Abstract
Objective. The activation of the complement system results in the generation of split products with pro-inflammatory properties. The objective of this study was to determine whether preeclampsia and small-for-gestational age (SGA) are associated with changes in the maternal plasma concentrations of anaphylatoxins C3a, C4a and C5a.
Methods. A cross-sectional study was conducted in the following groups: (a) normal pregnant women (n = 134); (b) women who delivered an SGA neonate (n = 53); (c) preeclampsia with (n = 52) and without SGA (n = 54). Maternal plasma anaphylatoxin concentrations were determined by enzyme-linked immunoassay.
Results. (1) Women with preeclampsia with or without SGA had a significantly higher median plasma C5a concentration than that of normal pregnant women and those with SGA alone (all P < 0.01); (2) women with SGA alone did not have an increase in plasma C5a concentration; (3) in contrast, the median maternal plasma concentration of C4a was lower in women with preeclampsia and SGA than that of those with a normal pregnancy (P = 0.001); (4) no changes in C3a were observed among the study groups.
Conclusion. Preeclampsia is associated with increased plasma concentration of C5a, regardless of the presence or absence of an SGA fetus. In contrast, there was no difference in the plasma C3a, C4a and C5a concentration in patients with SGA.
Introduction
Preeclampsia and small-for-gestational age (SGA) are two of the ‘great obstetrical syndromes’ [Citation1] and considered leading causes of maternal and perinatal morbidity and mortality [Citation2,Citation3]. Both conditions share similar mechanisms of disease such as abnormal physiologic transformation of the spiral arteries [Citation4–12], chronic uteroplacental ischemia [Citation13–27], increased trophoblast apoptosis [Citation28,Citation29], anti-angiogenic state [Citation30–66] and endothelial cell dysfunction [Citation67–87]. Common risk factors observed in both syndromes include advance maternal age [Citation88–90], renal disease [Citation91–93], systemic lupus erythematous [Citation94,Citation95] and chronic hypertension [Citation96–99].
In addition, activation of the innate immune system and an exaggerated maternal systemic inflammatory response has been described in preeclampsia [Citation67,Citation71,Citation73,Citation75,Citation100–106] and, to a lesser extent, in SGA [Citation72,Citation75,Citation104,Citation106–108]. Evidence to support this view includes the following: (1) leukocyte activation [Citation72,Citation73], (2) increased maternal serum concentration of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha [Citation108] and (3) increased plasma markers of endothelial cell activation [Citation75,Citation76].
As part of innate immunity, the complement system participates in recognition and elimination of microorganism and foreign cells and in the inflammatory response. It also constitutes a bridge between innate and adaptive immunity [Citation109]. Complement activation by the classical, alternative or lectin pathway results in the generation of split products C3a, C4a and C5a with pro-inflammatory properties. These glycopeptides, also referred as ‘anaphylatoxins’, induce vascular permeability [Citation110–112], smooth muscle contraction [Citation110,Citation112,Citation113] and chemotaxis of inflammatory cells [Citation114–116]. In addition, proteolytic enzymes from phagocytic cells can also cleave C5 and release C5a [Citation117–120]. The importance of complement activation in pregnancy complications has become evident from recent studies on animal models of antiphospholipid antibody syndrome and pregnancy loss, demonstrating that complement activation, especially C5a and C3a, are directly related to vascular injury, growth restriction and fetal demise [Citation52,Citation121–129].
Normal pregnancy is characterized by increased complement components in maternal circulation [Citation130–132]. Indeed, higher plasma anaphylatoxins concentrations are found during normal pregnancy compared to nonpregnant women [Citation133]. Previous studies have described an enhanced deposition of native complement proteins, split products and membrane attack complex (MAC) in trophoblast tissue of patients with preeclampsia [Citation134,Citation135]. However, the association between complement system and preeclampsia, through the determination of various complement proteins, has been addressed in the past with conflicting results [Citation136–139]. Although preeclampsia and SGA share common features, there is a paucity of data on maternal plasma complement activation in women with SGA with or without preeclampsia. This study was designed to determine if the maternal plasma anaphylatoxin C3a, C4a and C5a concentrations in women with SGA neonates are different from those with preeclampsia.
