Abstract
Objective. Aquaporin 9 (AQP9) is a water channel protein characterized by its high permeability to nutrients such as lactate and glycerol, as well as urea and other small solutes. These unique properties of AQP9 suggest that this molecule may play a role in the modulation of nutrient flux through the fetal membranes in conditions associated with increased metabolic demand, such as spontaneous labor and inflammation. The objective of this study was to determine the expression of AQP9 in the chorioamniotic membranes from women with and without term labor, as well as those with preterm prelabor rupture of membranes (PPROM) with and without histologic chorioamnionitis.
Study design. A cross-sectional study was performed which included patients in the following groups: (1) term not in labor (TNL; n = 14); (2) term, spontaneous labor (n = 14); and (3) PPROM with (n = 20) and without (n = 17) histologic chorioamnionitis. AQP9 mRNA expression in fetal membranes was quantified using quantitative real-time reverse transcription-polymerase chain reaction and analyzed with a linear model including gestational age as a covariate.
Results. (1) AQP9 mRNA expression was identified in all chorioamniotic membrane specimens; (2) AQP9 expression in fetal membranes was significantly higher in spontaneous term labor when compared with TNL (fold change 3.6; p = 0.01); and (3) Among patients with PPROM, the presence of histologic chorioamnionitis was associated with a higher expression of AQP9 in the chorioamniotic membranes compared with those from women without histologic chorioamnionitis (fold change 8.7; p < 0.001).
Conclusion. Aquaporin 9 mRNA expression is higher in the fetal membranes from patients with spontaneous term labor and those with PPROM and histologic chorioamnionitis. These findings are novel, and suggest a role for aquaporin 9 in membrane-mediated transfer of nutrients to support the increased metabolic demands associated with the host immune response of the terminal pathway of parturition and histologic chorioamnionitis.
Introduction
Spontaneous human parturition at term is well-recognized to involve systemic and local activation of the inflammatory cascade [Citation1–9]. Indeed, term labor is associated with an acute inflammatory gene expression profile in the chorioamniotic membranes, an organ which, in part, regulates amniotic fluid composition, volume, and intramembranous flow [Citation10,Citation11]. Deployment of inflammatory mediators demands increased energy utilization [Citation12], as does labor, which requires the utilization of substantial metabolic substrate in order to maintain uterine contractility. Numerous studies have demonstrated elevated concentrations of glucose and free fatty acids in the maternal serum of women in labor compared with those not in labor [Citation13–16] and alterations of metabolic mediators in both pregnancy and labor have been described [Citation17–22]. Furthermore, myometrial glycogen storage content is lower prior to the onset of labor [Citation23]. In addition to physiologic events, inflammation has also been implicated to play a major role in the development of pregnancy complications such as preterm prelabor rupture of membranes (PPROM), in which 25-39% of cases have evidence of intra-amniotic infection and/or inflammation [Citation24]. Therefore, similar to normal term labor, pregnancies complicated by PPROM and infection may also require increased cellular energy availability to maintain the host response.
First isolated by Preston and Agre in 1991 [Citation25], aquaporin membrane proteins play a significant role in the regulation of amniotic fluid composition and volume [Citation26]. Aquaporins are a family of integral membrane proteins whose primary function is to dictate solute-independent transmembrane water transport [Citation27]. Classified according to their permeability characteristics [Citation28], this family of water channels includes aquaporin 9, which allows passage of solutes such as urea and glycerol. Importantly, in the human liver, purified aquaporin 9 has been described to significantly increase tissue permeability to glycerol 63 fold, as well as increasing urea permeability 90 fold [Citation29]. Furthermore, in a knockout mouse model, investigators demonstrated increased plasma concentrations of glycerol and triglycerides associated with an inability of glycerol to enter the hepatocyte, preventing gluconeogenesis [Citation30]. These findings support the role of aquaporin 9 in metabolism. Aquaporin 9 expression has been reported in human organs and cells including the liver [Citation31], brain [Citation32], testis [Citation33], osteoclast cells [Citation34], placenta [Citation35], peripheral leukocytes [Citation31], and has been localized in human chorioamniotic membranes [Citation36]. Given the significant role of this aquaglyceroporin in solute transport, aquaporin 9 may be involved both in the maintenance of amniotic fluid composition, as well as in the supply of precursor substrates for cellular metabolism.
