Abstract
Objective: To determine whether maternal plasma concentrations of placental growth factor (PlGF), soluble endoglin (sEng), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and -2 could identify patients at risk for developing preeclampsia (PE) requiring preterm delivery. Study design: Patients presenting with the diagnosis “rule out PE” to the obstetrical triage area of our hospital at <37 weeks of gestation (n=87) were included in this study. Delivery outcomes were used to classify patients into four groups: I) patients without PE or those with gestational hypertension (GHTN) or chronic hypertension (CHTN) who subsequently developed PE at term (n = 19); II): mild PE who delivered at term (n = 15); III): mild disease (mild PE, GHTN, CHTN) who subsequently developed severe PE requiring preterm delivery (n = 26); and IV): diagnosis of severe PE (n = 27). Plasma concentrations of PlGF, sEng, sVEGFR-1 and -2 were determined at the time of presentation by ELISA. Reference ranges for analytes were constructed by quantile regression in our laboratory (n = 180; 1046 samples). Comparisons among groups were performed using multiples of the median (MoM) and parametric statistics after log transformation. Receiver operating characteristic curves, logistic regression and survival analysis were employed for analysis. Results: The mean MoM plasma concentration of PlGF/sVEGFR-1, PlGF/sEng, PlGF, sVEGFR-1 and -2, and sEng in Group III was significantly different from Group II (all p < 0.05). A plasma concentration of PlGF/sVEGFR-1 ≤ 0.05 MoM or PlGF/sEng ≤0.07 MoM had the highest likelihood ratio of a positive test (8.3, 95% CI 2.8–25 and 8.6, 95% CI 2.9–25, respectively), while that of PlGF ≤0.396 MoM had the lowest likelihood ratio of a negative test (0.08, 95% CI 0.03–0.25). The association between low plasma concentrations of PlGF/sVEGFR-1 (≤0.05 MoM) as well as that of PlGF/sEng (≤0.07 MoM) and the development of severe PE remained significant after adjusting for gestational age at presentation, average systolic and diastolic blood pressure, and a history of chronic hypertension [adjusted odds ratio (OR) = 27 (95% CI 6.4–109) and adjusted OR 30 (95% CI 6.9–126), respectively]. Among patients who presented <34 weeks gestation (n = 59), a plasma concentration of PlGF/sVEGFR-1 < 0.033 MoM identified patients who delivered within 2 weeks because of PE with a sensitivity of 93% (25/27) and a specificity of 78% (25/32). This cut-off was associated with a shorter interval-to-delivery due to PE [hazard ratio = 6 (95% CI 2.5–14.6)]. Conclusions: Plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in the obstetrical triage area. These observations support the value of these biomarkers in the clinical setting for the identification of the patient at risk for disease progression requiring preterm delivery.
Introduction
Preeclampsia (PE) remains one of the leading causes of maternal mortality/morbidity worldwide [Citation1–9]. Neonates born to mothers with PE are at risk for complications because of prematurity and small-for-gestational age [Citation10–13]. Moreover, recent studies also suggest that patients with PE are at risk for death from cardiovascular disease and stroke later in life [Citation14–19]. The only effective treatment for PE is delivery. Several preventive measures such as supplementation with vitamin C and vitamin E [Citation20–26], aspirin [Citation27–34], or calcium [Citation35,Citation36] also fail to consistently prevent subsequent development of PE.
One in 10 pregnant women develops some signs and symptoms observed in PE, and only about 20% of such patients are eventually diagnosed to have PE [Citation37–39]. Women with signs and symptoms observed in PE (e.g. headache, abdominal pain, edema, etc.) are usually referred to an obstetrical triage area where they undergo evaluation for maternal and fetal involvement. The standard work-up includes blood pressure determination, urine analysis for protein and the determination for uric acid, platelet counts and liver enzymes in peripheral blood [Citation39–42]. However, the diagnostic performance of these tests is controversial and several studies report poor performance in the prediction of PE in women with suspected gestational hypertension [Citation43–45]. Furthermore, many symptoms associated with PE, such as headache, epigastric pain and visual disorders, are subjective and non-specific. Consequently, many patients with signs and symptoms observed in PE are hospitalized for observation. Those who are diagnosed with PE at preterm gestations undergo long-term hospitalization or frequent monitoring as outpatients (in some cases of mild PE), if they qualify for expectant management. The lack of adequate biomarkers to predict disease progression and/or adverse maternal/perinatal outcomes [Citation10,Citation46] makes clinical decision-making a challenge for physicians (e.g. hospitalization, intensive surveillance, repeated blood test, induction of labor, etc.). This clinical uncertainty results in excessive utilization of the laboratory, ultrasound, antepartum testing, etc. and is a cause for additional charges to the health care system [Citation47–51]. Therefore, a new set of tests with a better prognostic performance in the identification of patients who will develop severe PE requiring preterm delivery or those who will develop maternal/fetal complications is highly desirable and urgently needed.
A test with a low false positive rate has the potential to reduce the costs of intensive monitoring of these patients to avoid unnecessary hospitalization, whereas that with a low false negative rate can potentially decreases the likelihood of complications. Early diagnosis may allow prevention of the complications of PE by timely delivery [Citation52].
