For this analysis, samples obtained at the time of diagnosis were excluded. The dependent variable in the logistic model was the presence of PTL. Different cut-offs for high plasma sEng concentration in various GA intervals as well as unadjusted and adjusted odds ratios are displayed in . High plasma sEng concentrations at 28–31 and 32–36 weeks of gestation conferred the risk of spontaneous PTL and delivery with an odds ratio of 3.1 (95% CI: 1.3–7.2) and 6.6 (95% CI: 1.5–28.6), respectively, after adjusting for maternal age, BMI, smoking, nulliparity, previous preterm delivery, GA at blood sampling and sample storage time. There was no significant association between the development of spontaneous PTL and high plasma sEng concentrations at any other GA intervals () after adjusting for potential confounders. Similar analysis was applied to sVEGFR-1, PlGF and sVEGFR-2, all of these high (above the 3rd quartile) or low (below the 1st quartile) plasma angiogenic factor concentrations showed no significant association with the development of spontaneous PTL (data not shown).
Table III. Unadjusted and adjusted odds ratio for the identification of spontaneous preterm labor by plasma sEng concentrations above the 3rd quartile for various gestational age intervals in pre-clinical samples.
Cox-regression analysis was applied to determine the association of high or low plasma angiogenic factor concentrations and the duration from blood sampling to delivery interval while adjusting for GA at blood sampling, maternal age, BMI, smoking, nulliparity, previous preterm delivery and sample storage time. Among patients with PTL prior to the diagnosis and normal pregnant women at 32–37 weeks, high plasma sEng concentrations, high plasma sVEGFR-1 concentrations and low plasma PlGF concentrations were associated with shorter duration to delivery [hazard ratio 1.5 (95% CI: 1.02–2.2), 1.7 (95% CI: 1.1–2.5) and 2.4 (95% CI: 1.7–3.6), respectively. In contrast, when the analysis was restricted to normal pregnant women who delivered at term, only plasma sVEGFR-1 and PlGF were associated with shorter duration of the venipuncture to delivery interval with similar hazard ratios. High plasma sEng concentrations and low plasma sVEGFR-2 concentrations were not associated with a shorter duration to delivery interval [hazard ratios of 0.8 (95% CI: 0.5–1.1) and 0.7 (95% CI: 0.5–1.1), respectively].
Plasma sEng concentrations are elevated prior to the clinical manifestation of PTL: longitudinal approach
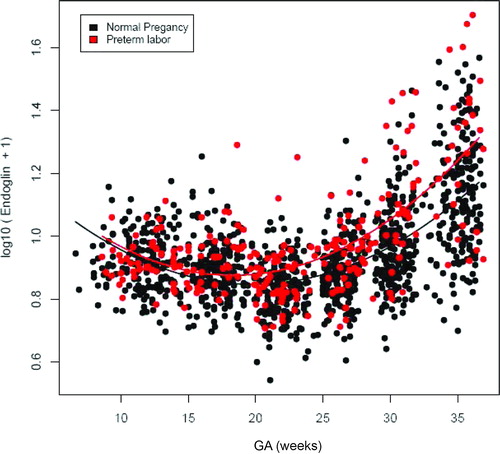
Patients who subsequently delivered preterm had a significantly different profile (plasma concentration over time) of plasma sEng concentration from patients with normal pregnancies after adjusting for GA at blood sampling, maternal age, BMI, nulliparity, history of preterm delivery, smoking and duration of sample storage (p = 0.04; ). Plasma sEng concentrations were higher in women destined to develop PTL than in normal pregnant women from approximately 15 weeks of gestation. The difference became statistically significant (p < 0.05) at 27.7 weeks of gestation (see Statistics section), and it was more pronounced as delivery approached ().
Table IV. Mixed-effect model comparing the profiles of plasma sEng and sVEGFR-1 concentrations in relation to gestational age between patients with PTL and normal pregnant women adjusting for confounders (see text).
Figure 1. Profile of plasma soluble endoglin (sEng) concentrations (ng/ml) in relation to GA in normal pregnant women and patients with spontaneous PTL and delivery. Patients destined to developed PTL had a significantly different profile (plasma concentration over time) of plasma sEng concentration from patients with normal pregnancies after adjusting for GA at blood sampling, maternal age, body mass index, nulliparity, a history of preterm delivery, smoking and duration of sample storage (p = 0.04). Plasma sEng concentration was higher in patients destined to develop PTL than in normal pregnant women from 15 to 20 weeks of gestation. The difference became statistical significance at 27.7 weeks and was more pronounce as delivery approached.