In addition to regulating amniotic fluid volume and solute concentration, aquaporin 9 activity has also been implicated as a fundamental component of the innate immune response. Loitto et al. [Citation37] have reported a series of experiments which demonstrate that aquaporin 9 expression is essential for neutrophil response to chemotactic gradients. Indeed, the authors demonstrated that aquaporin 9 activity is required for neutrophil lamellipodium development, and thus, neutrophil motility. The incubation of isolated human neutrophils with anti-aquaporin 9 antibody resulted in an inability of the treated leukocytes to respond to chemoattractant stimulation. In addition, the authors investigated the distribution of aquaporin 9 on stimulated neutrophils and found that this water channel localized to the leading edges of the cell in a pattern similar to that of N-formyl chemoattractant receptors.
Although the physiologic roles of aquaporin 9 in metabolism and the immune response have been characterized, limited data are available for its role in human pregnancy. Our group has previously reported microarray data demonstrating significant upregulation of aquaporin 9 expression in the chorioamniotic membranes from patients at term in labor [Citation1,Citation38]. However, the differential expression of aquaporin 9 in human fetal membranes during conditions characterized by increased metabolic demand (such as labor) or infection and inflammation has not been confirmed. The objectives of this study were: (1) to confirm if the expression of aquaporin 9 in chorioamniotic membranes changes with spontaneous term labor; and (2) to compare the expression of aquaporin 9 in fetal membranes between cases of PPROM with and without histologic chorioamnionitis.
Materials and methods
Study design and population
This cross-sectional study was designed to investigate the differential expression of the AQP9 gene in the fetal membranes of patients in the following groups: (1) Term, not in labor (n = 14); (2) Term, spontaneous labor (n = 14); (3) PPROM without evidence of infection (n = 17); and (4) PPROM with histologic chorioamnionitis (n = 20). Women at term with and without labor did not have evidence of histologic chorioamnionitis. Patients presenting with medical complications, multiple pregnancies, and fetal congenital or chromosomal abnormalities were excluded. All patients provided written informed consent prior to the collection of samples. The collection and utilization of samples for research purposes were approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS, Bethesda, Maryland) and the Human Investigation Committee of Wayne State University (Detroit, Michigan). Many of these samples have been employed to study the biology of inflammation and labor in both normal pregnant women, and those with complicated pregnancies.
Definitions
Spontaneous term labor with intact membranes was defined as the presence of regular uterine contractions that occurred at a frequency of at least 2 in every 10 min associated with cervical changes which led to spontaneous delivery at ≥37 weeks of gestation. PPROM was defined as prelabor rupture of membranes occurring at less than 37 weeks of gestation, diagnosed by speculum examination of vaginal pooling, nitrazine, and ferning tests. Amniocentesis was performed at the discretion of the treating physician by trans-abdominal amniocentesis under ultrasonographic guidance in a subset of patients with PPROM for the determination of the microbiologic state of the amniotic cavity. Amniotic fluid was cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain for microorganisms were also performed in amniotic fluid shortly after collection. Intra-amniotic infection was defined by positive amniotic fluid cultures for microorganisms and intra-amniotic inflammation by an amniotic fluid WBC count of ≥100 cells/ml. The results of the amniotic fluid analyses were used for clinical management.
Placental histopathological examinations
Chorioamniotic membranes containing attached maternal decidua were obtained from placentas delivered by spontaneous vaginal delivery or cesarean section at Hutzel Women's Hospital (Detroit, MI). Tissue samples were fixed in 10% neutral buffered formalin overnight and embedded in paraffin. Five micrometer paraffin sections were stained with hematoxylin and eosin and examined using bright-field light microscopy. Histopathological examinations were performed based on the diagnostic criteria previously described [Citation39] by pathologists who were blinded to the clinical information. Histologic chorioamnionitis was diagnosed in the presence of acute inflammation using previously described criteria [Citation40,Citation41].