A growing body of evidence suggests that an imbalance of angiogenic/anti-angiogenic factors is involved in the pathophysiology of PE [Citation53–75]. The changes in concentrations of the angiogenic factor placental growth factor (PlGF), and the anti-angiogenic factors soluble vascular endothelial growth factor receptor (sVEGFR)-1 and -2 as well as soluble endoglin (sEng) in maternal circulation precede the manifestations of disease with a higher magnitude in preterm than in term PE [Citation55,Citation57,Citation76–80]. Most studies examining the value of these biomarkers have focused on the prediction of disease in the first and second trimesters. The results of such studies largely suggest that an imbalance between angiogenic and anti-angiogenic factors increases the likelihood of preterm PE, but not of term PE [Citation65,Citation75,Citation78,Citation81–84]. However, not all studies have uniform results [Citation85,Citation86]. Yet, even if these biomarkers had excellent predictive values for preterm PE, a major issue is that there is not a clear intervention that can prevent the subsequent development of the disease. Thus, the value of such biomarkers remains either a “research procedure” or a method of risk assessment to identify patients who may benefit from more intensive surveillance[Citation75,Citation78,Citation81–86]. As of yet, there are no studies evaluating the role of plasma angiogenic and anti-angiogenic factors in predicting the outcome of patients with suspected PE admitted to the obstetrical triage area.
The objective of this study was to examine whether plasma concentrations of angiogenic/anti-angiogenic factors in patients presenting with the diagnosis “rule out PE” to the obstetrical triage area with preterm gestations have prognostic value in the identification of patients who would require preterm delivery because of PE.
Materials and methods
Study design
A retrospective cohort study was conducted by searching our clinical database and bank of biologic samples. Patients who were sent to the obstetrical triage area for the diagnosis of “suspected PE” and had blood samples taken prior to medications between 20-36 weeks of gestation were included (n = 87). Exclusion criteria were: 1) known major fetal or chromosomal anomaly; and 2) multiple gestations.
Delivery outcomes were reviewed and used to retrospectively classify the patients into four groups: Group I: patients without PE or those with gestational hypertension or chronic hypertension who subsequently developed mild PE at ≥37 weeks (n = 19); Group II: mild PE or those with gestational hypertension or chronic hypertension who developed mild PE before 37 weeks and either remained stable until term or delivered preterm spontaneously (n = 15); Group III: mild disease (mild PE, gestational hypertension, chronic hypertension) who subsequently developed severe PE requiring preterm delivery (n = 26); and Group IV: severe PE at admission (n = 27).
Reference ranges for sVEGFR-1, sEng, PlGF, sVEGFR-2, PlGF/sVEGFR-1 and PlGF/sEng were constructed from a separate set of uncomplicated pregnant women (n = 180) who were enrolled in a longitudinal study and delivered at term. Pregnancies were considered to be “uncomplicated” if patients did not have major obstetrical, medical or surgical complications during pregnancy, and delivered a term neonate whose birth weight was between the 10th-90th percentile using the reference range of Alexander et al. [Citation87].
All women were enrolled at Hutzel Women’s Hospital, Detroit, MI and followed until delivery. All patients provided written informed consent for the collection and use of samples for research purposes under the protocols approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS).
Clinical definitions
PE was defined as the new onset of hypertension that developed after 20 weeks of gestation and proteinuria [Citation40]. Hypertension was defined as systolic ≥140 or diastolic blood pressure ≥90 mm Hg, measured at two occasions, 4 h–1 week apart. Proteinuria was defined as a urine protein of ≥300 mg in a 24-h urine collection, or two random urine specimens obtained 4 h–1 week apart containing ≥1+ by dipstick or one dipstick demonstrating ≥2+ protein [Citation88]. Gestational hypertension was defined as hypertension without proteinuria after 20 weeks of gestation. Chronic hypertension was defined as women with hypertension before 20 weeks of gestation or those who reported a history of hypertension. Chronic hypertension with superimposed PE was diagnosed in women with a diagnosis of chronic hypertension with a sudden increase in blood pressure or proteinuria [Citation40].
Severe hypertension was defined as systolic blood pressure of at least 160 and/or diastolic blood pressure of at least 110 mm Hg. Severe proteinuria was diagnosed by a 24-hour urine sample containing 5 g protein or two random urine specimens with 3+ protein by dipstick. Severe PE was defined as severe hypertension with proteinuria, mild hypertension with severe proteinuria or mild PE with one of the following [Citation40]: 1) development of neurological symptoms (severe headache, scotoma, epigastric pain); 2) oliguria (<500 mL/24 hours); 3) pulmonary edema; 4) thrombocytopenia (platelet counts <100 000/mm3 in the absence of other known causes); 5) evidence of hepatic dysfunction (increased aspartate aminotransferase level of 70 IU/L and/or increased alanine aminotransferase level of 70 IU/L); 6) HELLP (hemolysis, elevated liver enzymes, low platelet) syndrome; 7) small-for-gestational age fetuses which was defined as estimated fetal weight of <10th percentile for gestational age confirmed by neonatal birth weight; or 8) eclampsia. The diagnosis of HELLP syndrome required the presence of thrombocytopenia, evidence of hepatic dysfunction and hemolysis (lactate dehydrogenase >600 IU/L or the presence of schistocytes on the peripheral blood smear).
