Abstract
Objective. To investigate the accuracy of cervicovaginal fetal fibronectin in predicting preterm birth in women with multiple pregnancies.
Methods. Systematic review and meta-analysis of predictive test accuracy. Cohort or cross-sectional studies were identified through searches in databases, reference lists, proceedings, and reviews. Study selection, quality assessment, and data extraction were performed. We constructed summary receiver operating characteristic curves and calculated pooled sensitivities and specificities using a bivariate, random-effects meta-regression model. We also calculated summary likelihood ratios and post-test probabilities of preterm birth.
Results. Fifteen studies (11 in asymptomatic women and 4 in women with symptoms of preterm labor) involving 1221 women with multiple pregnancies were included. Among asymptomatic women with multiple or twin pregnancies, the pooled sensitivities, specificities, and positive and negative likelihood ratios for predicting preterm birth before 32, 34, and 37 weeks' gestation ranged from 33% to 45%, 80% to 94%, 2.0 to 5.5, and 0.68 to 0.76, respectively. Among women with twin pregnancies and threatened preterm labor, the test was most accurate in predicting spontaneous preterm birth within 7 days of testing (pooled sensitivity, specificity, and positive and negative likelihood ratios of 85%, 78%, 3.9, and 0.20, respectively).
Conclusions. Cervicovaginal fetal fibronectin provides moderate to minimal prediction of preterm birth in women with multiple pregnancies. The test is most accurate in predicting spontaneous preterm birth within 7 days of testing in women with twin pregnancies and threatened preterm labor.
Introduction
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide [Citation1]. In the United States, the preterm birth rate has risen from 9.4% in 1981 to 12.8% in 2006 [Citation2]. This increase has been linked to rising indicated preterm births in singletons [Citation3] and preterm delivery of artificially conceived multiple pregnancies [Citation1].
The number of multiple births has increased over the last several decades in the United States. From 1980 to 2006, the multiple birth rate increased 75% from 19.3 to 33.7 per 1000 live births [Citation2]. The increase in multiple births, particularly higher order multiples, has been attributed to older age at childbearing and the growing availability and use of assisted reproductive technologies (ART) such as in vitro fertilization, and other non-ART fertility treatments such as ovulation-inducing drugs and intrauterine insemination [Citation4].
Babies born from multiple pregnancies are more likely to be born prematurely than those from single pregnancies. Martin et al. [Citation2] have recently reported that the percent of singletons born less than 37 weeks of gestation was 11.1% compared to that of multiples at 61.9%. The rate of preterm birth less at than 32 weeks of gestation was much more common among multiples (13.3%) than among singletons (1.6%).
The identification of women with multiple pregnancies who are at higher risk for preterm birth could lead to intensified maternal and fetal surveillance as well as earlier interventions that could decrease the severity of adverse perinatal outcomes associated with preterm birth in such women. Several tests have been proposed to predict spontaneous preterm birth in multiple pregnancies, including cervicovaginal fetal fibronectin testing [Citation5]. Fetal fibronectin is a glycoprotein produced in fetal tissues that is mainly found in amniotic fluid, placental tissue, and the extracellular substance of the decidua basalis next to the placental intervellous space. Fetal fibronectin acts as a ‘cellular glue’ and is believed to mediate implantation and placental–uterine attachment throughout gestation [Citation6]. Fetal fibronectin can be measured in cervicovaginal secretions early in pregnancy and at term, but is rarely detectable between 20 and 37 weeks' gestation in normal pregnancies that are delivered at term [Citation7]. It has been postulated that damage to the fetal membranes may release fetal fibronectin into the cervix and vagina [Citation8]. This proposed pathway for release has led to the hypothesis that measurement of fetal fibronectin in cervicovaginal secretions might be used as a predictive test for preterm labor.
Previous meta-analyses [Citation9–14] have concluded that cervicovaginal fetal fibronectin testing might be clinically useful in the prediction of preterm birth, with an emphasis given to the high negative predictive value of the test, particularly in women who are symptomatic of threatened preterm labor and delivery within 7–10 days of sampling. However, only one meta-analysis reported on the predictive accuracy of fetal fibronectin for preterm birth in asymptomatic women with multiple pregnancies [Citation14]. The literature search for this review was done in March 2002. Since then, substantial new evidence has emerged, allowing for more robust and specific inferences for clinical practice.
We undertook a systematic review and meta-analysis of all available studies to investigate the accuracy of cervicovaginal fetal fibronectin in predicting preterm birth in women with multiple pregnancies.
Methods
The systematic review was conducted following a prospectively prepared protocol and reported using widely recommended guidelines for systematic reviews of diagnostic test accuracy [Citation15].