Material and methods
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples including 293 women in four groups: (1) normal pregnant women (n = 134); (2) women with SGA neonates (n = 53); (3) patients with preeclampsia with SGA (n = 52) and (4) patients with preeclampsia without SGA (n = 54). Eligible patients were approached at the Detroit Medical Center/Hutzel Women's Hospital in Detroit/Michigan. All women provided written informed consent prior to the collection of the samples. The collection of samples was approved by the Human Investigation Committees and its utilization for research purposes by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously used to study the biology of inflammation, hemostasis and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Women with normal pregnancies met the following criteria: no medical, obstetrical or surgical complications, not in labor, gestational age that ranged from 20 weeks until term and delivery of a normal term infant with a normal birth weight [Citation140]. Patients in this group were enrolled from either a labor-delivery unit (in cases of scheduled cesarean section) or our antenatal clinic and followed until delivery. Preeclampsia was defined as hypertension (systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg on at least two occasions, 4 h to 1 week apart) and proteinuria (≥300 mg in a 24-h urine collection or one dipstick measurement of ≥2+). Severe preeclampsia was defined as severe hypertension (diastolic blood pressure ≥110 mmHg) and proteinuria, or mild hypertension and severe proteinuria (a 24 h urine sample that contained 3.5 g protein or one urine specimen of ≥3+ protein by dipstick measurement). SGA was defined as estimated fetal weight below the 10th percentile for gestational age, confirmed by neonatal birth weight using the reference range proposed by Alexander et al. [Citation140].
Blood collection and human anaphylatoxins immunoassays
Samples of peripheral blood were collected into tubes containing ethylene diamine tetraacetic acid (EDTA). Samples were centrifuged and stored at −70°C. Specific and sensitive complement C3a, C4a and C5a enzyme-linked immunoassays (ELISA) were performed as previously described [Citation133,Citation141].
Statistical analysis
The Kolmogorov–Smirnov test was used to test for normal distribution of the data. Because the maternal plasma concentrations of C3a, C4a and C5a were not normally distributed, nonparametric tests were used for analyses. Kruskal–Wallis with post-hoc Mann–Whitney U tests were performed when indicated to determine the difference of the median among and between groups, and Bonferroni correction was applied to adjust for multiple comparisons. Chi-square test was used for comparison of proportions. The statistical package used was SPSS 12 (SPSS, Chicago, IL). A probability value <0.05 was considered significant.
Results
displays the demographic and clinical characteristics of women in each group. Patients with SGA had the highest proportion of smoking among the study groups. The normal pregnancy group had a significantly lower rate of nulliparous women than the other groups. The median birth weight of patients with preeclampsia with and without SGA, as well as that of those in the SGA group, was lower than that of normal pregnancy (all comparisons P < 0.05). The median birth weight of neonates from the SGA group was lower than that of patients with preeclampsia in the absence of an SGA (P < 0.05). No significant differences were observed in the median gestational age at the time of blood collection among groups.
Table I. Clinical characteristics of the study population.
displays the median plasma concentrations of complement splits products C3a, C4a and C5a among the study groups. Women with preeclampsia, regardless of the presence or absence of an SGA neonate, had a higher median plasma concentration of C5a than normal pregnant women and women with an SGA alone [all P < 0.01; ]. Among patients with preeclampsia, there was no significant difference in the median plasma concentration of C5a between those with and without an SGA neonate (P = 0.9). Similarly, there was no difference in the median plasma concentrations of C5a between patients with an SGA neonate and normal pregnant women (P = 0.1). In contrast, the median plasma C4a concentration in women with preeclampsia who delivered an SGA neonate was lower than that of normal pregnant women [P = 0.001; ], and no significant differences in the median plasma concentration of C4a were observed among other groups. Also, there was no difference in the median plasma concentration of C3a among the study groups [].
Table II. Plasma concentration of C3a, C4a and C5a of the study population.