Sample collection and immunoassays
Venipuncture was performed on admission to the triage area and the blood was collected into tubes containing EDTA. Samples were centrifuged and stored at −70°C. Maternal plasma concentrations of PlGF, sVEGFR-2, sEng and sVEGFR-1 were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). All immunoassays utilized the quantitative sandwich enzyme immunoassay technique, and their concentrations in maternal plasma were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation (CV) obtained were as follows: PlGF, 6.02 and 4.8%, respectively; sVEGFR-2, 2 and 4%, respectively; sEng, 2.3 and 4.6%, respectively; and sVEGFR-1, 1.4 and 3.9%, respectively. The sensitivity of the assays were as follows: PlGF, 9.52 pg/ml; sVEGFR-2, 19.01 pg/ml; sEng, 0.08 ng/ml and sVEGFR-1, 16.97 pg/ml. The validation of these assays has been described in previous studies [Citation89].
Statistical analysis
Reference range for PlGF, sEng, sVEGFR-1 and sVEGFR-2 using the quantile regression model
Data were log transformed to achieve normality. A quantile regression model [Citation90] was used to estimate the q-th conditional quantile function of the response (log analyte concentration), given the covariate (gestational age). To allow for a non-linear relation between the analyte level and gestational age, a moving window approach was used by iteratively fitting one model for every discrete gestational age encountered in the dataset. At each iteration, only samples having the exact same gestational age were assigned a full weight of 1.0, whereas data points further away (in either direction) received smaller weights decreasing as dictated by a Gaussian distribution. The processes were repeated for each of the quantile of interest: 0.025, 0.05, 0.5, 0.95, and 0.975, hence constructing a 95% and 90% confidence interval around the median. When the concentration of an analyte fell below the limit of detection of the assay, the concentration was scored to correspond to 99% of the lowest detected concentrations in all samples. This was done to avoid dealing with zero values.
Analysis of the data
Comparisons among groups were performed using multiples of the median (MoM) derived from the observed over the expected median of the analyte concentration at each gestational age. After logarithmic transformation, analysis of variance (ANOVA) and post-hoc tests with Bonferroni correction for multiple comparisons were performed. The contingency table and χ2 tests were used to compare the proportion among and between groups. Receiver operating characteristic (ROC) curves were used to determine the cut-offs at which each biomarker best identified the outcomes (i.e. severe PE and delivery within 14 days). Multiple logistic regression (backward-stepwise) was applied to estimate the association between angiogenic/anti-angiogenic factor concentrations and these outcomes, while adjusting for potential confounders (gestational age at presentation, average systolic and diastolic blood pressure measured in the obstetrical triage area, and a history of chronic hypertension). Survival analysis and Cox proportional hazard models were utilized to examine the relationship between sampling-to-delivery interval and plasma MoM concentrations of PlGF/sVEGFR-1 while adjusting for the above-mentioned potential confounders. Patients who delivered preterm due to causes other than PE had the interval between triage and delivery treated as a censored observation. Analysis was conducted with SPSS V.15 (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered significant.
Results
Demographic and clinical characteristic of the study groups
The demographic, clinical and obstetrical characteristics of the study population are presented in and . There was no significant difference in the mean gestational age at presentation or at blood sampling among groups (ANOVA p = 0.2). The severe PE group (Group IV) had the highest mean systolic and diastolic blood pressure among groups (ANOVA p < 0.001). There was no significant difference in the mean systolic and diastolic blood pressure between patients with mild PE who delivered at term (Group II) and those who subsequently developed severe PE and delivered preterm (Group III) (). Fourteen patients (73.7%) in Group I delivered at term, whereas the other five delivered preterm because of: spontaneous preterm labor/delivery (n = 3), chronic hypertension with oligohydramnios (n = 1) and gestational hypertension with non-reassuring fetal heart rate tracing (n = 1). All patients in Group II developed PE before 37 weeks. Three delivered after spontaneous preterm labor and the rest remained stable until delivery at term ().
Table I. Demographic and clinical characteristics of the study population.
Table II. Obstetrical and neonatal outcomes of the study population.
Thirteen patients had maternal complications including placental abruption (n = 5), pulmonary edema (n = 4), HELLP (n = 2) and eclampsia (n = 2). All except one were in Groups III and IV (). As expected, the mean gestational age at delivery and the mean birth weight in Groups III and IV were lower than in Groups I and II (p < 0.001).
The demographic and clinical characteristics of uncomplicated pregnant women (n=180) whose samples (n=1,046) were used to constructed reference ranges for plasma concentrations of angiogenic/anti-angiogenic factors are displayed in . The majority of patients (83%) were African-American and approximately one-third were nulliparous. Plasma samples were collected from each patient in the following eight intervals: 1) 6-9.9 weeks; 2) 10-14.9 weeks; 3) 15-19.9 weeks; 4)20-23.9 weeks; 5) 24-27.9 weeks; 6) 28-31.9 weeks; 7) 32-36.7 weeks; 8) 37 weeks of gestation or more.
Table III. Demographic and clinical characteristics of uncomplicated pregnant women whose samples were used to construct the reference range (n = 180)
Maternal plasma concentrations of angiogenic/anti-angiogenic factors are associated with the severity and the subsequent development of PE requiring preterm delivery
– display the mean plasma concentration of each analyte and their ratios in MoM units. This allows comparison among groups without adjustment for gestational age at venipuncture. The mean MoM plasma concentration of angiogenic/anti-angiogenic factors tested in this study was significantly different between patients with mild PE who subsequently developed severe PE, requiring preterm delivery (Group III), and those who remained stable until term (Group II) (each p < 0.05; ). There was no significant difference in the mean MoM plasma concentration of any angiogenic/anti-angiogenic factors tested in this study between patients with mild PE who subsequently developed severe PE requiring preterm delivery (Group III) and those presented with severe PE (Group IV) (each p > 0.05). The mean interval from diagnosis of mild PE to diagnosis of severe PE requiring preterm delivery was 17 ± 18 days (median 10 days, inter-quartile range 6–19 days).