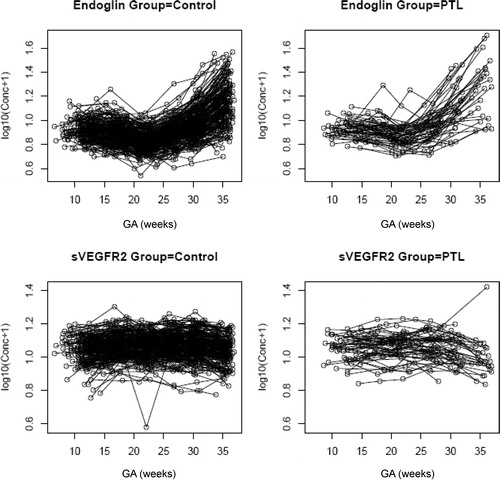
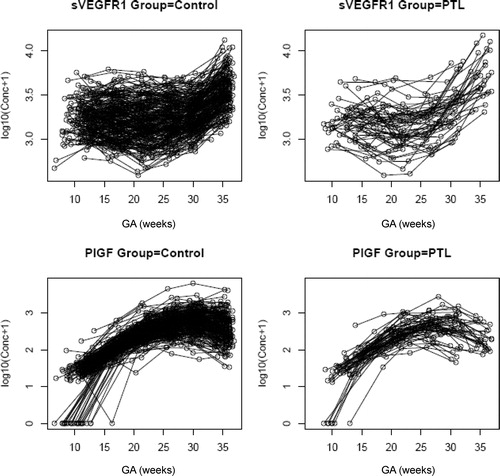
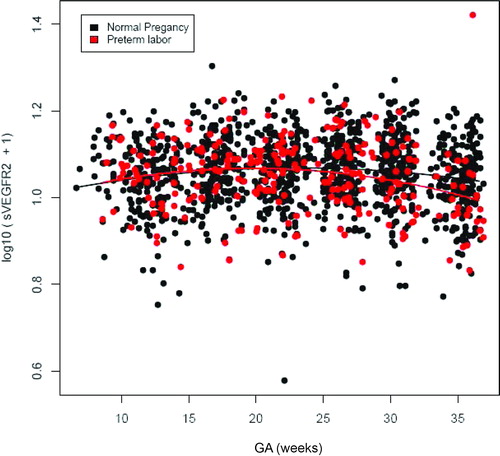
Patients who subsequently delivered preterm also had a significantly different profile of plasma sVEGFR-1 concentration from that of normal pregnant women (p = 0.003; ). Plasma sVEGFR-1 concentrations in patients with PTL were lower than those in normal pregnant women from 10 to 28 weeks of gestation. After that GA, plasma concentrations of sVEGFR-1 became higher until the time of delivery (). In contrast, there was no significant difference in the profiles of plasma concentrations of PlGF and sVEGFR-2 between patients with PTL and normal pregnant women (p > 0.05; ). However, upon observations of the profiles of sVEGFR-1, PlGF and sVEGFR-2, there was an increase in plasma sVEGFR-1 concentrations, but a decrease in plasma PlGF and sVEGFR-2 concentrations just prior to the time of diagnosis of ‘true PTL’ (, respectively). Individual changes in maternal plasma concentration of sEng, sVEGFR-2, sVEGFR-1 and PlGF in normal pregnant women and patients destined to develop PTL and delivery in relation to GA were displayed in and .
Table V. Mixed-effect model comparing the profiles of plasma PlGF and sVEGFR-2 concentrations in relation to gestational age between patients with PTL and normal pregnant women adjusting for confounders (see text).
Figure 2. Profile of plasma sVEGFR-1 concentrations (pg/ml) in relation to GA in normal pregnant women and patients with spontaneous PTL and delivery. Patients destined to developed PTL had a significantly different profile (plasma concentration over time) of plasma sVEGFR-1 concentration from patients with normal pregnancies after adjusting for GA at blood sampling, maternal age, body mass index, nulliparity, a history of preterm delivery, smoking and duration of sample storage (p = 0.003). Plasma sVEGFR-1 concentrations in patients with PTL were slightly lower than those in normal pregnant women from 10 to 28 weeks of gestation. After this GA, plasma sVEGFR-1 concentrations became higher until delivery.
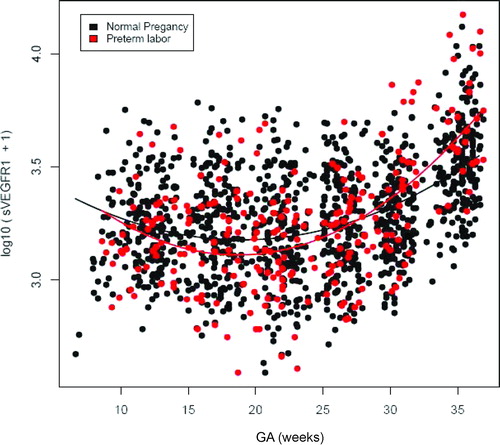
Figure 3. Profile of plasma placenta growth factor (PlGF) concentrations (pg/ml) in relation to GA in normal pregnant women and patients with spontaneous PTL and delivery. There was no significant difference in the profile (plasma concentration over time) of plasma PlGF concentration between patients with PTL and normal pregnant women after adjusting for GA at blood sampling, maternal age, body mass index, nulliparity, a history of preterm delivery, smoking and duration of sample storage (p = 0.3).