Figure 1. Median plasma anaphylatoxins concentration of normal pregnant women, women with small for gestational age neonates (SGA) and women with preeclampsia with and without SGA. (A) The median plasma C5a concentration was higher in patients with preeclampsia with SGA (median: 19.7 ng/ml; range: 4.5–119 ng/ml) or without SGA (median: 19.7 ng/ml; range: 4.3–94.1 ng/ml) than normal pregnant women (median: 12.4 ng/ml; range: 1.2–87.1 ng/ml) or women with isolated SGA (median: 14.3 ng/ml; range: 1.1–30.5 ng/ml). (B) In contrast, women with preeclampsia and SGA had a median plasma C4a concentration lower than normal pregnant women (preeclampsia and SGA median: 5696 ng/ml; range: 389.9–35,690 ng/ml vs. normal pregnancy median: 10125.4 ng/ml; range: 850.7–32,640 ng/ml; P = 0.001). No differences in plasma C4a concentration were observed among other groups. (C) There were no significant differences in the C3a plasma concentration among the study groups.
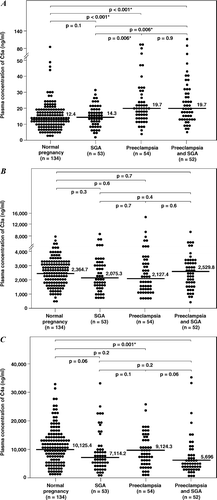
A subanalysis conducted in patients with mild and severe preeclampsia demonstrated that there was no difference in the median plasma concentration of the complement splits products C3a, C4a and C5a according to the severity of the disease (P > 0.05).
Discussion
Principal findings of the study
This study demonstrates that patients with preeclampsia, with or without SGA neonates, had higher maternal median plasma C5a concentrations than normal pregnant women and those with SGA by itself. In contrast, preeclampsia with SGA is characterized by lower median plasma C4a concentrations than normal pregnancy.
The complement system and preeclampsia
Previous studies of complement system in preeclampsia found no differences [Citation136,Citation142,Citation143] in serum complement hemolytic activity (CH50) in women with preeclampsia. In contrast, Haeger et al. [Citation144] reported in a cross-sectional study that plasma C3a and C5a concentrations in patients with preeclampsia were higher than in normal pregnant women at the time of delivery. The same authors reported in a longitudinal study [Citation139] that patients with preeclampsia had increased plasma C5a concentrations but not C3a at delivery [Citation139]. Moreover, patients with severe preeclampsia or with HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count) were found to have higher plasma C3a and C5a concentrations at delivery than the control group [Citation145,Citation146]. In addition, other complement proteins including C1-INH, C4, C3, C3d, C5 and MAC have been described to be reduced [Citation138,Citation147,Citation148], increased [Citation143] or unchanged [Citation137–139,Citation147,Citation149] in patients with preeclampsia. These results differed probably due to the variety of complement components measured, and the different methods employed in their determination. Recently, Lynch et al. [Citation150] reported in a prospective study that women who had elevated plasma fragment Bb (>90th percentile) before 20 weeks of gestation were more likely to develop preeclampsia than those who had a fragment Bb plasma concentration below the 90th percentile.
Complement C5a and preeclampsia
Preeclampsia is associated with phenotypic and metabolic changes in granulocytes and monocytes that suggest leukocyte activation [Citation72,Citation73,Citation101] The high concentrations of C5a in plasma of patients with preeclampsia could be attributed to leukocyte activation, because proteolytic enzymes (e.g. elastase, serine protease) in leukocytes can cleave complement C5 directly [Citation117–120]. The anaphylatoxin C5a exerts its activities through transmembrane receptor C5aR/CD88 [Citation151]. Several biological and pro-inflammatory effects of C5a on white blood cells have been reported. C5a can induce the release of elastase from neutrophils [Citation146], increase respiratory burst with the generation of reactive oxygen species [Citation152–155], induce the expression of adhesion molecules [Citation156] and delay neutrophil apoptosis [Citation157]. It is noteworthy that these markers of leukocyte activation have been described to be increased in women with preeclampsia [Citation73,Citation146,Citation158–160]. The actions of C5a on neutrophils and the generation of C5a by these cells would constitute a positive feedback loop that potentiates the inflammatory response in patients with preeclampsia. In addition, C5a induces the expression and/or the release of pro-inflammatory cytokines such as Interleukin (IL)- 1 [Citation161–163], IL-6 [Citation164], IL-8 [Citation165] and TNF-alpha [Citation162,Citation163] in mononuclear cells and neutrophils. Moreover, it increases endothelial gene expression of IL-6 [Citation166]. On the other hand, IL-6 and IL-1 increases the expression of C5aR on endothelial cells and monocytes, respectively [Citation167,Citation168]. Interestingly, an increase plasma TNF-alpha, IL-6 and IL-8 concentrations has been reported in patients with preeclampsia [Citation19,Citation169–173].