Figure 1. Plasma concentration of sVEGFR-1 in Multiple of Median (MoM) unit. The mean MoM plasma concentration of sVEGFR-1 was significantly higher in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p = 0.002). Comparisons among groups were performed after logarithmic transformation.
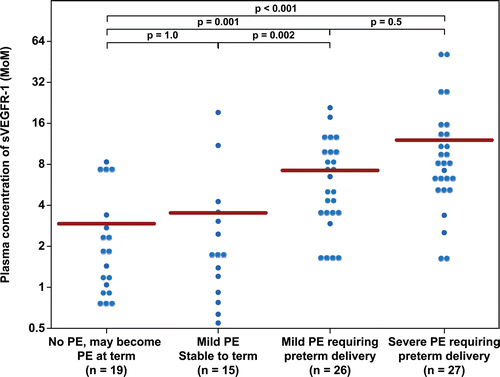
Figure 2. Plasma concentration of sEng in Multiple of Median (MoM) unit. The mean MoM plasma concentration of sEng was significantly higher in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p = 0.008). Comparisons among groups were performed after logarithmic transformation.
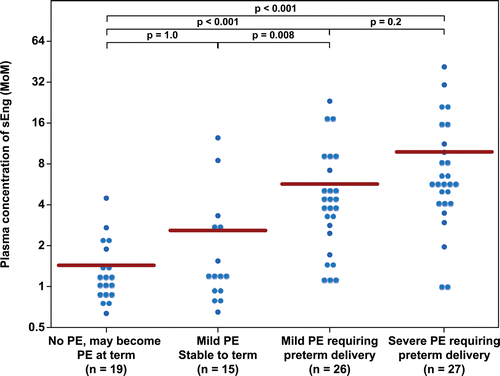
Figure 3. Plasma concentration of PlGF in Multiple of Median (MoM) unit. The mean MoM plasma concentration of PlGF was significantly lower in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p = 0.005). Comparisons among groups were performed after logarithmic transformation.
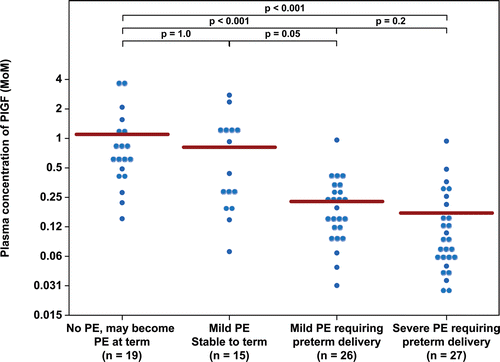
Figure 4. Plasma concentration of sVEGFR-2 in Multiple of Median (MoM) unit. The mean MoM plasma concentration of sVEGFR-2 was significantly lower in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p = 0.001). Comparisons among groups were performed after logarithmic transformation.
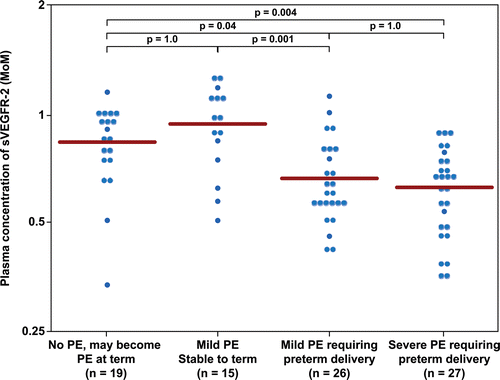
Figure 5. Plasma concentration of PlGF/sVEGFR-1 ratio in Multiple of Median (MoM) unit. The mean MoM plasma concentration of PlGF/sVEGFR-1 ratio was significantly lower in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p < 0.001). Comparisons among groups were performed after logarithmic transformation.
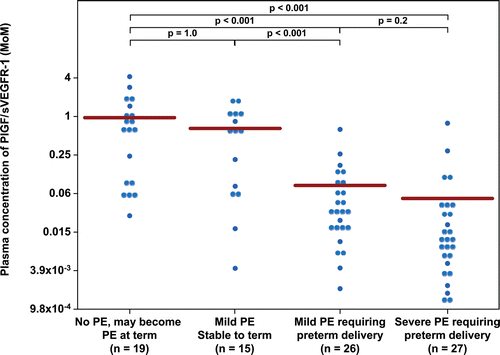
Figure 6. Plasma concentration of PlGF/sEng ratio in Multiple of Median (MoM) unit. The mean MoM plasma concentration of PlGF/sEng ratio was significantly lower in patients with mild preeclampsia who subsequently developed severe preeclampsia than those who remained stable until term (p = 0.002). Comparisons among groups were performed after logarithmic transformation.