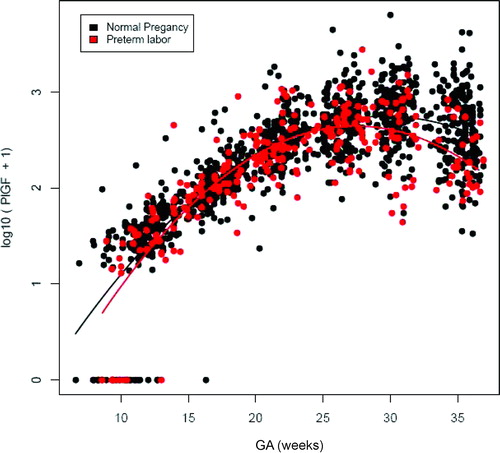
Figure 4. Profile of plasma sVEGFR-2 concentrations (ng/ml) in relation to GA in normal pregnant women and patients with spontaneous PTL and delivery. There was no significant difference in the profile (plasma concentration over time) of plasma sVEGFR-2 concentration between patients with PTL and normal pregnant women after adjusting for GA at blood sampling, maternal age, body mass index, nulliparity, a history of preterm delivery, smoking and duration of sample storage (p = 0.1).
Plasma concentrations of sEng are elevated prior to the clinical manifestation of PTL: (backward analysis)
To examine the relationship between plasma concentrations of angiogenic/antiangiogenic factors and the interval-to-clinical diagnosis of PTL leading to preterm delivery, plasma samples of patients with PTL at different GAs were stratified according to the interval from blood sampling to clinical diagnosis into seven groups: (1) at clinical diagnosis; (2) 2–14 days before diagnosis; (3) 15–35 days before diagnosis; (4) 36–70 days before diagnosis; (5) 71–105 days before diagnosis; (6) 106–140 days before diagnosis; and (7) more than 140 days before diagnosis. Plasma samples from normal pregnant women were matched for GA with the plasma samples of patients with PTL at different GAs according to the intervals pre-specified above (e.g. 2–14 days before diagnosis, etc.).
The median plasma sEng concentration was significantly higher in patients who developed PTL than in normal pregnant women at clinical diagnosis, at 2–14 days, 15–35 days, 36–70 days and at 106–140 days before the clinical diagnosis (all p < 0.05; ). No significant differences in the median plasma sEng concentration were observed between patients who developed PTL and the normal control group, both at 71–105 days before the diagnosis and more than 140 days before clinical diagnosis of PTL (both p > 0.05, ).
Table VI. Plasma concentrations of angiogenic factors in normal pregnancy and preterm labor who delivered preterm.
In contrast, the median plasma concentration of sVEGFR-1 was higher and that of sVEGFR-2 was lower in patients with PTL than that of normal pregnant women at clinical diagnosis and at 15–35 days (median 27 days) before the clinical diagnosis (all p < 0.05 ). Women with PTL had a median plasma concentration of PlGF lower than that of normal pregnant women at clinical diagnosis (p < 0.001) and at 2–14 days (median days) before the diagnosis. However, the difference did not reach statistical significance at interval 2–14 days (p = 0.054; ).
Women with high plasma sEng concentration (above the 3rd quartile) had a high rate of placental pathologic findings consistent with maternal under perfusion
Among patients with PTL, placental pathology results were available in 30 cases (57%). Four (13%) cases of pathologic findings in the placenta were consistent with lesions indicative of maternal hypoperfusion, and 5 (17%) cases were consistent with histologic chorioamnionitis and/or funisitis. Pathologic findings consistent with maternal hypoperfusion, but not inflammation, were associated with high plasma concentrations of sEng at GA intervals of 15–19 and 28–31 weeks (p < 0.05; ). Either high plasma concentrations of sVEGFR-1 or low plasma concentrations of PlGF as well as sVEGFR-2 were not associated with pathologic findings consistent with maternal hypoperfusion (all p > 0.05; data not shown).
Table VII. Proportions of PTL patients who had plasma sEng concentrations above the 3rd quartile stratified by placental pathology.
DISCUSSION
Principal findings
(1) A subset of mothers destined to develop spontaneous PTL and delivery had higher plasma sEng concentrations than those who had a normal pregnancy. This difference was detectable several weeks prior to the clinical diagnosis of PTL; (2) the median plasma sEng concentration in patients destined to develop PTL and delivery was elevated from 15 to 20 weeks of gestation onwards, and became significantly different from normal pregnant women at 28 weeks of gestation or approximately 5–10 weeks prior to the diagnosis of PTL, and the difference was more pronounced as PTL and delivery approached; (3) among normal pregnant women and patients destined to develop PTL and delivery, high plasma sEng, high plasma sVEGFR-1 and low plasma PlGF concentrations at 32–37 weeks were associated with a shorter duration to delivery interval after adjusting for potential confounders including GA at blood sampling; (4) backward analysis suggests that plasma concentrations of PlGF and sVEGFR-2 were lower and those of sVEGFR-1 were higher than those of normal pregnant women less than 5 weeks prior to the diagnosis of PTL and delivery; and (5) plasma concentrations of sEng and sVEGFR-1 were higher and those of PlGF and sVEGFR-2 were lower in patients diagnosed with PTL and delivery within 1 day than in normal pregnant women who delivered at term.