Literature search
We searched PubMed, Embase, Cinahl, Lilacs, and Medion (all from inception to 30 September 2010), using a combination of keywords and text words related to fibronectin and preterm birth. Proceedings of the Society for Maternal–Fetal Medicine and international meetings on preterm birth and twin or multiple pregnancy, reference lists of identified studies, textbooks, previously published systematic reviews, and review articles were also searched. In addition, we contacted investigators involved in the field to locate unpublished studies. No language restrictions were applied. All searches were carried independently by the two authors and results were merged. For studies that resulted in multiple publications, the data from the publication with the largest sample size were used and supplemented if additional information appeared in the other publications.
Study selection
The systematic review focused on cohort or cross-sectional studies that evaluated the accuracy of cervicovaginal fetal fibronectin testing to predict spontaneous preterm birth in asymptomatic or symptomatic pregnant women with multiple pregnancies. The studies had to provide the necessary information to construct 2 × 2 tables. Unless data for multiples were extractable separately, studies basing their results on mixed (singleton and multiple) pregnancies were not considered for inclusion in the review.
Case–control studies were excluded because they overestimate predictive accuracy [Citation16]. Studies were also excluded if they were case series or reports, editorials, comments or reviews without original data, or if accuracy test estimates were not published and sufficient information to calculate them could not be retrieved despite writing to the corresponding author.
All published studies deemed suitable were retrieved and reviewed independently by the two authors to determine inclusion. Disagreements were resolved through consensus.
Quality assessment
We generated the quality assessment criteria for evaluating studies included in the review by using 4 of the 14 items of the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool [Citation17]. Each item was scored as ‘yes', ‘no’, or ‘unclear’. The remaining 10 QUADAS items were not used because they were not relevant to our review.
The items of the QUADAS tool evaluated in studies included in the systematic review and their interpretation were as follows:
Representative spectrum of patients: this item was scored ‘yes' when pregnant women with multiple pregnancies were consecutively selected in a prospective way. Convenience sampling, such as arbitrary recruitment or nonconsecutive recruitment, was scored as ‘no’.
Description of the test: if the study reported sufficient details of the execution of the fetal fibronectin test, such as the particular laboratory or analytical method used and cut-off level for an abnormal result, then this item was scored as ‘yes'. In other cases, this item was scored as ‘no’.
Blinding of index test result: this item was scored ‘yes' if the study clearly stated that clinicians managing the patient did not have knowledge of the fetal fibronectin test results. If this did not appear to be the case, this item was scored as ‘no’.
Reporting of study withdrawals: in case there were withdrawals from the study, this item was scored as ‘yes' if withdrawals were explained or if a flow diagram of study participants was reported. If it appeared that some of the participants who entered the study did not complete the study and these patients were not accounted for, then this item was scored as ‘no’.
If there was insufficient information available to make a judgment of these items, then they were scored as ‘unclear’. We did not calculate a summary score estimating the overall quality of an article since the interpretation of such summary scores is problematic and potentially misleading [Citation18].
The methodological quality of included studies was assessed individually by the two reviewers who were not associated with any of the studies. When differences of opinion existed, a consensus was reached.
Data extraction
Potentially relevant articles were acquired and data were extracted in duplicate from all reports and recorded on a piloted form independently by the two reviewers. There was no blinding of authorship. Information was extracted on study characteristics (recruitment of women, prospective or retrospective data collection, blinding of test results, completeness of follow-up, and reporting of withdrawals), participants (inclusion and exclusion criteria, number of pregnant women with twins, triplets, quadruplets, and higher order pregnancies, demographic characteristics, and country and date of publication), and description of fetal fibronectin test (gestational age at sampling, frequency of test, sampling site, analytical method used, and cut-off level).
We then extracted numbers of true positive, false positive, true negative, and false negative results separately for studies of asymptomatic women with multiple (twins, triplets, quadruplets, or higher order) and twin pregnancies, and women with twin pregnancies and threatened preterm labor. When predictive accuracy data were not extractable, we attempted to contact the corresponding author by e-mail to obtain the additional data.
In studies where serial fetal fibronectin samples were collected, we considered any positive result as a positive result overall. Studies reporting spontaneous preterm birth before 35 weeks' gestation were included into the group of studies with spontaneous preterm birth before 34 weeks' gestation in our data synthesis because of the relatively similar neonatal outcomes. In the same way, studies reporting spontaneous preterm birth before 36 weeks' gestation were considered with those reporting spontaneous preterm birth before 37 weeks' gestation.