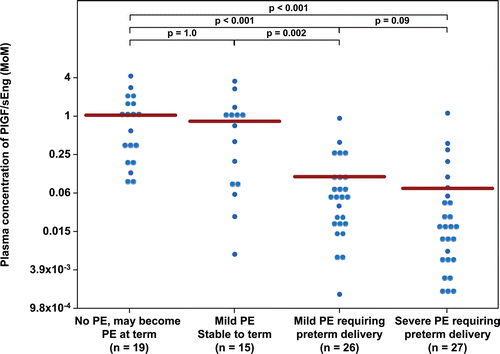
displays the MoM cut-offs (derived from ROC curves) for each angiogenic/anti-angiogenic factor and the diagnostic performance of each analyte for the identification of patients who developed severe PE (Group III +IV). A plasma concentration of PlGF/sEng ≤0.07 MoM had a sensitivity of 76% (40/53) and a specificity of 91% (31/34), while that of PlGF ≤0.396 MoM had a sensitivity of 94% (50/53) and a specificity of 71% (24/34) for the identification of patients who developed severe PE (Group III + IV). A plasma concentration of PlGF/sVEGFR-1 ≤ 0.05 MoM or PlGF/sEng ≤ 0.07 MoM had the highest likelihood ratio of a positive test (8.3, 95% CI 2.8–25 and 8.6, 95% CI 2.9–25, respectively), while that of PlGF ≤ 0.396 MoM had the lowest likelihood ratio of a negative test (0.08, 95% CI 0.03–0.25). The probability that a patient will develop severe PE after a positive test for PlGF/sVEGFR-1 ratio and the PlGF/sEng ratio was similar (93%), whereas that of a negative test for PlGF was 11% ().
Table IV. Diagnostic performance of plasma concentrations of angiogenic/anti-angiogenic factors for the identification of patients who subsequently develop severe preeclampsia (Group III and IV).
The association between low plasma concentrations of PlGF/sVEGFR-1 (≤0.05 MoM) as well as that of PlGF/sEng (≤0.07 MoM) and the development of severe PE remained significant after adjusting for gestational age at presentation, average systolic and diastolic blood pressure in the triage area, and a history of chronic hypertension [adjusted odds ratio (OR) = 27 (95% CI 6.4–109) and adjusted OR 30 (95% CI 6.9–126), respectively].
Among 42 patients who had a plasma PlGF/sVEGFR-1 concentration below 0.05 MoM, 3 patients (7%) did not subsequently develop severe PE; 2 (0.003 MoM, 0.015 MoM) from Group II and 1 (0.027 MoM) from Group I. The first patient was referred to the triage area complaining of a headache and with a new-onset hypertension (142/99, 146/100 mmHg) at 32 4/7 weeks. She was hospitalized for 3 days, and her 24-hour urine sample showed a total protein of 512 mg. Two days after discharge, she returned to the hospital with a diagnosis of spontaneous preterm labor and went on to deliver a neonate with a birth weight of 1790 g (13th percentile). The second patient had underlying chronic hypertension and diabetes class B and was referred to the obstetrical triage area at 35 6/7 weeks for borderline high blood pressure (117/71, 147/77 mmHg) and edema in the lower extremities. Her 24-hour urine collection showed a total protein of 944 mg. She developed preterm prelabor rupture of membranes the next day and subsequently delivered by C-section for non-reassuring fetal heart rate tracing. The last patient was referred to the obstetrical triage area at 33 weeks for high blood pressure (169/100, 157/87 mmHg) and a urine dip-stick was negative for protein. She delivered spontaneously the next day with an appropriate-weight-for gestational-age neonate. These three patients also had very high plasma concentrations of sEng (2.1, 7.6 and 11.2 MoM) and sVEGFR-1 (7.5, 10 and 18 MoM).
Among 45 patients who had a plasma PlGF/sVEGFR-1 concentration above 0.05 MoM, 14 patients (31%) subsequently developed severe PE; 10 from Group III and 4 from Group IV. Five of these fourteen (36%) were presented after 34 weeks of gestation and among the other 9 patients, only 2 had plasma concentrations of these biomarkers above the 5th centile (0.532 MoM and 0.579 MoM, respectively).
The first patient had an elevated blood pressure from 15 weeks of gestation onward and, thus, was diagnosed to have chronic hypertension. She was sent to the obstetrical triage area with a blood pressure of 142/87 mmHg at 27 3/7 weeks. She was hospitalized for 3 days and her 24-hour urine collection showed a total protein of 752 mg, which increased to 1280 mg in 1 month. At 36 3/7 weeks, the patient was hospitalized and diagnosed to have severe PE because of headache, high blood pressure (175/85), and an estimated fetal weight of less than the 5th percentile. A male baby, weight 2110 g (4th percentile), was delivered by Cesarean section for an arrest of dilatation after induction of labor. Another patient presented at 26 weeks with new-onset high blood pressure of 152/67 mmHg. She was hospitalized for 3 days and her 24-hour urine collection showed a total protein of 525 mg, and the estimated fetal weight was < 10th percentile. Her blood pressure was stable until 36 6/7 weeks when she underwent induction of labor for headache, excessive weight gain and intrauterine growth restriction. A female baby, weight 2390 g (6th percentile), was delivered by Cesarean section due to non-reassuring fetal heart rate tracing. The duration from blood sampling to delivery was 64 and 74 days, respectively.
display plasma concentrations of each analyte according to gestational age at presentation in each group of patients on the reference ranges derived from 180 uncomplicated pregnant women. By examining plasma angiogenic/anti-angiogenic factor concentrations in each group, the earlier the gestational age at blood sampling, the better the discrimination of patients in Groups III and IV from those in Groups I and II.