Plasma concentrations of pro-angiogenic and anti-angiogenic factors change prior to the diagnosis of spontaneous PTL and delivery
The findings that women destined to develop PTL and delivery had an increase in plasma sEng and sVEGFR-1 concentrations, a decrease in plasma PlGF and sVEGFR-2 concentrations several weeks prior to the diagnosis of PTL and delivery are consistent with a study of Tsai et al. (published in an abstract form) who reported that plasma concentrations of sEng and sVEGFR-1 concentrations were elevated and those of PlGF were decreased before the diagnosis of spontaneous PTL and delivery [Citation82]. These observations suggest that an imbalance of angiogenesis is involved in the pathophysiology of a subset of patients with spontaneous preterm parturition. Moreover, these findings support the view that the differential expression of angiogenesis–related genes or proteins in gestational tissues observed in studies comparing patients with and without labor is not a consequence of wound healing or tissue remodeling associated with the physical stress of labor, but is more likely to be casual in the preparation for labor [Citation83].
Plasma sEng concentrations are elevated 5–10 weeks prior to the diagnosis of spontaneous PTL and delivery
Among the studied angiogenic factors, plasma sEng concentration is the earliest one to increase in patients destined to deliver preterm. Although forward cross-sectional analysis suggested that this change began at 15–20 weeks of gestation, it became statistically different from that of normal pregnant women at 28 weeks of gestation after adjusting for potential confounders. Backward analysis indicated that the pattern of elevation of plasma sEng concentration was bimodal. The first elevation was at 15–20 weeks prior to PTL and a second elevation was observed at 5–10 weeks prior to preterm delivery. It is likely that the elevation in plasma sEng concentrations in patients with PTL is not a signal to initiate labor, but rather reflects perturbation in blood supply to the placenta.
Eng is expressed on vascular endothelial cells, vascular smooth muscle cells, cytotrophoblasts, syncytiotrophoblasts, uterine stromal cells, monocytes and hematopoietic stem cells [Citation74,Citation84]. Elevated plasma sEng concentrations have been reported in obstetrical conditions with perturbation of blood supply to the uterus such as preeclampsia [Citation38–45,Citation48,Citation49,Citation51,Citation52,Citation56] and pregnancies with fetal growth restriction [Citation57–59]. Evidence suggests that over-expression of Eng on trophoblasts could inhibit trophoblast differentiation and invasion during placentation [Citation85,Citation86]. Systemic elevation of plasma sEng concentrations in patients with PTL might reflect the changes of this protein locally in the feto-maternal interface and prevent the remodelling of spiral arteries resulting in reduced blood supply to the placenta. The remodelling process of the spiral arteries, although begins in early pregnancy, continues until the third trimester as late normalisation of abnormal uterine artery Doppler velocimetry has been documented [Citation87]. Consistent with this hypothesis, in this study we observed an association between high plasma sEng concentrations and the presence of pathological lesions in the placenta that were consistent with poor maternal perfusion. The finding of an elevation of plasma sEng concentrations in patients destined to develop PTL observed herein was of a lesser magnitude than that observed in patients destined to develop preeclampsia and that of pregnancies destined to be diagnosed to have an SGA neonate. Thus, it seems that all these conditions have in common a degree of utero-placental ischemia. This interpretation is consistent with the findings in the placental bed of patients with PTL [Citation33].
Alternatively, an elevation of plasma sEng concentrations, particularly for the second elevation, in patients with PTL could indicate the changes in gestational tissues in preparation for labor. TGF-β plays a pivotal role in cyclic growth and remodelling in uterine epithelial [Citation88], stromal [Citation89], endothelial [Citation90] and myometrial cells [Citation91,Citation92]. Because Eng is a co-receptor of TGF-β, it can modulate the function of TGF-β1 as well as TGF-β3. TGF- β1 has been proposed to participate in human parturition by up-regulating the ryanodine-sensitive intra-cellular Ca2+ release channel, a contraction-associated protein [Citation93,Citation94]. By triggering the synthesis of this protein, the smooth muscle cells of the uterus are transformed from quiescent (phase 0) into activated state (phase I) and primed to response to endogenous uterotonin (eg: oxytocin, prostaglandins) [Citation95]. Moreover, the protein expression of TGF-β1 in myometrium is elevated during pregnancy and further increased after labor, while TGF-β receptor type I and II protein expression in myometrium increased before labor and down-regulated after term labor [Citation94]. The temporal change of TGF-β and its receptor protein expression in myometrium suggests that the TGF-β system plays a role in preparation of myometrium at term [Citation91,Citation94]. Moreover, TGF-β1 has been demonstrated to inhibit the production of prostaglandin E2 by human decidual [Citation96] and amnion cells [Citation97]. Although Eng is highly expressed at the surface of mouse uterine stromal cells and has been shown to modulate the TGF- β-induced cell proliferation [Citation89], it remains to be determined if Eng is expressed in human myometrium, uterine cervix, choriodecidua and amnion cells, components of the common pathways of parturition [Citation1].