All the data were extracted independently by the two reviewers and any disagreements were resolved by discussion among them.
Statistical analysis
Data were synthesized separately for studies on asymptomatic women with spontaneous preterm birth before 32, 34, and 37 weeks' gestation. For women with twin pregnancies and threatened preterm labor, we synthesized data for spontaneous preterm birth before 34 and 37 weeks' gestation, and within 7–14 days of testing.
Data extracted from each study were arranged in 2 × 2 contingency tables. When these tables contained cells for which the value was 0, we added 0.5 to those cells to allow for the calculation of variances [Citation19]. We calculated the sensitivity and specificity for each study and plotted them in receiver operating characteristic (ROC) plots. Then we constructed summary ROC curves for each outcome using a bivariate random-effects approach [Citation20] and calculated area under the summary ROC curve with their corresponding 95% confidence intervals (CIs) [Citation21]. This measure allows comparing predictive accuracy of the test for different outcomes. Two-sided p < 0.05 was considered to be statistically significant.
A bivariate, random-effects meta-regression model was used to calculate pooled estimates of sensitivity and specificity with 95% CIs [Citation20]. The bivariate model incorporates and estimates the correlation that might exist between estimates of sensitivity and specificity within studies, which allows producing more valid results. Thereafter, we derived likelihood ratios with 95% CIs from the pooled sensitivities and specificities for each outcome reported [Citation22]. Likelihood ratios indicate by how much a given test result raises or lowers the probability of having the disease and thus allows interpretation of the results in terms of clinical importance [Citation23]. The likelihood ratio of a positive test is the ratio of the probability of a positive fetal fibronectin test result in women who subsequently have a spontaneous preterm birth to the probability of the positive fetal fibronectin test result in women who subsequently do not have a preterm birth (sensitivity/1 – specificity). The likelihood ratio of a negative test is the ratio of the probability of a negative fetal fibronectin test result in women who subsequently have a spontaneous preterm birth to the probability of the negative fetal fibronectin test result in women who subsequently do not have a preterm birth ([1 – sensitivity]/specificity). Likelihood ratios for a positive test result above 10 and likelihood ratios for a negative test result below 0.1 have been noted as providing convincing predictive evidence. Moderate prediction can be achieved with likelihood ratio values of 5–10 and 0.1–0.2, whereas those below 5 and above 0.2 would give only minimal prediction [Citation23].
We used likelihood ratios generated from meta-analyses to determine post-test probabilities of spontaneous preterm birth before 32, 34, and 37 weeks' gestation, and within 7 and 14 days of testing for positive and negative fetal fibronectin test results as follows [Citation23]:
Estimates of pretest probabilities of preterm birth <32, <34, and <37 weeks' gestation, and within 7 and 14 days of testing were obtained from the global prevalence of these outcomes across the studies.
Heterogeneity of the results among studies was investigated through visual examination of forest plots of sensitivities and specificities, and ROC plots. In addition, heterogeneity was assessed by means of the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance [Citation24]. Statistical heterogeneity was defined as an I2 statistic value of 50% or more [Citation24]. We explored potential sources of heterogeneity by performing meta-regression analysis of subgroups defined a priori [Citation25]. These were study setting (those conducted in the United States versus those conducted in other countries), sample size (<100 versus ≥100 in studies of asymptomatic women and <50 versus ≥50 in studies of symptomatic women), the study's year of publication (before 2000 versus at or after 2000), pregnancy plurality (studies in multiples versus studies in twins), method for measuring fetal fibronectin (quantitative versus qualitative), and sampling frequency (single versus serial). In addition, we planned to examine the impact of study quality on estimation of predictive accuracy according to individual quality items and an overall quality level incorporating these items (those that met all 4 methodological criteria versus those that met <4 criteria).
We assessed publication and related biases visually by examining the symmetry of funnel plots (log diagnostic odds ratios versus the inverse of variance) and statistically by using the Egger's regression test [Citation26]. p < 0.1 indicated significant asymmetry.