Recently, Mellembakken et al. [Citation174] demonstrated that C5aR (CD88) was decreased in neutrophils from patients with preeclampsia and suggested that it may reflect an enhanced C5a-C5aR interaction. This proposal would be in accordance to our result because high concentration of C5a would saturate white blood cells C5aR [Citation175]. Moreover, decay accelerating factor (DAF), a complement regulatory protein, was increased in neutrophils from patients with preeclampsia [Citation174]. Furthermore, in women with preeclampsia, membrane cofactor protein (MCP) (CD46), complement protectin (CD59) and CR-1 (CD35) were higher in leukocytes obtained from the uterine vein than those obtained from the antecubital vein [Citation174]. Following these results, the authors proposed that complement activation occurs in the uteroplacental compartment and the upregulation of complement regulatory proteins is a protective mechanism for complement attack.
Collectively, these observations suggest that C5a may play a role in the mechanisms of leukocyte activation and intravascular inflammation underlying the maternal syndrome of preeclampsia. Although SGA is also associated with leukocyte activation and inflammation [Citation72], no changes were observed in the plasma concentration of C5a.
Endothelium, coagulation and complement
The endothelium plays an active role in the inflammatory response. Endothelial activation/dysfunction is considered central to the pathophysiology of preeclampsia [Citation20,Citation67]. In fact, women with preeclampsia have increased circulating markers of endothelial cell activation [Citation67,Citation80,Citation176]. C5a increases the gene expression of E-selectin, V-CAM, I-CAM and upregulates P-selectin adhesion molecules on endothelial cells. Furthermore, elevated concentrations of tissue factor (TF) have been found in patient with preeclampsia [Citation177–179]. Both, in vitro and in vivo studies have demonstrated that C5a induces TF mRNA expression by endothelial cells [Citation180] and increases the procoagulant activity of alveolar macrophages by 5- to 6-fold through TF activation [Citation181]. Therefore, C5a represents one of the links between inflammation and the coagulation system, both of which are enhanced in preeclampsia.
Complement and SGA
Enhance complement activation was not observed in women with SGA. The causes and the significance of low median plasma C4a concentration found in women with preeclampsia and SGA are unknown. We expected to have elevated complement activation in the SGA group because it has been demonstrated in murine models of pregnancy loss that complement activation is a critical mediator of embryo injury and growth restriction [Citation129]. In a model, where maternal T-cells specific for paternal antigens triggered complement activation, embryo demise and growth restriction was observed [Citation52]. Moreover, extensive deposition of C3 at the maternal–fetal interface and macrophage infiltration was detected despite adequate Crry expression (a murine complement regulatory protein) [Citation52]. Surprisingly, when complement C3 was blocked with Crry-Ig it prevented embryo loss and growth restriction [Citation52]. In addition, in a murine model of antiphospholipid pregnancy loss, the blockage of C5 cleavage with anti-C5 monoclonal antibody and C5 deficient mice (C5 –/–) prevented fetal growth restriction and pregnancy loss [Citation125]. Similarly, fetal resorption and growth restriction were prevented when the C5a receptor was neutralized with an antagonist peptide [Citation125].