Plasma concentration of PlGF, sVEGFR-2 is decreased and that of sVEGFR-1 is increased less than 5 weeks prior to the diagnosis of spontaneous PTL and delivery
Although longitudinal analysis suggested that the profile of plasma sVEGFR-1 concentration in patients destined to deliver preterm was different from that of normal pregnant women, we did not observe a statistically significant difference between the two groups at any GA intervals using a forward cross-sectional analysis. Moreover, longitudinal and forward cross-sectional analysis also suggests that there was no difference in the profiles of plasma PlGF and sVEGFR-2 between the two groups. In contrast, backward analysis demonstrated that plasma concentrations of PlGF and sVEGFR-2 are lower and those of sVEGFR-1 are higher than normal pregnant women less than 5 weeks prior to the diagnosis of PTL and delivery. We suspect that the reason for this apparent discrepancy is related to the study design. In this study, maternal blood was sampled every 4 weeks, and this interval may have been too long to detect any rapid changes in plasma concentrations of these angiogenic/anti-angiogenic factors which start a short period of time prior to delivery. By the time ‘true PTL’ was diagnosed, the plasma concentrations of sVEGFR-1 were already higher and those of PlGF and sVEGFR-2 were already lower in patients with PTL and delivery within 1 day than in normal pregnant women who delivered at term. Interestingly, among normal pregnant women at 32–36 weeks of gestation, high plasma sVEGFR-1 and low plasma PlGF concentrations, but not high plasma sEng and low plasma sVEGFR-2 concentrations, were associated with shorter duration to delivery after adjusting for GA at blood sampling. These observations indicate that the changes in plasma sVEGFR-1 and PlGF, but not those of sEng and sVEGFR-2, observed in patients destined to deliver preterm in the last 4–5 weeks might follow the same pattern as those in normal pregnancy. Future studies should be designed to allow blood sampling once a week in the last 4–6 weeks before term (longitudinal study) to confirm this hypothesis. We have previously reported that there was no significant difference in the median delta plasma sVEGFR-1 concentration between patients presenting with PTL and intact membranes and normal pregnancy as well as among PTL subgroups including patients with PTL who delivered at term gestation, those who delivered preterm without intra-amniotic infection (IAI) and those who delivered preterm with IAI [Citation63]. The difference in the study design (cross-sectional vs. longitudinal) and the endpoint of both studies (preterm delivery at any GA after blood sampling vs. preterm delivery within 1 day of diagnosis) could explain the apparent discrepancy in the results of our studies.
Clinical implication
The observations in this study could explain, at least in part, the false positive results of angiogenic/anti-angiogenic markers in the prediction of preeclampsia, especially when using plasma sEng concentration in the second trimester. It is of interest that unlike the angiogenic/anti-angiogenic profile observed in preeclampsia, there was no significant change in the maternal plasma concentration of other angiogenic (PlGF) or anti-angiogenic factors (sVEGFR-1 or sVEGFR-2) in the second trimester in patients who subsequently had spontaneous preterm delivery. Therefore, we propose that the changes in maternal plasma angiogenic and anti-angiogenic factors are stereotypic for each of the ‘Great Obstetrical Syndromes' [Citation98], and that the profile and magnitude of different angiogenic and anti-angiogenic factor concentrations at a specific time in gestation is associated with the development of different complications of pregnancy, such as: (1) early onset preeclampsia [Citation44,Citation46,Citation49,Citation51]; (2) pregnancies with small for GA fetuses (SGA) [Citation49,Citation51]; (3) term preeclampsia [Citation44,Citation46,Citation49,Citation51]; and (4) PTL.
Preeclampsia, PTL with intact membranes, preterm premature rupture of membranes (PROM), fetal death and pregnancies with SGA fetuses are all considered the ‘Great Obstetrical Syndromes’ resulting from multiple etiologies [Citation98]. Moreover, it is now apparent that the same mechanisms of disease (e.g. intravascular inflammation, an anti-angiogenic state, etc.) may be shared by several of the ‘Great Obstetrical Syndromes’ at the time of the onset of the disease or even before. Intravascular inflammation (previously implicated in the pathophysiology of preeclampsia [Citation99–104]) has subsequently been reported in SGA [Citation105], PTL with intact membranes [Citation106] and preterm PROM [Citation107]. Moreover, unexplained fetal death was associated with changes in the adaptive limb of the maternal immune response consistent with prior antigenic exposure [Citation108]. Thus, there is similarity between the observations made with flow cytometry in these ‘Great Obstetrical Syndromes’, and those that we report by examining the concentrations of angiogenic and anti-angiogenic factors (e.g. preeclampsia [Citation38–45,Citation47–56], pregnancies with SGA fetuses and abnormal Doppler velocimetry [Citation57–59], ‘mirror syndrome’ [Citation61], TTTS [Citation62] and unexplained fetal death [Citation63].