Figure 7. Plasma concentrations of sVEGFR-1 (ng/ml) in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
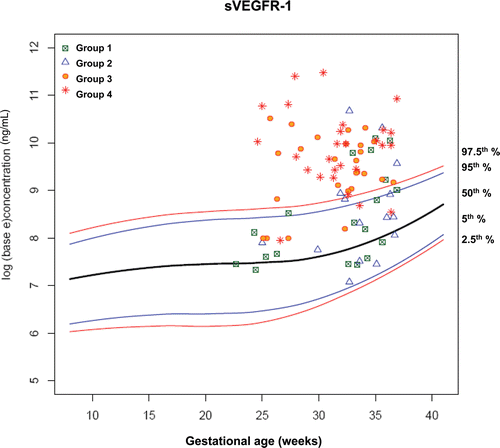
Figure 8. Plasma concentrations of sEng (ng/ml) in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
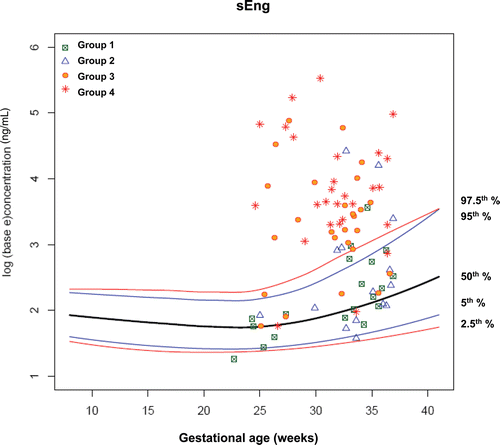
Figure 9. Plasma concentrations of PlGF (ng/ml) in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
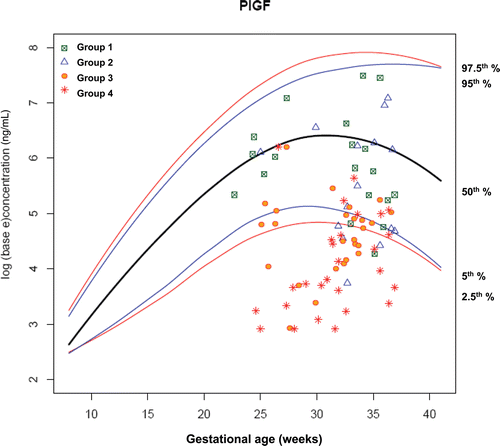
Figure 10. Plasma concentrations of sVEGFR-2 (ng/ml) in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
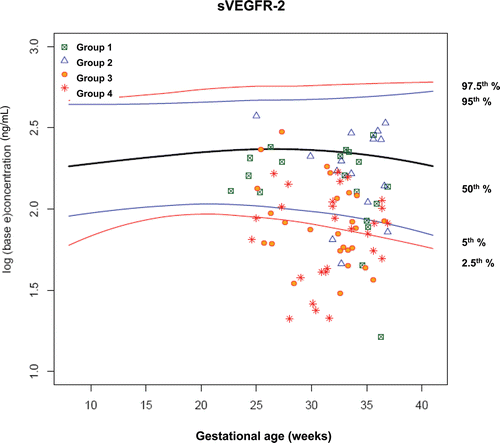
Figure 11. Plasma concentrations of PIGF/sVEGFR-1 ratio in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
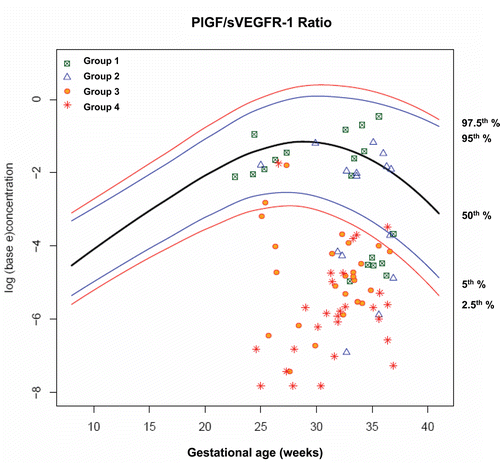
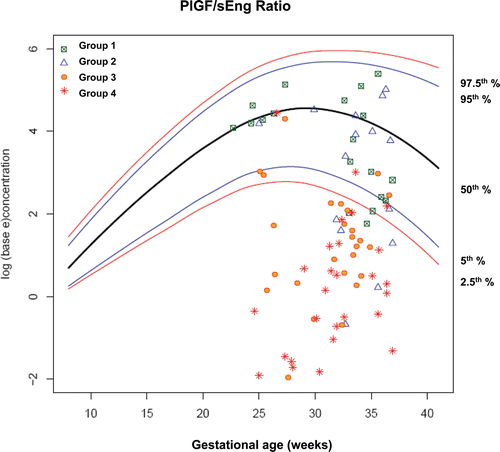
Figure 12. Plasma concentrations of PlGF/sEng ratio in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) derived from quantile regression of 1046 samples obtained from 180 uncomplicated pregnant women.