The bivariate models were fitted using the NLMIXED procedure (SAS 9.1 for Windows [SAS Institute, Inc., Cary, NC]) The summary ROC curves were constructed using the RevMan (Review Manager) 5.0.20. The remaining analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
Results
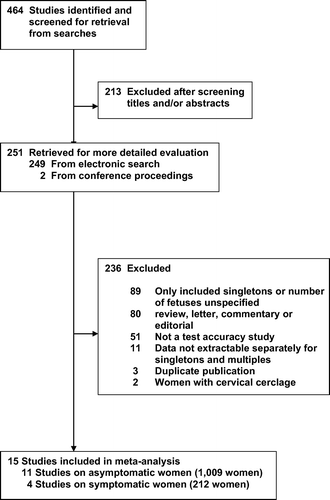
shows details of the search strategy. Of the 464 articles identified through the searches, we retrieved the full text of 251 articles for more detailed evaluation. Two-hundred thirty-six studies were excluded, mainly because they did not include multiple pregnancies (37%) or lacked original data (34%). A total of 15 studies (11 providing data on asymptomatic women and 4 on women with symptoms of preterm labor) involving 1221 women with multiple pregnancies (1133 twins, 57 triplets, 2 quadruplets, and 29 unspecified) met the inclusion criteria for the review [Citation27–41]. Agreement regarding inclusion of the studies was 100% (κ = 1.00).
The main characteristics of included studies are depicted in . Ten studies (67%) were performed in the United States [Citation28,Citation29,Citation32,Citation33,Citation35–39 ,Citation41], and one each in Italy [Citation27], Sweden [Citation30], Brazil [Citation31], United Kingdom [Citation34], and France [Citation40]. The sample size ranged from 29 [Citation28] to 169 [Citation36] in asymptomatic women and from 38 [Citation38] to 87 [Citation41] in symptomatic women. Cervicovaginal fetal fibronectin was measured using enzyme-linked immunoabsorbent assay quantitative method (nine studies [Citation28–30 ,Citation33,Citation35–39]), membrane immunoassay qualitative method (three studies [Citation27,Citation31,Citation34]), and rapid TLi system qualitative method (one study [Citation41]). Two studies [Citation32,Citation40] did not report their method for measuring fetal fibronectin. All studies considered a cervicovaginal fetal fibronectin concentration of 50 ng/ml or greater a positive test result. Of the 11 studies in asymptomatic women, 9 (82%) looked at serial testing at 22–34 weeks' gestation, weekly or every 2–3 weeks [Citation27–32 ,Citation35–37]. Two studies in asymptomatic women [Citation33,Citation34] and four in symptomatic women [Citation38–41] examined single testing at 20–35 weeks' gestation. In women without symptoms, three studies evaluated cervicovaginal fetal fibronectin tests to predict preterm birth before 32 weeks' gestation [Citation29,Citation33,Citation37], nine evaluated preterm birth before 34 weeks' gestation [Citation27–31 ,Citation34–37], and six evaluated preterm birth before 37 weeks' gestation [Citation27,Citation29–32 ,Citation37]. In women with symptoms of preterm labor, three studies evaluated preterm birth within 7 days of testing [Citation38,Citation39,Citation41], and two each evaluated preterm birth within 14 days of testing [Citation38,Citation41], and before 34 [Citation40,Citation41] and 37 [Citation38,Citation40] weeks' gestation.
Table I. Characteristics of studies included in the systematic review.
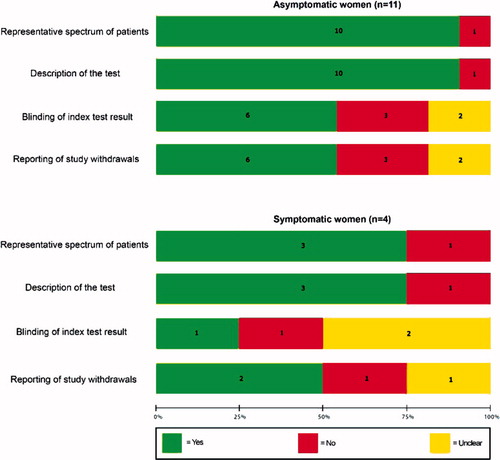
The methodological quality of included studies is shown in . Only six studies (40%), five among asymptomatic women and one among symptomatic women, fulfilled all four methodological criteria. The remaining 10 studies had at least one methodological flaw. The most common shortcomings were the failure to blind investigators to fetal fibronectin test results and the report of loss to follow-up or exclusions.
Figure 2. Methodological quality of studies included in the systematic review. Data presented as percentages across all included studies. Figures in the stacks represent number of studies.
show summary ROC curves of cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in asymptomatic women with multiple and twin pregnancies, and symptomatic women with twin pregnancies. Among asymptomatic women with multiple pregnancies, the area under the summary ROC curve to predict spontaneous preterm birth before 37 weeks' gestation (0.82) was larger than the area under the summary ROC curves to predict spontaneous preterm birth before 32 (0.78) and 34 (0.71) weeks' gestation, although the differences were not statistically significant (). Similar summary ROC curves were obtained for asymptomatic women with twin pregnancies (). Among women with twin pregnancies and threatened preterm labor, the area under the summary ROC curve was greatest for the prediction of spontaneous preterm birth within 7 days of testing (0.85), followed by preterm birth within 14 days of testing (0.74), preterm birth before 34 weeks (0.70), and preterm birth before 37 weeks (0.57) (). Only the difference between the areas under the summary ROC curves for the prediction of preterm birth within 7 days of testing and preterm birth before 37 weeks was statistically significant (p = 0.03).