Preeclampsia and SGA
Despite that preeclampsia and SGA share many maternal and placental pathological features, these obstetrical syndromes have different phenotypes [Citation160,Citation182]. However, it is not clear why some women will manifest the maternal phenotype of the disease (preeclampsia) with or without fetal involvement, while others will have only the fetal phenotype (SGA). The difference in the complement split products between SGA and preeclampsia observed herein is in agreement with previous studies suggesting that SGA and preeclampsia have different biological profiles [Citation160,Citation179,Citation183,Citation184]. Preeclampsia is primarily a systemic maternal disease that is, in some cases, associated with fetal growth restriction. In contrast, SGA is primarily a fetal disease in which the systemic changes in the maternal compartment may not be as prominent as in preeclampsia.
In summary, the increased plasma concentration of C5a in patients with preeclampsia with or without SGA provides additional evidence supporting the view that preeclampsia is characterized by activation of the innate immune system [Citation73]. Whether the increase in C5a plasma concentration precedes the development of preeclampsia or is a consequence of a systemic intravascular inflammation remains to be determined.
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Romero R. Prenatal medicine: the child is the father of the man. Prenat Neonat Med 1996;1:8–11.
- Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 2004;191:481–487.
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregn 2003;22:203–212.
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J Pathol 1970;101:vi.
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972;1:177–191.
- Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol 1977;4:573–593.
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 1977;84:656–663.
- De Wolf F, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol 1980;87:678–685.
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049–1059.
- Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991;98:648–655.
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–674.
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 2002;187:1416–1423.
- Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens 1976;5:115–127.
- Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 1981;88:695–705.
- Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, Teague MJ. New doppler technique for assessing uteroplacental blood flow. Lancet 1983;1:675–677.
- Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol 1991;42(Suppl):S14–S20.
- Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol 1993;100:989–994.
- Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultrasound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol 1996;7:182–188.
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 1997;37:240–249.
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 1998;179:1359–1375.
- Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks' gestation. Obstet Gynecol 2000;96:559–564.
- Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol 2001;18:441–449.
- Myatt L. Role of placenta in preeclampsia. Endocrine 2002;19:103–111.
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 2002;9:147–160.
- Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta 2003;24:540–548.
- Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol 2004;2:53.
- Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2004;18:383–396.
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 2002;186:158–166.
- Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol 1997;177:1395–1401.
- Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol 1997;104:223–228.
- Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, Gull I, Lessing JB. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol 1997;38:302–306.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–1544.
- Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001;184:1267–1272.
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 2002;160:1405–1423.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547.
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004;89:770–775.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol 2004;191:1240–1246.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18.
- Malamitsi-Puchner A, Boutsikou T, Economou E, Sarandakou A, Makrakis E, Hassiakos D, Creatsas G. Vascular endothelial growth factor and placenta growth factor in intrauterine growth-restricted fetuses and neonates. Mediators Inflamm 2005;2005:293–297.
- Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 2005;57:1R–7R.
- Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab 2005;90:4895–4903.
- Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2005;122:33–39.
- Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005;26:563–573.
- Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int 2006;69:621–624.
- Boutsikou T, Malamitsi-Puchner A, Economou E, Boutsikou M, Puchner KP, Hassiakos D. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum Dev 2006;82:235–239.
- Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol 2006;195:201–207.
- Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–613.
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006;203:2165–2175.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005.
- Padavala S, Pope N, Baker P, Crocker I. An imbalance between vascular endothelial growth factor and its soluble receptor in placental villous explants of intrauterine growth-restricted pregnancies. J Soc Gynecol Investig 2006;13:40–47.
- Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255–259.
- Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med 2006;354:1857–1858.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–649.
- Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–57.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab 2007;92:2831–2834.
- Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol 2008;198:175–176.
- Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J Clin Endocrinol Metab 2007;92:2672–2679.
- Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol 2007;197:174–175.
- Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregn 2004;23:101–111.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M, et al Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol 2007;196:326–313.
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989;161:1200–1204.
- Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol 1992;166:962–968.
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:5–15.
- Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:17–31.
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506.
- Sabatier F, Bretelle F, D'ercole C, Boubli L, Sampol J, Dignat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol 2000;183:1558–1563.
- Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–797.