Among patients who presented to the obstetrical triage area before 34 weeks of gestation, 46% (27/59) delivered within 2 weeks due to PE. displays the MoM cut-offs (derived from ROC curves) for each angiogenic/anti-angiogenic factor and the diagnostic performance of each analyte for the identification of patients who delivered within 2 weeks because of PE. A plasma concentration of PlGF/sVEGFR-1 ≤ 0.033 MoM identified patients who delivered within 2 weeks because of PE with the largest area under the ROC curve (0.88), a sensitivity of 93% (25/27) and a specificity of 78% (25/32).
Table V. Diagnostic performance of plasma concentrations of angiogenic/anti-angiogenic factors in patients who presented at <34 weeks for the identification of those who subsequently delivered within 14 days due to preeclampsia.
The association between low plasma concentrations of PlGF/sVEGFR-1 (≤0.033 MoM) and delivery within 2 weeks due to PE remained significant after adjusting for gestational age at presentation, average systolic and diastolic blood pressure and a history of chronic hypertension [adjusted OR 45 (95% CI 8.4–236)]. This cut-off was also associated with a shorter interval-to-delivery due to PE after adjusting for the above-mentioned potential confounders [PlGF/sVEGFR-1 > 0.033 MoM: n = 27, censored 5; median survival 41 days, inter-quartile range (IQR) 22–85 days vs. PlGF/sVEGFR-1 ≤ 0.033 MoM: n = 32, censored 3, median survival 6 days, IQR 3-10 days; p < 0.001; hazard ratio = 6 (95% CI 2.5–14.6); ].
Figure 13. Survival curve of patients who had plasma concentration of PlGF/sVEGFR-1 ratio ≤ 0.033 MoM and >0.033 MoM. This cut-off was associated with a shorter interval-to-delivery due to preeclampsia [PlGF/sVEGFR-1 > 0.033 MoM: n = 27, censored 5; median survival 41 days, interquartile range (IQR) 22–85 days vs. PlGF/sVEGFR-1 ≤ 0.033 MoM: n = 32, censored 3, median survival 6 days, IQR 3-10 days; p < 0.001; hazard ratio = 6 (95% CI 2.5–14.6)].
![Figure 13. Survival curve of patients who had plasma concentration of PlGF/sVEGFR-1 ratio ≤ 0.033 MoM and >0.033 MoM. This cut-off was associated with a shorter interval-to-delivery due to preeclampsia [PlGF/sVEGFR-1 > 0.033 MoM: n = 27, censored 5; median survival 41 days, interquartile range (IQR) 22–85 days vs. PlGF/sVEGFR-1 ≤ 0.033 MoM: n = 32, censored 3, median survival 6 days, IQR 3-10 days; p < 0.001; hazard ratio = 6 (95% CI 2.5–14.6)].](/cms/asset/5fd0f620-828f-4e7b-a21a-0250bc523c92/ijmf_a_589932_f0013_b.gif)
Development of grading criteria using plasma concentrations of angiogenic/anti-angiogenic factors
Upon examination of the distribution of PlGF/sVEGFR-1 and PlGF/sEng in each group, plasma concentrations of these biomarkers between patients in Groups I+II and those in Groups III+IV were overlapping in the middle part of the scattergram. Thus, the decision was made to divide these biomarkers into 3 zones including: Zone 1, which included the majority of patients in Groups I and II; Zone 2, the overlapping zone; and Zone 3, which included the majority of patients in Groups III and IV.
The cut-off points for Zone 3 (PlGF/sVEGFR-1 ≤ 0.05 MoM or PlGF/sEng ≤ 0.07 MoM) were derived from the ROC curves for the identification of patients in Groups III and IV (see ), because these cut-offs had a high specificity (91%) for severe PE in preterm gestations. The cut-off points for Zone 1 (PlGF/sVEGFR-1 ≥ 0.35 MoM or PlGF/sEng ≥ 0.30 MoM) was derived from the ROC curves for the identification of patients in Groups I and II before 34 weeks of gestation because these cut-offs had a high specificity (95%) for patients without severe PE. Plasma concentrations of PlGF/sVEGFR-1 ≥ 0.35 (rounded up from 0.3602 MoM which was derived from the ROC curve) and PlGF/sEng ≥ 0.30 (rounded up from 0.3008 MoM which was derived from the ROC curve) had a similar sensitivity of 78% (14/18) and a similar specificity of 95% (39/41) for the identification of patients in Groups I and II ( and ).
Figure 14. Plasma concentrations of PIGF/sVEGFR-1 ratio in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) and the cut-offs (dash line) according to the 3-zone classification.
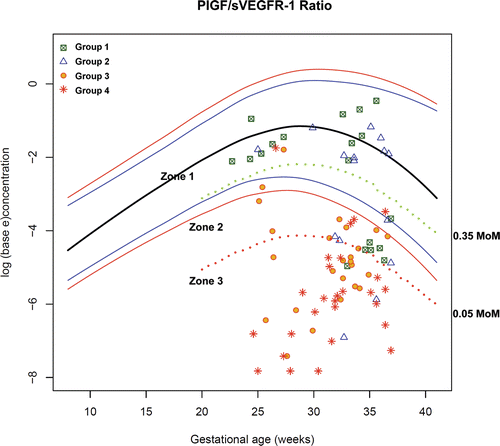
Figure 15. Plasma concentrations of PlGF/sEng ratio in patients from each study group plotted against a reference range (2.5th, 5th, 50th, 95th, and 97.5th percentile) and the cut-offs (dash line) according to the 3-zone classification.