Strengths and limitations
Several studies have documented the differentially regulated gene or protein expression in myometrium [Citation83,Citation109–112], cervix [Citation113–115] and chorioamniotic membranes [Citation116–119] or terminal villi in the placenta [Citation120] after term [Citation111,Citation112,Citation121] and preterm parturition [Citation110,Citation114,Citation122]. Other than genes encoding proteins involved in prostaglandin synthesis and in the control of the inflammatory response (chemokines, cytokines, etc.), angiogenesis-related genes have been identified as differentially regulated in labor (in the common pathway of parturition) supporting a role of angiogenesis in parturition [Citation83,Citation113]. Although the changes of angiogenic factors in maternal plasma might not reflect protein expression in gestational tissues, the advantage of studying maternal plasma is that we can examine the temporal changes in angiogenic factors systemically prior to spontaneous parturition. Longitudinal sampling of tissues could not be performed in human gestational tissues in an on-going pregnancy due to an obvious reason.
The limitation of this study is that patients with PTL were carefully selected and might not represent PTL patients in general population. We applied strict criteria for the diagnosis of ‘true PTL’ as an endpoint and excluded blood samples that were obtained within 1 day of an episode of false labor. This was done to avoid the possibility of changes in the plasma angiogenic factor determination related to an episode of false PTL or preterm contractions, since either one of these two may change the profile of these angiogenic factors. Further studies are required to test whether this is the case or not. We also excluded patients who delivered after induction of labor and included only cases with at least three serial samples of plasma. The majority (87%) of patients with PTL in this study delivered after 32 weeks, which represents the majority of preterm birth in general population [Citation4]. However, it can be argued that an abnormality of blood vessel development may be more relevant as a mechanism of disease in late preterm birth than in early preterm birth. Previous studies by our group [Citation123–126], as well as those reported by others [Citation3,Citation127–130], indicate that the lower the GA at delivery, the higher the rate of infection/inflammation.
In this study, we examined the association between the maternal plasma concentration of angiogenic/anti-angiogenic factors and covariates [e.g. pregnancy outcome (normal pregnancy or PTL), gestational age, previous preterm delivery, etc.] using longitudinal data. The analysis cannot be performed via classical generalized linear models or repeated measure analysis of variance. Using a simple linear model on these longitudinal data would over-state the significance of the covariates, while the repeated measure analysis could not be used due to missing samples in some patients. In contrast, the linear mixed effects model analysis, used herein, is among the statistical tools able to handle the repeated, yet correlated data, with occasional missing observations from the same individuals by allowing each individual to have its own “random effect” on the baseline analyte concentrations. The ability of these models to fit the observed data is improved over classical linear models (F test p-value <0.0001 for all 4 analytes). Both the group effect magnitude and significance presented in Tables (IV and V) were extracted from the linear mixed effects model analysis.
We have incorporated all data points included in the linear mixed-effects model analysis in the figures to enable the reader to visualize the main trends in the raw data of each study group on a logarithmic scale. The curves in the figures (one for each clinical group) represent a quadratic fit of the analyte concentrations based on the gestational age alone. The purpose of these over all curves is to depict each group's average concentration at a given gestational age.
Finally, we have presented the changes of plasma concentrations of angiogenic/anti-angiogenic factors in PTL across all gestational age ranges. However, this was based on the assumption that the early (e.g. <32 weeks of gestation) and the late PTL groups had similar profiles of angiogenic/anti-angiogenic factor concentrations. Of note, 87% of patients with PTL in this study delivered after 32 weeks of gestation. A larger study that is specifically designed to address the question of whether early PTL and delivery has a different profile of angiogenic/anti-angiogenic factor concentrations compared to late preterm delivery is warranted.
In conclusion, our study demonstrates that plasma sEng concentration in women destined to develop PTL and delivery was elevated from 15 to 20 weeks of gestation and became significantly higher compared to normal pregnant women approximately 5–10 weeks prior to the clinical manifestation of the disease. In contrast, the changes in plasma concentrations of sVEGFR-1, PlGF and sVEGFR-2 were detectable within 5 weeks prior to spontaneous PTL and delivery. These changes were more pronounced as the patient approached the time of PTL and delivery. These observations support the view that an imbalance of angiogenic/anti-angiogenic factors participates in the pathophysiology of preterm parturition in a subset of patients.
Acknowledgements
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113(Suppl 3):17–42.
- Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008;371:164–175.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep 2008;57:1–32.
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824.
- Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids 1989;37:183–186.
- Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci 1991;622:355–375.
- Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–1528.
- Dudley DJ, Chen CL, Branch DW, Hammond E, Mitchell MD. A murine model of preterm labor: inflammatory mediators regulate the production of prostaglandin E2 and interleukin-6 by murine decidua. Biol Reprod 1993;48:33–39.
- Yoon BH, Park KH, Koo JN, Kwon JH, Jun JK, Syn HC, Romero R. Intra-amniotic infection of twin pregnancies with preterm labor. Am J Obstet Gynecol 1997;3:127–130.
- Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–550.
- Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev 2002;60:S19–S25.
- Kloeck FK, Jung H. In vitro release of prostaglandins from the human myometrium under the influence of stretching. Am J Obstet Gynecol 1973;115:1066–1069.