Total RNA extraction
Fetal membranes were dissected from placentas, rinsed thoroughly with a sterile ice-cold phosphate buffered saline solution (Sigma Chemical Company, St Louis, MO), cut into small pieces, placed in RNAlater solution (Ambion, Austin, TX), and stored at 4°C for no longer than 2 weeks. Total RNA was isolated with a modification of the standard guanidinium isothiocyanate–cesium chloride method. Briefly, tissues were homogenized with a PRO200 rotor-stator homogenizer (Pro Scientific, Monroe, CT) in the presence of 4 mol/l guanidinium isothiocyanate, 0.1 mol/l mercaptoethanol, 0.5% sarkosyl, and 5 mmol/l sodium citrate (pH 7). Solid CsCl was added to the samples (final concentration of 0.25 g/ml), and the samples were centrifuged in an ultracentrifuge, according to the protocol. RNA pellets were extracted with chloroform: isoamylalcohol and the RNA was precipitated with ethanol and glycogen (Roche Molecular Biochemicals, Indianapolis, IN) as a carrier. Before the first use, the RNA was pelleted and resuspended in water that contained RNasin (Promega Corp, Madison, WI).
Quantitative real-time reverse transcription-polymerase chain reaction
A total of 2.5 μg of RNA from each sample and a positive control sample was reverse transcribed using Superscript II reverse transcriptase, random hexamer primers and oligo(dT) primers (Invitrogen Life Technologies, Rockville, MD). The standard curve was run with the AQP9 mRNA and the 18S ribosomal RNA housekeeping gene to determine the quantity of cDNA needed for an approximate cycle threshold (Ct) of 25. Subsequently, cDNA derived from an equivalent of 75 ng RNA from each sample was run in triplicate on 96-well plates to obtain technical replicates for the target and reference assays. A ‘calibrator’ sample was run in triplicate in all plates to account for plate effects. In addition, a negative control containing no RNA, and 12.5 ng of human genomic DNA were also tested in duplicates. Samples from the study groups were randomly allocated on the plates; the AQP9 and 18S rRNA assays were run with the same allocation on the parallel plates. The quantitative real-time reverse transcription-polymerase chain reactions (qRT-PCR) were assembled based on the TaqMan Universal PCR Master Mix protocol (Applied Biosystems, Foster City, CA) using the 18S rRNA TaqMan gene expression assay (Hs99999901_s1; Applied Biosystems) for the quantification of the housekeeping gene and self-designed primers and probe for AQP9 (forward primer: 5′-AGCAGCGAACAGGGAATGAC-3′; reverse primer: 5′-ACTGTACAAATGCCGTTCCAATT-3′; probe: 5′-TTCCACCAGAAGACGATTAAGCCACAGC-3′). Data were collected by the ABI Prism 7700 Sequence Detection System (Applied Biosystems).
Statistical analysis
Demographic and clinical characteristics of the study groups were compared using the Pearson's chi-square test and the Fisher's exact test for proportions and the Mann–Whitney U test for continuous variables. The statistical package used was SPSS v.12.0 (SPSS, Chicago, IL). Quantitative RT-PCR data were analyzed using the R statistical software [Citation42].
Gene expression levels were profiled in the sample groups by qRT-PCR experiments. The RT reactions were run on 96-well plates. Samples from the study groups were randomly allocated on the plates, and only one target gene and the 18S reference assay were run in parallel on each given plate. Each reaction was repeated to obtain technical replicates for both the target assay and the reference assay. A ‘calibrator’ patient sample was placed on all plates to account for eventual plate effects. Briefly, the delta–delta method [Citation43,Citation44] was used to generate an outcome variable, Y, which is a surrogate of the log2 concentration of the target gene in each patient sample, already corrected for eventual plate effects.
A linear model was employed in which Y values were fitted using the group variable and the gestational age as predictors without including the interaction term between these two variables. The coefficients of the two predictors in the linear model were estimated together with their significant p-values.
The outcome variable, Y, also included a positive constant to render the Y values positive for convenient data plotting. A False Discovery Rate adjustment [Citation45] of resulting p-values was performed to account for all parallel tests. For each pair-wise comparison, the group effect was considered significant if the adjusted p-values were <0.05 and the magnitude of change was at least 2-fold (one Ct unit difference). For the gestational age effect, adjusted p-values <0.05 were considered significant.
Results
Demographic, clinical, and histopathological data
Sixty-five women were included in this study. Demographic and clinical characteristics of the study groups are displayed in .
Table I. Demographic and clinical characteristics of the study groups.