- Poston L, Chappell LC. Is oxidative stress involved in the aetiology of pre-eclampsia? Acta Paediatr Suppl 2001;90:3–5.
- Bretelle F, Sabatier F, Blann A, D'ercole C, Boutiere B, Mutin M, Boubli L, Sampol J, Dignat-George F. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG 2001;108:1277–1282.
- Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG 2002;109:836–839.
- Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta 2002;23:359–372.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–1594.
- Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ, Kingdom JC. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol 1994;101:485–487.
- Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, Kalache K, Edwin S, Bujold E, Gomez R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002;12:19–27.
- Austgulen R, Lien E, Vince G, Redman CW. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 1997;71:53–58.
- Coata G, Pennacchi L, Bini V, Liotta L, Di Renzo GC. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med 2002;12:28–34.
- Kim SY, Ryu HM, Yang JH, Kim MY, Ahn HK, Lim HJ, Shin JS, Woo HJ, Park SY, Kim YM, et al Maternal serum levels of VCAM-1, ICAM-1 and E-selectin in preeclampsia. J Korean Med Sci 2004;19:688–692.
- Chavarria ME, Lara-Gonzalez L, Garcia-Paleta Y, Vital-Reyes VS, Reyes A. Adhesion molecules changes at 20 gestation weeks in pregnancies complicated by preeclampsia. Eur J Obstet Gynecol Reprod Biol 2008;137:157–164.
- Daniel Y, Kupferminc MJ, Baram A, Geva E, Fait G, Lessing JB. A selective increase in plasma soluble vascular cell adhesion molecule-1 levels in preeclampsia. Am J Reprod Immunol 1999;41:407–412.
- Budak E, Madazli R, Aksu MF, Benian A, Gezer A, Palit N, Yildizfer F. Vascular cell adhesion molecule-1 (VCAM-1) and leukocyte activation in pre-eclampsia and eclampsia. Int J Gynaecol Obstet 1998;63:115–121.
- Krauss T, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol 1997;177:443–449.
- Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol 2004;104:727–733.
- Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol 2006;23:325–328.
- Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997;177:1003–1010.
- Chao AS, Huang JY, Lien R, Kung FT, Chen PJ, Hsieh PC. Pregnancy in women who undergo long-term hemodialysis. Am J Obstet Gynecol 2002;187:152–156.
- Germain S, Nelson-Piercy C. Lupus nephritis and renal disease in pregnancy. Lupus 2006;15:148–155.
- Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC III. Chronic renal disease in pregnancy. Obstet Gynecol 2006;108:1531–1539.
- Carmona F, Font J, Cervera R, Munoz F, Cararach V, Balasch J. Obstetrical outcome of pregnancy in patients with systemic Lupus erythematosus. A study of 60 cases. Eur J Obstet Gynecol Reprod Biol 1999;83:137–142.
- Moroni G, Quaglini S, Banfi G, Caloni M, Finazzi S, Ambroso G, Como G, Ponticelli C. Pregnancy in lupus nephritis. Am J Kid Dis 2002;40:713–720.
- Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregn Childbirth 2004;4:17.
- Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol 1997;40:704–714.
- Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol 1994;171:410–416.
- Zetterstrom K, Lindeberg SN, Haglund B, Hanson U. Chronic hypertension as a risk factor for offspring to be born small for gestational age. Acta Obstet Gynecol Scand 2006;85:1046–1050.
- Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol 1998;105:632–640.
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–86.
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000;21:597–602.
- Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol 2003;59:153–160.
- Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol 2003;59:29–37.
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta 2009;30(Suppl A):S38–S42.
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002;11:362–367.
- Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol 1996;106:127–133.
- Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand 2003;82:1099–1102.
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol 2004;5:981–986.
- Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med 1968;127:371–386.
- Schumacher WA, Fantone JC, Kunkel SE, Webb RC, Lucchesi BR. The anaphylatoxins C3a and C5a are vasodilators in the canine coronary vasculature in vitro and in vivo. Agents Actions 1991;34:345–349.
- Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a: the third anaphylatoxin of the human complement system. Proc Natl Acad Sci USA 1979;76:5299–5302.