- Laudanski T, Rocki W. The effects on stretching and prostaglandin F2alpha on the contractile and bioelectric activity of the uterus in rat. Acta Physiol Pol 1975;26:385–393.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 1997;138:5398–5407.
- Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol 1990;10:347–350.
- Romero R, Mazor M, Avila C, Quintero R, Munoz H. Uterine “allergy”: A novel mechanism for preterm labor. Am J Obstet Gynecol 1991;164:375.
- Garfield RE, Bytautiene E, Vedernikov YP, Marshall JS, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am J Obstet Gynecol 2000;183:118–125.
- Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–1361.
- Ludmir J, Samuels P, Brooks S, Mennuti MT. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol 1990;75:906–910.
- Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol 1995;172:1097–1103.
- Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length <or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000;182:1458–1467.
- Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report. Gynecol Obstet Invest 1992;33:183–184.
- Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol 1990;97:149–154.
- Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol Reprod 1996;55:992–995.
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, et al Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–2385.
- Liggins GC. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol 1968;42:323–329.
- Jones SA, Challis JR. Steroid corticotrophin-releasing hormone, ACTH and prostaglandin interactions in the amnion and placenta of early pregnancy in man. J Endocrinol 1990;125:153–159.
- Challis JR. CRH, a placental clock and preterm labour. Nat Med 1995;1:416.
- Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, et al The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks' gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1996;175:1286–1292.
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks' gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol 1999;180:S257–S263.
- Combs CA, Katz MA, Kitzmiller JL, Brescia RJ. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol 1993;169:215–223.
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189:1063–1069.
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–1142.
- Fonseca E, Yu CK, Singh M, Papageorghiou AT, Nicolaides KH. Relationship between second-trimester uterine artery Doppler and spontaneous early preterm delivery. Ultrasound Obstet Gynecol 2006;27:301–305.
- Brar HS, Medearis AL, DeVore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: prediction of successful tocolysis. Am J Obstet Gynecol 1988;159:947–950.
- Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol 1993;168:585–591.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–1544.
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–182.
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–1022.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18.
- Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–989.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–649.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005.
- Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255–259.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008;21:41–52.
- Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol 2007;196:239–236.
- Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol 2007;109:1368–1374.
- Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol 2007;197:211–214.
- Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, et al The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–287.
- Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303–309.
- Savvidou MD, Noori M, Anderson JM, Hingorani AD, Nicolaides KH. Maternal endothelial function and serum concentrations of placental growth factor and soluble endoglin in women with abnormal placentation. Ultrasound Obstet Gynecol 2008;32:871–876.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol 2006;108:338–344.
- Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–613.
- Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, et al Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol 2008;198:382–388.
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, Erez O, Bujold E, Goncalves LF, et al Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507.
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242–248.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676.
- Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 2008;41:278–286.
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336–30343.
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005;94:209–231.
- Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 2006.
- Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol 2008;28:322–328.
- Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res 2004;2:315–326.
- Lin P, Sankar S, Shan S, Dewhirst MW, Polverini PJ, Quinn TQ, Peters KG. Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ 1998;9:49–58.
- McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2002;43:474–482.
- Ten DP, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008;11:79–89.
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439.
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–442.
- Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 1999;126:3015–3025.
- Cheung CY. Vascular endothelial growth factor: possible role in fetal development and placental function. J Soc Gynecol Investig 1997;4:169–117.
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26(Suppl A):S114–S117.
- Tsai J, Klebanoff MA, Qian C, Yu K, Levine RJ. Circulating angiogenic factors in spontaneous preterm labor and delivery. Am J Obstet Gynecol 2009;197:S7.
- Haddad R, Romero R, Gould BR, Tromp G, Gotsch F, Edwin SS, Zingg HH. Angiogenesis gene expression in mouse uterus during the common pathway of parturition. Am J Obstet Gynecol 2008;198:539–548.
- Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab 2008;93:260–266.
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 1997;138:4977–4988.
- Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest 1999;103:1641–1650.
- Soregaroli M, Valcamonico A, Scalvi L, Danti L, Frusca T. Late normalisation of uterine artery velocimetry in high risk pregnancy. Eur J Obstet Gynecol Reprod Biol 2001;95:42–45.
- Dumont N, O'Connor-McCourt MD, Philip A. Transforming growth factor-beta receptors on human endometrial cells: identification of the type I, II, and III receptors and glycosyl-phosphatidylinositol anchored TGF-beta binding proteins. Mol Cell Endocrinol 1995;111:57–66.
- Ouellette MJ, Hamel L, Tremblay N, Lamothe M, St-Jacques S. Characterization of endoglin on mouse uterine stromal cells. J Reprod Fertil 1999;117:229–239.
- Godkin JD, Dore JJ. Transforming growth factor beta and the endometrium. Rev Reprod 1998;3:1–6.
- Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. The expression of transforming growth factor beta in pregnant rat myometrium is hormone and stretch dependent. Reproduction 2007;134:503–511.
- Kuscu NK, Lacin S, Vatansever S, Yildirim Y, Var A, Uyanik BS, Koyuncu F. Immunolocalization of transforming growth factor-beta 3 in pregnant human myometrium. Acta Obstet Gynecol Scand 2001;80:1079–1083.