The diagnosis of histologic chorioamnionitis was based on the presence of either a maternal or fetal inflammatory response. Maternal inflammatory response was identified by neutrophilic infiltration of the chorioamniotic membranes, whereas a fetal inflammatory response was defined as the presence of chorionic vasculitis, umbilical phlebitis and/or arteritis identified by fetal neutrophil infiltrates. Among 20 patients with PPROM and histologic chorioamnionitis, 7 (35%) had only a maternal inflammatory response, 2 (10%) had a fetal inflammatory response, and 11 (55%) had both.
Amniocentesis was performed in 19/37 (51%) of all patients with PPROM. A positive amniotic fluid culture or WBC count of >100 cells/ml was detected in 42% (8/19) of these patients. Of note, all specimens with evidence of intraamniotic infection/inflammation were in the subset of patients with PPROM and histologic chorioamnionitis. No women in the group with PPROM alone had evidence of intraamniotic infection and/or inflammation (0/7), whereas of the 12 patients in the group with PPROM and histologic chorioamnionitis who underwent amniocentesis, 8/12 (66%) patients had evidence of intraamniotic infection/inflammation (66% versus 0%; p = 0.01). Positive amniotic fluid cultures were observed in 6/12 (50%) of these patients, and intramniotic inflammation in 2/12 (16%) of this population. Microorganisms identified in amniotic fluid cultures included the following: Ureaplasma urealyticum (three), Mycoplamsma hominis (one), Prevotella species (one), Candida albicans (one), Gardnerella vaginalis (two), and Peptostreptococcus species (one). Amniotic fluid cultures from 2 patients grew multiple organisms. One patient was positive for U. urealyticum, M. hominis, and G. vaginalis while the second patient had an amniotic fluid culture positive for G. vaginalis and Peptostreptococcus species.
Aquaporin 9 expression in human chorioamniotic membranes
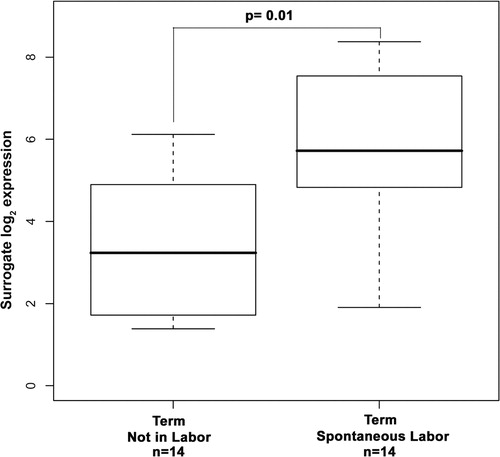
AQP9 mRNA expression was detectable in all fetal membrane specimens. In order to assess the association between normal term spontaneous labor and AQP9 mRNA expression in fetal membranes, samples from women after uncomplicated term delivery were compared with those from women undergoing elective term cesarean section, not in labor. AQP9 mRNA expression was 3.6-fold higher in the fetal membranes from women in spontaneous labor when compared with those who were not in labor at term (p = 0.01; ).
Figure 1. Box and whisker plot of aquaporin 9 mRNA expression in chorioamniotic membranes from women at term. Aquaporin 9 mRNA expression was significantly higher in the chorioamniotic membranes of women at term in labor when compared with the fetal membranes of women at term not in labor (fold-change 3.6; p = 0.01).
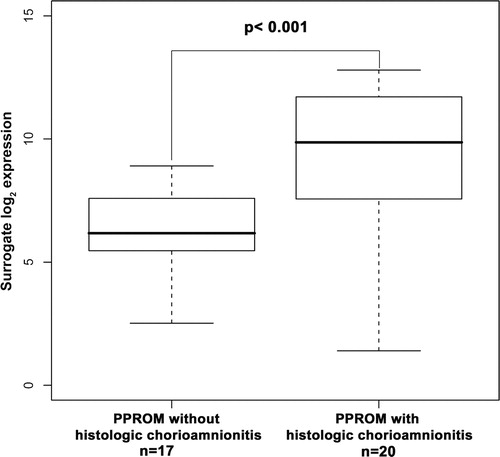
The association between inflammation and chorioamniotic membrane expression of AQP9 mRNA was explored through comparison of patients with PPROM with and without histologic chorioamnionitis. PPROM with histologic chorioamnionitis was associated with a significantly increased AQP9 expression in fetal membranes when compared with those of patients with PPROM without histologic evidence of inflammation (fold change 8.7; p < 0.001; ).