- Dias DS, Lepow IH. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med 1967;125:921–946.
- Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med 1995;181:2119–2127.
- Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood 1997;89:2863–2870.
- Shin HS, Snyderman R, Friedman E, Mellors A, Mayer MM. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science 1968;162:361–363.
- Vogt W. Cleavage of the fifth component of complement and generation of a functionally active C5b6-like complex by human leukocyte elastase. Immunobiology 2000;201:470–477.
- Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology 1996;195:334–346.
- Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol 2002;161:1849–1859.
- Ward PA, Hill JH. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol 1970;104:535–543.
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 2000;287:498–501.
- Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, et al Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med 2002;195:211–220.
- Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis 2002;61(Suppl 2):ii46–ii50.
- Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, Molina H. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity 2003;19:813–822.
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, et al Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003;112:1644–1654.
- Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, et al A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 2005;42:87–97.
- Molina H. Complement regulation during pregnancy. Immunol Res 2005;32:187–192.
- Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood 2007;110:2423–2431.
- Girardi G. Guilty as charged: all available evidence implicates complement's role in fetal demise. Am J Reprod Immunol 2008;59:183–192.
- Baines MG, Millar KG, Mills P. Studies of complement levels in normal human pregnancy. Obstet Gynecol 1974;43:806–810.
- Hopkinson ND, Powell RJ. Classical complement activation induced by pregnancy: implications for management of connective tissue diseases. J Clin Pathol 1992;45:66–67.
- Johnson U, Gustavii B. Complement components in normal pregnancy. Acta Pathol Microbiol Immunol Scand C 1987;95:97–99.
- Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 2005;17:239–245.
- Sinha D, Wells M, Faulk WP. Immunological studies of human placentae: complement components in pre-eclamptic chorionic villi. Clin Exp Immunol 1984;56:175–184.
- Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE, Pecorari D. Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clin Exp Immunol 1990;80:236–240.
- Prall RH, Kantor FS. Serum complement in eclamptogenic toxemia. Am J Obstet Gynecol 1966;95:530–533.
- Armstrong NP, Teisner B, Redman CW, Westergaard JG, Folkersen J, Grudzinskas JG. Complement activation, circulating protease inhibitors and pregnancy-associated proteins in severe pre-eclampsia. Br J Obstet Gynaecol 1986;93:811–814.
- Mellembakken JR, Hogasen K, Mollnes TE, Hack CE, Abyholm T, Videm V. Increased systemic activation of neutrophils but not complement in preeclampsia. Obstet Gynecol 2001;97:371–374.
- Haeger M, Unander M, Bengtsson A. Complement activation in relation to development of preeclampsia. Obstet Gynecol 1991;78:46–49.
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Richani K, Romero R, Soto E, Espinoza J, Nien JK, Chaiworapongsa T, Refuerzo J, Blackwell S, Edwin SS, Santolaya-Forgas J, et al Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat Med 2005;33:296–305.
- Kitzmiller JL, Stoneburner L, Yelenosky PF, Lucas WE. Serum complement in normal pregnancy and pre-eclampsia. Am J Obstet Gynecol 1973;117:312–315.
- Tedder RS, Nelson M, Eisen V. Effects on serum complement of normal and pre-eclamptic pregnancy and of oral contraceptives. Br J Exp Pathol 1975;56:389–395.
- Haeger M, Bengtson A, Karlsson K, Heideman M. Complement activation and anaphylatoxin (C3a and C5a) formation in preeclampsia and by amniotic fluid. Obstet Gynecol 1989;73:551–556.
- Haeger M, Unander M, Bengtsson A. Enhanced anaphylatoxin and terminal C5b-9 complement complex formation in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol 1990;76:698–702.
- Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol 1992;79:19–26.
- Buyon JP, Cronstein BN, Morris M, Tanner M, Weissmann G. Serum complement values (C3 and C4) to differentiate between systemic lupus activity and pre-eclampsia. Am J Med 1986;81:194–200.
- Halbmayer WM, Hopmeier P, Mannhalter C, Heuss F, Leodolter S, Rubi K, Fischer M. C1-esterase inhibitor in uncomplicated pregnancy and mild and moderate preeclampsia. Thromb Haemost 1991;65:134–138.