Figure 3. Summary receiver operating characteristic (ROC) curves of cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in asymptomatic women with multiple pregnancies. The area of each circle, rectangle and diamond is proportional to study's sample size.
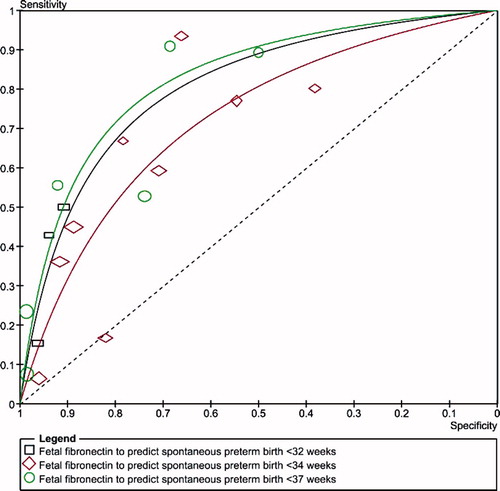
Figure 4. Summary receiver operating characteristic (ROC) curves of cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in asymptomatic women with twin pregnancies. The area of each circle, rectangle and diamond is proportional to study's sample size.
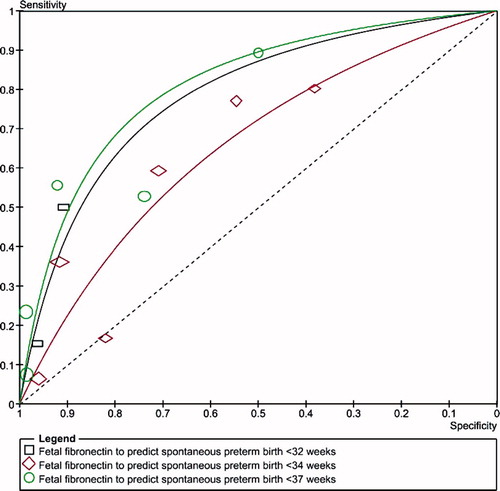
Figure 5. Summary receiver operating characteristic (ROC) curves of cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in women with twin pregnancies and threatened preterm labor. The area of each circle, rectangle and diamond is proportional to study's sample size.
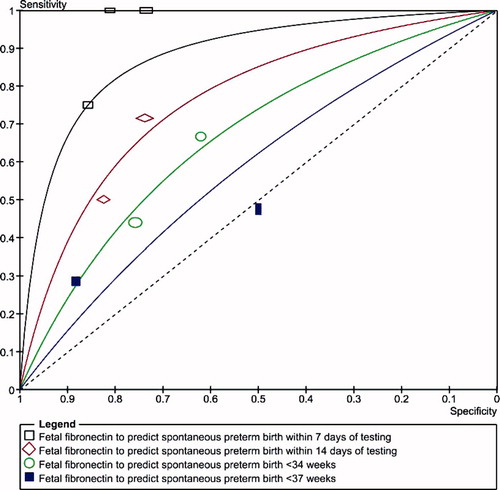
and show pooled estimates of sensitivity, specificity, and positive and negative likelihood ratios of cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in women with multiple pregnancies. Among asymptomatic women with multiple pregnancies, the pooled sensitivities and specificities ranged between 35% and 45%, and 81% and 94%, respectively. The best summary likelihood ratio for a positive test result was 5.5 for predicting preterm birth before 32 weeks' gestation, with corresponding summary likelihood ratio for a negative test result of 0.69. The test was less accurate to predict preterm birth before 34 and 37 weeks' gestation (summary positive and negative likelihood ratios around 2.5 and 0.70, respectively). Similar pooled estimates of predictive accuracy were obtained for studies in asymptomatic women with twin pregnancies (). Among women with twin pregnancies and threatened preterm labor, cervicovaginal fetal fibronectin was most accurate in predicting spontaneous preterm birth within 7 days of testing (pooled sensitivity, specificity, and positive and negative likelihood ratios of 85%, 78%, 3.9, and 0.20, respectively) (). For delivery within 14 days of testing, and preterm birth before 34 and 37 weeks' gestation, the test had a minimal predictive accuracy (summary positive and negative likelihood ratios between 1.8 and 2.7, and 0.48 and 0.73, respectively).