Figure 2. Box and whisker plot of aquaporin 9 mRNA expression in chorioamniotic membranes from women diagnosed with preterm prelabor rupture of membranes (PPROM). Chorioamniotic membranes from women with PPROM and histologic chorioamnionitis demonstrated a significantly higher expression of aquaporin 9 mRNA when compared with women with PPROM alone (fold-change 8.7; p < 0.001).
Discussion
Principle findings of this study
(1) Aquaporin 9 mRNA expression was detected in all fetal membrane specimens; (2) the expression of aquaporin 9 was significantly higher in the chorioamniotic membranes of women at term in spontaneous labor when compared with women at term not in labor; and (3) the presence of histologic chorioamnionitis is associated with increased expression of aquaporin 9 among patients with PPROM.
What is Aquaporin 9?
First described in 1991 by Agre and colleagues [Citation25], aquaporins are widely distributed integral membrane channels that contain transmembrane α-helices and are universally permeable by water. Thirteen mammalian aquaporins have been described, which are loosely classified according to their permeability characteristics. Variation in peptide sequences determines the pore size of the resultant tetramer, and therefore the specificity of each water channel [Citation46]. Aquaporin 9 is of interest as it is permeable to urea, glycerol, and small solutes. Among this family of water channels, aquaporin 9 demonstrates the greatest homology to aquaporins 3 and 7, and the least molecular likeness to aquaporin 1 [Citation31]. As well as being expressed in organs such as the human liver [Citation31], brain [Citation32], osteoclast cells [Citation34], placenta [Citation35], and peripheral leukocytes [Citation31], aquaporin 9 has also been shown to be regulated by testosterone and estrogen in the male reproductive tract [Citation47,Citation48]. A number of studies have investigated the molecular regulation of this aquaglyceroporin. Pietrement et al. [Citation49] co-localized both cystic fibrosis transmembrane conductance regulator (CFTR) and sodium/hydrogen exchanger regulatory factor1 (NHERF1) in an apical complex with aquaporin 9 on the epididymis. Furthermore, the addition of a CFTR inhibitor markedly decreased aquaporin 9-dependent glycerol permeability. These findings suggest an important mechanism by which CFTR participates in the regulation of aquaporin 9 function. In addition, Shengbiao et al. [Citation50] recently described an elegant investigation of aquaporin 9 regulation using a primary amnion cell culture line. The authors present data supporting the upregulation of aquaporin 9 via second-messenger cAMP through the alternative protein kinase A independent pathway.
The role of Aquaporin 9 in energy homeostasis
A compelling body of evidence supports the role of aquaporin 9 in metabolism – specifically, glucose homeostasis. In a rat model, Carbrey et al. [Citation29] demonstrated a significant increase in aquaporin 9 expression in rat liver hepatocytes following a period of starvation. Following the administration of insulin and re-feeding of the animals, aquaporin 9 expression returned to baseline. Furthermore, the expression of aquaporin 9 fluctuated in accordance with the animal's nutritional status and circulating concentration of insulin. Therefore, given its permeability to glycerol and the findings noted above, aquaporin 9 has been implicated to play a key role in the transport of glycerol into the liver to support starvation-induced gluconeogenesis. This was confirmed by a knockout mouse model [Citation30] which demonstrated that the liver is the main expression site of aquaporin 9 and that knockout mice developed increased plasma concentrations of glycerol and triglycerides and were subsequently unable to respond to fasting with hepatic gluconeogenesis due to impaired entry of glycerol. In addition, these findings were further exaggerated in obese mice lacking aquaporin 9 and leptin receptor function. The aforementioned evidence substantiates the role of aquaporin 9 in normal glucose metabolism as well as in the pathologic substrate utilization seen in diabetes mellitus. Indeed, among obese women (body mass index ≥30 kg/m2), those with Type 2 diabetes mellitus have a significantly decreased expression of hepatic aquaporin 9 when compared with those with normoglycemia [Citation51].
In addition to its singular role in the liver, aquaporin 9 has also been identified in the periventricular region of the brain; specifically in ependymal cells, ventricular astrocytes, endothelial cells of pial vessels and importantly, in catecholaminergic neurons, which play a key role in neural energy homeostasis [Citation33,Citation52]. Because of its substantive glycerol permeability, aquaporin 9 has been hypothesized to contribute to brain energy metabolism as well as cerebral spinal fluid regulation [Citation33,Citation53]. Indeed, in a recent study, Badaut et al. [Citation54] describe a rat model in which the expression of aquaporin 9 increases in catecholaminergic neurons during diabetic conditions where plasma insulin concentration is low. These findings further support the important role of aquaporin 9 in metabolic balance.