- Awad SS, Lamb HK, Morgan JM, Dunlop W, Gillespie JI. Differential expression of ryanodine receptor RyR2 mRNA in the non-pregnant and pregnant human myometrium. Biochem J 1997;322:777–783.
- Hatthachote P, Morgan J, Dunlop W, Europe-Finner GN, Gillespie JI. Gestational changes in the levels of transforming growth factor-beta1 (TGFbeta1) and TGFbeta receptor types I and II in the human myometrium. J Clin Endocrinol Metab 1998;83:2987–2992.
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrinol Rev 2000;21:514–550.
- Bry K, Lappalainen U. Interleukin-4 and transforming growth factor-beta 1 modulate the production of interleukin-1 receptor antagonist and of prostaglandin E2 by decidual cells. Am J Obstet Gynecol 1994;170:1194–1198.
- Bry K, Lappalainen U, Hallman M. Interleukin-1 binding and prostaglandin E2 synthesis by amnion cells in culture: regulation by tumor necrosis factor-alpha, transforming growth factor-beta, and interleukin-1 receptor antagonist. Biochim Biophys Acta 1993;1181:31–36.
- Romero R. Prenatal medicine: the child is the father of the man. Prenat Neonatal Med 1996;1:8–11.
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506.
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–86.
- Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–797.
- Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001;185:1118–1123.
- Bujold E, Chaiworapongsa T, Romero R, Gervasi MT, Espinoza J, Goncalves LF, Berman S, Yoon BH, Kim YM. Neonates born to pre-eclamptic mothers have a higher percentage of natural killer cells (CD3-/CD56+16+) in umbilical cord blood than those without pre-eclampsia. J Matern Fetal Neonatal Med 2003;14:305–312.
- Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in pre-eclampsia. Am J Obstet Gynecol 2002;187:889–893.
- Chaiworapongsa T, Gervasi MT, Espinoza J, Bujold E, Kim YM, Blackwell SC, Romero R. Preeclampsia and SGA differ by the extent of monocyte but not neutrophil, metabolic activity and oxidative burst. Am J Obstet Gynecol 2002;187:S220.
- Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1124–1129.
- Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, et al Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:171–175.
- Blackwell S, Romero R, Chaiworapongsa T, Refuerzo J, Gervasi MT, Yoshimatsu J, Espinoza J, Berman S, Yoon BH. Unexplained fetal death is associated with changes in the adaptive limb of the maternal immune response consistent with prior antigenic exposure. J Matern Fetal Neonatal Med 2003;14:241–246.
- Wu WX, Zhang Q, Ma XH, Unno N, Nathanielsz PW. Suppression subtractive hybridization identified a marked increase in thrombospondin-1 associated with parturition in pregnant sheep myometrium. Endocrinology 1999;140:2364–2371.
- Charpigny G, Leroy MJ, Breuiller-Fouche M, Tanfin Z, Mhaouty-Kodja S, Robin P, Leiber D, Cohen-Tannoudji J, Cabrol D, Barberis C, et al A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol Reprod 2003;68:2289–2296.
- Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, Adashi EY, Whiting D. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol 2005;193:404–413.
- Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS.Med. 2006;3:e169.
- Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–786.
- Klimaviciute A, Calciolari J, Bertucci E, Belin-Tornblom S, Stjernholm-Vladic Y, Bystrom B, Petraglia F, Ekman-Ordeberg G. Corticotropin-releasing hormone, its binding protein and receptors in human cervical tissue at preterm and term labor in comparison to non-pregnant state. Reprod Biol Endocrinol 2006;4:29.
- Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, Khalek N, Camacho N, Mittal P, Yoon BH, et al Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2007;197:250–257.
- Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol 2002;187:728–734.
- Daneshmand SS, Chmait RH, Moore TR, Bogic L. Preterm premature rupture of membranes: vascular endothelial growth factor and its association with histologic chorioamnionitis. Am J Obstet Gynecol 2002;187:1131–1136.
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394–424.
- Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, Kim JS, Lee DC, Erez O, Gotsch F, et al Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J Pathol 2009;217:113–121.
- Kramer BW, Kaemmerer U, Kapp M, Herbst D, Marx A, Berg D, Groneck PA, Speer CP. Decreased expression of angiogenic factors in placentas with chorioamnionitis after preterm birth. Pediatr Res 2005;58:607–612.
- Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009;200:104–111.
- Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, Mazaki-Tovi S, Than NG, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006;113(Suppl 3):118–135.
- Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–1091.
- Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–816.
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventative strategies. Sem Neonatal. 2002;7:259–274.
- Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56.
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–357.
- Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks' gestation or less. Obstet Gynecol 2001;98:1080–1088.
- Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–1528.
- Gupta M, Mestan KK, Martin CR, Pearson C, Ortiz K, Fu L, Stubblefield P, Cerda S, Kasznica JM, Wang X. Impact of clinical and histologic correlates of maternal and fetal inflammatory response on gestational age in preterm births. J Matern.Fetal Neonatal Med. 2007;20:39–46.