Table II. Pooled estimates for cervicovaginal fetal fibronectin in predicting spontaneous preterm birth in asymptomatic women with multiple pregnancies.
Table III. Pooled estimates for cervicovaginal fetal fibronectin in predicting spontaneous preterm birth in women with twin pregnancies and threatened preterm labor.
Pooled estimates of pretest and post-test probabilities of having spontaneous preterm birth after a negative and positive test result are summarized in . The overall prevalences (pretest probabilities) of spontaneous preterm birth before 32, 34 and 37 weeks' gestation in studies that included asymptomatic women with multiple pregnancies were 8.7% (range, 7.9%–9.0%), 22.8% (range, 16.1%–54.2%), and 48.5% (range, 35.6%–54.4%), respectively. Among women with twin pregnancy and threatened preterm labor, the overall prevalences of spontaneous preterm birth within 7 and 14 days of testing, and before 34 and 37 weeks' gestation were 7.7% (range, 2.6%–18.6%), 8.8% (range, 8.0%–10.5%), 30.5% (range, 28.7%–34.1%), and 74.4% (range, 55.3–90.9%), respectively. In asymptomatic women with multiple pregnancies, the pretest probability of preterm birth before 32 weeks' gestation increased from 8.7% to 34.4% with a positive result and decreased to 6.2% with a negative result. Similar results were obtained for asymptomatic women with twin pregnancies. In women with twin pregnancies and symptoms of preterm labor, the pretest probability of delivery within 7 days of testing increased from 7.7% to 24.5% with a positive result and decreased to 1.6% with a negative result. For preterm birth before 34 and 37 weeks' gestation in both asymptomatic and symptomatic women, and preterm birth within 14 days of testing in symptomatic women, the likelihood ratios only produced moderate to minimal changes in the pretest probabilities.
Table IV. Pooled estimates of pre-test probabilities, likelihood ratios, and post-test probabilities for cervicovaginal fetal fibronectin in the prediction of spontaneous preterm birth in women with multiple pregnancies.
There was graphical and statistical heterogeneity of predictive performance among studies as confirmed by I2 values greater than 50% in almost all meta-analyses performed. An explanation for heterogeneity was not provided by the study setting, sample size, study's year of publication, pregnancy plurality, method for measuring fetal fibronectin, or sampling frequency. In addition, pooled predictive accuracy estimates obtained from studies that met <4 methodological criteria did not differ significantly from those obtained from studies that met all four criteria.
All funnel plots showed no asymmetry, either visually or in terms of statistical significance (p > 0.10 for all, by Egger test), indicating that publication and related biases were not present.
Discussion
In this systematic review, we found evidence that cervicovaginal fetal fibronectin has limited accuracy in predicting spontaneous preterm birth in both asymptomatic and symptomatic women with multiple pregnancies because the likelihood ratios for positive and negative test results generated only minimal to moderate changes in the pretest probabilities of preterm birth. The test was most accurate in predicting spontaneous preterm birth before 32 weeks' gestation in asymptomatic women with multiple or twin pregnancies, and spontaneous preterm birth within 7 days of testing in women with twin pregnancies and threatened preterm labor.
The strength of our review and the validity of its findings lie in compliance with stringent criteria for performing a rigorous systematic review of predictive test accuracy. These included, among others, the use of a prospective protocol designed to address a research question; the methods used in the identification of relevant studies; no language restrictions; the strict assessment of methodological quality of included studies; the use of techniques recently recommended for meta-analysis of diagnostic and predictive tests, the investigation of sources of heterogeneity; and the quantitative summarization of the evidence. In addition, there was no evidence of publication and related biases in our review.
Only one previous systematic review evaluated the predictive accuracy of cervicovaginal fetal fibronectin for spontaneous preterm birth in multiple pregnancies. In 2003, Leitich and Kaider [Citation14] reported pooled sensitivities of 68% (95% CI, 52–83%) and 72% (95% CI, 36–100%), and pooled specificities of 55% (95% CI, 23–87%) and 63% (95% CI, 40–87%), for predicting preterm birth before 34 and 37 weeks of gestation, respectively, in asymptomatic women with multiple pregnancies and serial sampling. However, this review did not mention the number of studies and the total number of women included in the meta-analyses, did not describe methods used to assess the quality of the included studies, did not address publication and related biases, and did not appear to have made any attempts to contact original authors regarding the methods for their studies or for other additional information. In addition, no results were reported for women with multiple pregnancies and symptoms of preterm labor.