Normal pregnancy and aquaporin 9
The finding that increased expression of aquaporin 9 in the chorioamniotic membranes is associated with spontaneous term labor is novel. This observation is of importance as increased expression of aquaporin 9 would favor intramembranous solute passage, specifically of glycogen, a quickly mobilized energy depot to sustain an acute need for glucose. Although a causal relationship between parturition and aquaporin 9 expression in the fetal membranes cannot be established due to the cross-sectional nature of the index study, the broad permeability of the aquaporin 9 water channel combined with the known metabolic demands and the inflammatory signature of parturition, lends itself to hypothesize a potential relationship. Recently performed microarray analysis of human myometrial tissue by our group supports the link between aquaporin 9 and spontaneous labor. Comparison of differential gene expression between myometrium from women at term with and without labor revealed a 3.49-fold upregulation in aquaporin 9 expression in tissue from patients in labor (p < 0.001; unpublished data). Increased glycogen turnover in human myometrium with a resultant decrease in glycogen storage prior to the onset of labor has been previously described [Citation23]. Of interest, while differential expression of aquaporin 9 during pregnancy has been found in the chorioamniotic membranes and myometrium, recently published data suggest that aquaporin 9 may not play a major role in cervical changes associated with late gestation. Using a rat model to elucidate the expression of the aquaporin family in late gestation, Anderson et al. [Citation55] describe the expression of aquaporin 9 at day 15, with no expression detected in the cervix during late gestation or postpartum.
In addition to its potential influence on the metabolic state of the pregnancy unit, the importance of aquaporin 9 in normal pregnancy development is supported by a number of lines of investigation: (1) the permeability of chorioamniotic membranes is believed to play a critical role in the regulation of amniotic fluid volume during gestation [Citation56]. Transcellular movement of water and solutes across the membranes during pregnancy, the intramembranous pathway, is a major pathway of fluid transport, in which aquaporin channels play a primary role [Citation10]. Of the 13 currently recognized aquaporin water channels, aquaporins 1, 3, 8, and 9 have all been identified within the human placental unit [Citation36,Citation57–59]. Wang et al. [Citation36] performed a series of in vitro studies demonstrating aquaporin 9 mRNA expression in human amnion, chorion, and placenta. Of interest, no expression was noted in umbilical cord. Furthermore, the investigators localized aquaporin 9 production to the chorion cytotrophoblasts (strongest expression), the cytotrophoblast and syncytiotrophoblast surrounding chorionic villi, and epithelial cells of the amnion (weakest expression). Indeed, the authors note that aquaporin 9 was the only member of its family identified in human fetal membranes via Northern analysis, supporting their hypothesis that this aquaglyceroporin is involved in integral mechanisms of amniotic fluid homeostasis. In support of this view, Beall et al. [Citation60] report a negative correlation between placental aquaporin 9 expression and amniotic fluid volume in a mouse model; (2) a compelling body of evidence supports the concept that regulation of uterine luminal fluid is necessary for successful blastocyst implantation. Both surface epithelial cells and glandular epithelium have been implicated in this process, in which aquaporins have been shown to play a role. In a series of investigations using a rat model, Lindsay and Murphy [Citation61] have reported a role for aquaporins in luminal fluid changes. Aquaporin 9 was localized to the apical glandular epithelium with increased immunofluorescent staining after implantation and the authors suggest its role in the re-absorption of glandular contents; and (3) recently, in an animal model, Wiegman et al. [Citation32] described the differential and regional expression of aquaporin 9 in the rat brain during pregnancy. The authors found that aquaporin 9 distribution did not differ between the pregnant and the non-pregnant state, but that its expression decreased in the posterior cerebrum when comparing non-pregnant and late-pregnancy animals, and in the cerebellum in both mid-pregnant and late-pregnant rats compared with non-pregnant animals. Of interest, aquaporin 9 expression then increased in the cerebellum during the postpartum period when compared with pregnancy. The differential distribution of aquaporin 9 in the pregnant rat brain suggests time- and location-specific demands for metabolite and water transport within the pregnant brain along pregnancy, further supporting a global role of aquaporin 9 in metabolic adaptations occurring during pregnancy.