- Massobrio M, Benedetto C, Bertini E, Tetta C, Camussi G. Immune complexes in preeclampsia and normal pregnancy. Am J Obstet Gynecol 1985;152:578–583.
- Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol 2008;198:385–389.
- Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature 1991;349:614–617.
- Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett 1994;346:181–184.
- Goldstein IM, Roos D, Kaplan HB, Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest 1975;56:1155–1163.
- Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002;100:1869–1877.
- Wymann MP, Kernen P, Deranleau DA, Baggiolini M. Respiratory burst oscillations in human neutrophils and their correlation with fluctuations in apparent cell shape. J Biol Chem 1989;264:15829–15834.
- Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology 2000;46:209–222.
- Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int 2002;61:456–463.
- Greer IA, Haddad NG, Dawes J, Johnstone FD, Calder AA. Neutrophil activation in pregnancy-induced hypertension. Br J Obstet Gynaecol 1989;96:978–982.
- Halim A, Kanayama N, El Maradny E, Maehara K, Bhuiyan AB, Terao T. Correlated plasma elastase and sera cytotoxicity in eclampsia. A possible role of endothelin-1 induced neutrophil activation in preeclampsia-eclampsia. Am J Hypertens 1996;9:33–38.
- von Dadelszen P, Watson RW, Noorwali F, Marshall JC, Parodo J, Farine D, Lye SJ, Ritchie JW, Rotstein OD. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol 1999;181:408–414.
- Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol 1987;139:2635–2640.
- Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med 1988;168:443–448.
- Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood 1990;76:1631–1638.
- Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, et al Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J 2004;18:370–372.
- Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am J Pathol 1994;144:393–403.
- Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol 2004;164:849–859.
- Takabayashi T, Shimizu S, Clark BD, Beinborn M, Burke JF, Gelfand JA. Interleukin-1 upregulates anaphylatoxin receptors on mononuclear cells. Surgery 2004;135:544–554.
- Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, et al Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol 2002;169:5962–5970.
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol 1995;102:20–25.
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 1998;40:102–111.
- Laskowska M, Laskowska K, Leszczynska-Gorzelak B, Oleszczuk J. Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med 2007;20:527–532.
- Brewster JA, Orsi NM, Gopichandran N, Ekbote UV, Cadogan E, Walker JJ. Host inflammatory response profiling in preeclampsia using an in vitro whole blood stimulation model. Hypertens Pregn 2008;27:1–16.
- Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol 1994;84:937–940.
- Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002;39:155–160.
- Chenoweth DE, Hugli TE. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci USA 1978;75:3943–3947.
- Friedman SA, Taylor RN, Roberts JM. Pathophysiology of preeclampsia. Clin Perinatol 1991;18:661–682.
- Abdel Gader AM, Al-Mishari AA, Awadalla SA, Buyuomi NM, Khashoggi T, Al-Hakeem M. Total and free tissue factor pathway inhibitor in pregnancy hypertension. Int J Gynaecol Obstet 2006;95:248–253.
- Bellart J, Gilabert R, Angles A, Piera V, Miralles RM, Monasterio J, Cabero L. Tissue factor levels and high ratio of fibrinopeptide A:D-dimer as a measure of endothelial procoagulant disorder in pre-eclampsia. Br J Obstet Gynaecol 1999;106:594–597.
- Erez O, Romero R, Hoppensteadt D, Than NG, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Kim SS, Yoon BH, et al Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med 2008;21:855–869.
- Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost 1997;77:394–398.
- Sitrin RG, Kaltreider HB, Ansfield MJ, Webster RO. Procoagulant activity of rabbit alveolar macrophages. Am Rev Respir Dis 1983;128:282–287.
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol 2006;195:40–49.
- Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, et al Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol 2006;194:921–931.
- Grisaru-Granovsky S, Halevy T, Eidelman A, Elstein D, Samueloff A. Hypertensive disorders of pregnancy and the small for gestational age neonate: not a simple relationship. Am J Obstet Gynecol 2007;196:335.