The results of this systematic review are in agreement with those obtained in meta-analyses of cervicovaginal fetal fibronectin to predict preterm birth in women with singleton pregnancies, especially in women with symptoms of preterm labor. The meta-analysis by Honest et al. [Citation13] reported that, among asymptomatic women, the best summary likelihood ratios for positive and negative test results were 4.0 and 0.78, respectively, for predicting preterm birth before 34 weeks' gestation. Among symptomatic women, the best summary likelihood ratios for positive and negative test results were 5.4 and 0.25, respectively, for predicting delivery within 7–10 days of testing. Similar results were reported in the meta-analyses by Revah et al. [Citation11], and Leitich and Kaider [Citation14].
Some potential limitations of our review must be considered. First, like any systematic review, it is limited by the quality of included studies. This concern applies even more for studies on predictive accuracy in which deficiencies in methodologic quality have an impact on estimates of test performance. The quality of the included studies was good in some areas and poor in others, allowing introduction of different types of bias. The main areas where quality was poor were in the areas of blinding of test results and reporting of withdrawals. There is evidence to suggest that these biases can lead to a significant overestimation of predictive accuracy [Citation16,Citation42,Citation43]. However, both subgroup and meta-regression analyses did not show the blinding of test results or reporting of withdrawals to significantly affect predictive performance. Second, there was considerable heterogeneity in most of the meta-analyses performed. Despite the fact that potential sources of heterogeneity were investigated with advanced statistical techniques planned a priori, we were not able to explain the heterogeneous results for the pooled estimates. In view of the lack of satisfactory explanations for heterogeneity among studies, it may be reasonable to avoid meta-analysis. However, we used a random-effects meta-regression model that provides the most useful estimate for informing practice in the presence of unexplained heterogeneity. Third, the statistical power of some of our meta-analyses was limited by the small number of studies within each subgroup and the relatively small sample size of most included studies. Taking into account all of these methodological issues, results must be interpreted with caution.
Our meta-analysis suggests that only 1.6% of women with twin pregnancies and threatened preterm labor who test negative for cervicovaginal fetal fibronectin will deliver within the next week. This finding could be clinically important because these women could be cared for at a primary care center rather than transferred to a tertiary care center. Nevertheless, more rigorous studies evaluating predictive accuracy of this test and effects of its implementation on health service costs are needed before recommending its use in women with twin pregnancies.
Cervicovaginal fetal fibronectin is commonly used in labor and delivery units to help in the management of women with symptoms of preterm labor. A recent systematic review [Citation44] including five controlled trials that randomized 474 pregnant women (23 twin pregnancies) with symptoms of preterm labor found that preterm birth before 37 weeks of gestation was significantly decreased among women with management based on knowledge of cervicovaginal fetal fibronectin results (15.6%) versus controls without such knowledge (28.6%; risk ratio 0.54; 95% CI, 0.34–0.87). Further well-designed randomized controlled trials are required to evaluate the effectiveness of management of women with multiple or twin pregnancies and threatened preterm labor based on knowledge of cervicovaginal fetal fibronectin testing results for the prevention of preterm birth.
Acknowledgements
This research was supported by the Perinatology Research Branch: Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. We are very grateful to Dr. Percy Pacora for his assistance in obtaining the articles. We would like to thank Dr. Nathan Fox for assistance in providing unpublished data from your study and for clarifications of other queries.
References
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ. Births: final data for 2006. National vital statistics reports. Hyatsville, MD: National Center for Health Statistics; 2009. Vol. 57, No 7.
- Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol 2005;105:1084–1091.
- Reynolds MA, Schieve LA, Martin JA, Jeng G, Macaluso M. Trends in multiple births conceived using assisted reproductive technology, United States, 1997–2000. Pediatrics 2003;111:1159–1162.
- Chuileannáin FN, Brennecke S. Prediction of preterm labour in multiple pregnancies. Baillieres Clin Obstet Gynaecol 1998;12:53–66.
- Feinberg RF, Kliman HJ, Lockwood CJ. Is oncofetal fibronectin a trophoblast glue for human implantation? Am J Pathol 1991;138:537–543.
- Ascarelli MH, Morrison JC. Use of fetal fibronectin in clinical practice. Obstet Gynecol Surv 1997;52:S1–S12.
- Lockwood CJ, Senyei AE, Dische MR, Casal D, Shah KD, Thung SN, Jones L, Deligdisch L, Garite TJ. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669–674.
- Chien PF, Khan KS, Ogston S, Owen P. The diagnostic accuracy of cervico-vaginal fetal fibronectin in predicting preterm delivery: an overview. Br J Obstet Gynaecol 1997;104:436–444.