Aquaporin 9 and pregnancy complications
This is the first study to describe increased expression of aquaporin 9 mRNA in the chorioamniotic membranes of pregnancies complicated by PPROM with histologic chorioamnionitis, and the potential role of aquaporin 9 in the metabolic adaptation of human pregnancy to an inflammatory state. To date, there is no further data addressing the putative relationship between aquaporin 9 expression and pregnancy complications. Although labor itself appears to be associated with increased chorioamniotic membrane aquaporin 9 expression, the increased expression of this vital component of glucose homeostasis in PPROM with histologic chorioamnionitis compared with PPROM alone substantiates the hypothesis that aquaporin 9 functions as a link between inflammation and metabolism.
In conclusion, the results of the present study implicate a novel role for aquaporin 9 in normal term labor and PPROM with histologic chorioamnionitis. Furthermore, given its important role in energy homeostasis, aquaporin 9 may represent a novel metabolic pathway supporting the genesis of both a physiologic (normal labor) and a pathologic (histologic chorioamnionitis) state of inflammation in human pregnancy. Additional studies are needed to further elucidate the role of this unique aquaglyceroporin in human pregnancy and parturition.
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. The authors would like to acknowledge the invaluable contributions of the nursing staff of the Perinatology Research Branch and Hutzel Women's Hospital to this manuscript.
References
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394-e1–394-e14.
- Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction 2005;130:569–581.
- Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol 2003;1:122.
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–326.
- Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, et al Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–461.
- Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, et al Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–616.
- Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 2005;17:239–245.
- Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Gonzalez R, Adashi EY. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med 2003;14:51–56.
- Hamill N, Romero R, Gotsch F, Pedro KJ, Edwin S, Erez O, Than NG, Mittal P, Espinoza J, Friel LA, et al Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008;36:217–227.
- Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 1989;74:748–754.
- Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Regulation of amniotic fluid volume. Placenta 2007;28:824–832.
- Beisel WR. Metabolic response to infection. Annu Rev Med 1975;26:9–20.
- Jovanovic L. Glucose and insulin requirements during labor and delivery: the case for normoglycemia in pregnancies complicated by diabetes. Endocr Pract 2004;10(Suppl 2):40–45.
- Kashyap ML, Sivasamboo R, Sothy SP, Cheah JS, Gartside PS. Carbohydrate and lipid metabolism during human labor: free fatty acids, glucose, insulin, and lactic acid metabolism during normal and oxytocin-induced labor for postmaturity. Metabolism 1976;25:865–875.
- Maheux PC, Bonin B, Dizazo A, Guimond P, Monier D, Bourque J, Chiasson JL. Glucose homeostasis during spontaneous labor in normal human pregnancy. J Clin Endocrinol Metab 1996;81:209–215.
- Whaley WH, Zuspan FP, Nelson GH, Ahlquist RP. Alterations of plasma free fatty acids and glucose during labor. Am J Obstet Gynecol 1967;97:875–880.
- Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, Mittal P, Than GN, Nhan-Chang C, Chaiworapongsa T, et al Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med 2008;21:796–815.
- Gunasegaram R, Peh KL, Loganath A, Ng SC, Kottegoda SR, Ratnam SS. Cholesterol synthesizing enzymes in term human fetal amnion. J Perinat Med 1985;13:143–146.
- Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med 2008;21:902–916.
- Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–496.
- Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med 2007;35:522–531.
- Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than NG, Kim SK, Dong Z, Gotsch F, Mittal P, Chaiworapongsa T, et al Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009 In press.
- Steingrimsdottir T, Ronquist G, Ulmsten U, Waldenstrom A. Low myometrial glycogen content compared with rectus muscle in term pregnant women before labor. Gynecol Obstet Invest 1999;47:166–171.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113(Suppl 3):17–42.
- Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 1991;88:11110–11114.
- Liu H, Zheng Z, Wintour EM. Aquaporins and fetal fluid balance. Placenta 2008;29:840–847.
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 2004;5:687–698.
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 2003;555:72–78.
- Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA 2003;100:2945–2950.
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA 2007;104:3609–3614.
- Ishibashi K, Kuwahara M, Gu Y, Tanaka Y, Marumo F, Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem Biophys Res Commun 1998;244:268–274.