- Faron G, Boulvain M, Irion O, Bernard PM, Fraser WD. Prediction of preterm delivery by fetal fibronectin: a meta-analysis. Obstet Gynecol 1998;92:153–158.
- Revah A, Hannah ME, Sue-A-Quan AK. Fetal fibronectin as a predictor of preterm birth: an overview. Am J Perinatol 1998;15:613–621.
- Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol 1999;180:1169–1176.
- Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. Br Med J 2002;325:301.
- Leitich H, Kaider A. Fetal fibronectin – how useful is it in the prediction of preterm birth? BJOG 2003;110:66–70.
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149: 889–897.
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JHP, Bossuyt PM. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282: 1061–1066.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 2005;5:19.
- Sankey S, Weisfiels L, Fine M, Kapoor W. An assessment of the use of the continuity correction for sparse data in meta-analysis. Commun Stat Simul Comput 1996;25:1031–1056.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–990.
- Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002;21:1237–1256.
- Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med 2008;27: 687–697.
- Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994;271:703–707.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–560.
- Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525–1537.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analyses detected by a simple graphical test. Br Med J 1997;315:629–634.
- Tolino A, Ronsini S, Zullo F, Pellicano M, Regine V, Nappi C. Fetal fibronectin as a screening test for premature delivery in multiple pregnancies. Int J Gynaecol Obstet 1996;52:3–7.
- Morrison JC, Naef RW III, Botti JJ, Katz M, Belluomini JM, McLaughlin BN. Prediction of spontaneous preterm birth by fetal fibronectin and uterine activity. Obstet Gynecol 1996;87:649–655.
- Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S, Mercer BM, Meis PJ, Moawad AH, Das A, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol 1996;175:1047–1053.
- Wennerholm UB, Holm B, Mattsby-Baltzer I, Nielsen T, Platz-Christensen J, Sundell G, Hosseini N, Hagberg H. Fetal fibronectin, endotoxin, bacterial vaginosis and cervical length as predictors of preterm birth and neonatal morbidity in twin pregnancies. Br J Obstet Gynaecol 1997;104:1398–1404.
- Oliveira T, de Souza E, Mariani-Neto C, Camano L. Fetal fibronectin as a predictor of preterm delivery in twin gestations. Int J Gynaecol Obstet 1998;62:135–139.
- Ramirez M, Turrentine M. Comparison of fetal fibronectin and home uterine monitoring as predictors of preterm delivery in twin gestations. Am J Obstet Gynecol 1999;180S:104S.
- McMahon KS, Neerhof MG, Haney EI, Thomas HA, Silver RK, Peaceman AM. Prematurity in multiple gestations: identification of patients who are at low risk. Am J Obstet Gynecol 2002;186:1137–1141.
- Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol 2004;23:561–566.
- Ruiz RJ, Fullerton J, Brown CE. The utility of fFN for the prediction of preterm birth in twin gestations. J Obstet Gynecol Neonatal Nurs 2004;33:446–454.
- Roman AS, Koklanaris N, Paidas MJ, Mulholland J, Levitz M, Rebarber A. “Blind” vaginal fetal fibronectin as a predictor of spontaneous preterm delivery. Obstet Gynecol 2005;105:285–289.
- Fox NS, Saltzman DH, Klauser CK, Peress D, Gutierrez CV, Rebarber A. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies with the use of combined fetal fibronectin and cervical length. Am J Obstet Gynecol 2009;201:313.e1–e5.
- Peaceman AM, Andrews WW, Thorp JM, Cliver SP, Lukes A, Iams JD, Coultrip L, Eriksen N, Holbrook RH, Elliott J, et al. Fetal fibronectin as a predictor of preterm birth in patients with symptoms: a multicenter trial. Am J Obstet Gynecol 1997;177:13–18.
- Terrone DA, Rinehart BK, Kraeden U, Morrison JC. Fetal fibronectin in symptomatic twin gestations. Prim Care Update Ob Gyns 1998;5:179.
- Gonzalez N, Bige V, Kandoussi S, Graesslin O, Quereux C, Gabriel R. Ultrasonographic measurement of cervical length in twin pregnancies with preterm labor: comparison with singleton pregnancies [In French]. Gynecol Obstet Fertil 2004;32:122–127.
- Singer E, Pilpel S, Bsat F, Plevyak M, Healy A, Markenson G. Accuracy of fetal fibronectin to predict preterm birth in twin gestations with symptoms of labor. Obstet Gynecol 2007;109:1083–1087.
- Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 2004;140:189–202.
- Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174:469–476.
- Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev 2008;4:CD006843.