- Wiegman MJ, Bullinger LV, Kohlmeyer MM, Hunter TC, Cipolla MJ. Regional expression of aquaporin 1, 4, and 9 in the brain during pregnancy. Reprod Sci 2008;15:506–516.
- Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, miry-Moghaddam M, Frokiaer J, Nielsen S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 2000;276:1118–1128.
- Aharon R, Bar-Shavit Z. Involvement of aquaporin 9 in osteoclast differentiation. J Biol Chem 2006;281:19305–19309.
- Damiano A, Zotta E, Goldstein J, Reisin I, Ibarra C. Water channel proteins AQP3 and AQP9 are present in syncytiotrophoblast of human term placenta. Placenta 2001;22:776–781.
- Wang S, Chen J, Beall M, Zhou W, Ross MG. Expression of aquaporin 9 in human chorioamniotic membranes and placenta. Am J Obstet Gynecol 2004;191:2160–2167.
- Loitto VM, Forslund T, Sundqvist T, Magnusson KE, Gustafsson M. Neutrophil leukocyte motility requires directed water influx. J Leukoc Biol 2002;71:212–222.
- Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, Maymon E, Edwin S. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol 2004;191:1331–1338.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report 140. Placenta 2005;26(Suppl A):S114–S117.
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25.
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301.
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; http://cran.r-project.org/doc/manuals/refman.pdf
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–408.
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 1999;270:41–49.
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284.
- Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys 2006;39:361–396.
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 2005;97:385–395.
- Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van HA, Brown D, Breton S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod 2002;66:1716–1722.
- Pietrement C, Da SN, Silberstein C, James M, Marsolais M, Van HA, Brown D, Pastor-Soler N, Ameen N, Laprade R, et al Role of NHERF1, cystic fibrosis transmembrane conductance regulator, and cAMP in the regulation of aquaporin 9. J Biol Chem 2008;283:2986–2996.
- Shengbiao W, Amidi F, Shengli Y, Beall M, Ross MG. Cyclic adenosine monophosphate regulation of aquaporin gene expression in human amnion epithelia. Reprod Sci 2007;14:234–240.
- Catalan V, Gomez-Ambrosi J, Pastor C, Rotellar F, Silva C, Rodriguez A, Gil MJ, Cienfuegos JA, Salvador J, Vendrell J, et al Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes Surg 2008;18:695–701.
- Badaut J, Petit JM, Brunet JF, Magistretti PJ, Charriaut-Marlangue C, Regli L. Distribution of Aquaporin 9 in the adult rat brain: preferential expression in catecholaminergic neurons and in glial cells. Neuroscience 2004;128:27–38.
- miry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci 2003;4:991–1001.
- Badaut J, Brunet JF, Petit JM, Guerin CF, Magistretti PJ, Regli L. Induction of brain aquaporin 9 (AQP9) in catecholaminergic neurons in diabetic rats. Brain Res 2008;1188:17–24.
- Anderson J, Brown N, Mahendroo MS, Reese J. Utilization of different aquaporin water channels in the mouse cervix during pregnancy and parturition and in models of preterm and delayed cervical ripening. Endocrinology 2006;147:130–140.
- Anderson D, Yang Q, Hohimer A, Faber J, Giraud G, Davis L. Intramembranous absorption rate is unaffected by changes in amniotic fluid composition. Am J Physiol Renal Physiol 2005;288:F964–F968.
- Mann SE, Ricke EA, Yang BA, Verkman AS, Taylor RN. Expression and localization of aquaporin 1 and 3 in human fetal membranes. Am J Obstet Gynecol 2002;187:902–907.
- Wang S, Kallichanda N, Song W, Ramirez BA, Ross MG. Expression of aquaporin-8 in human placenta and chorioamniotic membranes: evidence of molecular mechanism for intramembranous amniotic fluid resorption. Am J Obstet Gynecol 2001;185:1226–1231.
- Wang S, Amidi F, Beall M, Gui L, Ross MG. Aquaporin 3 expression in human fetal membranes and its up-regulation by cyclic adenosine monophosphate in amnion epithelial cell culture. J Soc Gynecol Investig 2006;13:181–185.
- Beall MH, Wang S, Yang B, Chaudhri N, Amidi F, Ross MG. Placental and membrane aquaporin water channels: correlation with amniotic fluid volume and composition. Placenta 2007;28:421–428.
- Lindsay LA, Murphy CR. Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J Mol Histol 2007;38:87–95.