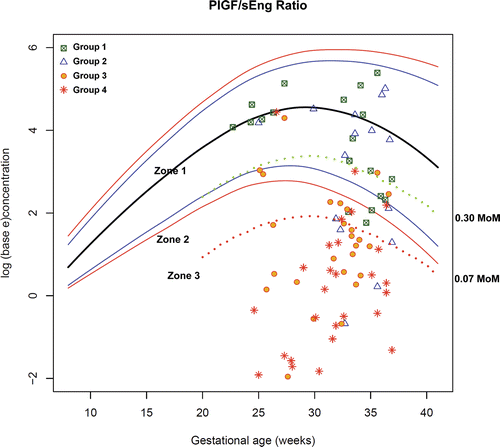
displays the rate of preterm delivery of patients presenting to the obstetrical triage area before 34 weeks with a diagnosis of “suspected preeclampsia” according to the proposed 3-zone classification of plasma MoM concentrations of PlGF/sVEGFR-1 and PlGF/sEng ratio. Among patients suspected to have PE and presented to the obstetrical triage area before 34 weeks with plasma concentrations of PlGF/sVEGFR-1 ≤ 0.05 MoM or PlGF/sEng ≤ 0.07 MoM (Zone 3), the rate of preterm delivery before 34 weeks was 79% (26/33). In contrast, among patients with plasma concentrations of PlGF/sVEGFR-1 ≥ 0.35 MoM (Zone 1), the rate of preterm delivery before 34 weeks was 6.3% (1/16). The only patient who delivered before 34 weeks in this group was referred to the obstetrical triage area for a headache, vaginal bleeding and high blood pressure (146/78 mmHg) at 33 3/7 weeks. She had a plasma PlGF/sVEGFR-1 concentration of 0.828 MoM and subsequently delivered spontaneously the next day. The rate of preterm delivery before 34 weeks in Zone 2 was 30% (3/10). The rates of preterm delivery (before 34 weeks, within 7 or 14 days) were correlated with the proposed 3-zone classification of plasma concentrations of PlGF/sVEGFR-1 and PlGF/sEng ratio (each p < 0.001; χ2 for trend ).
Table VI. Rate of preterm delivery in patients presenting to the obstetrical triage area at <34 weeks with a diagnosis of “suspected preeclampsia” according to the 3-zone classification of plasma angiogenic/anti-angiogenic factor concentrations.
displays the disposition of patients with “suspected PE” before 34 weeks of gestation from the obstetrical triage area according to the proposed 3-zone classification of plasma angiogenic/anti-angiogenic factor concentrations. Half of the patients (8/16) who were hospitalized had plasma PlGF/sVEGFR-1 ≥ 0.35 MoM or plasma PlGF/sEng ≥ 0.30 MoM (Zone I). Six of these were in Groups I and II, while the other two were in Groups III and IV. However, these two patients were stable until delivery at 36 4/7 and 37 weeks or 64 and 74 days after venipuncture (mentioned above).
Table VII. Disposition of patients with “suspected preeclampsia” before 34 weeks of gestation from the triage area according to the 3-zone classification of plasma angiogenic/anti-angiogenic factor concentrations.
Two patients were discharged from the obstetrical triage area although plasma concentrations of PlGF/sVEGFR-1 or PlGF/sEng were in Zone 3. The first patient was referred to the obstetrical triage area at 28 3/7 weeks because of an elevated blood pressure. Her blood pressure in the obstetrical triage area was 139–153/70–96 mmHg and proteinuria of 1+. She was discharged and the total protein in 24-hour urine sample was later found to be 1958 mg. She was induced 1 week later for worsening maternal disease (high blood pressure and central nervous system symptoms). Another patient was referred to the obstetrical triage area for headache and elevated blood pressure at 26 3/7 weeks. However, her blood pressure in the triage was 132/87 mmHg with a urine protein of 1+ and she was discharged. The total protein in her 24-hour urine sample was later found to be 1855 mg. This patient developed HELLP syndrome at 32 3/7 weeks.
displays obstetrical characteristics and maternal plasma MoM concentrations of PlGF/sVEGFR-1 as well as PlGF/sEng of 13 patients who had maternal complications. Ten patients (4 with abruptio placentae, 2 with HELLP syndrome, 3 with pulmonary edema and 1 with eclampsia) had plasma PlGF/sVEGFR-1 in Zone 3. Two patients were in Zone 2 (eclampsia and pulmonary edema) and one patient was in Zone 1 (gestational hypertension with placental abruption). This last patient had the longest interval from blood sampling to delivery (85 days).
Table VIII. Obstetrical characteristics and plasma concentrations of PlGF/sVEGFR-1 and PlGF/sEng ratio in patients with severe maternal complications.
Discussion
Principal findings of this study
1) Patients who presented to the obstetrical triage area with a diagnosis of “suspected PE” and subsequently were diagnosed with mild PE or severe PE requiring preterm delivery had significantly different mean plasma MoM concentrations of angiogenic/anti-angiogenic factors from those who did not require delivery until term; 2) plasma concentrations of sEng, PlGF, PlGF/sEng and PlGF/sVEGFR-1 performed very well in the identification of patients who developed severe PE requiring preterm delivery (area under the ROC curve of 87–90%); 3) among patients who presented before 34 weeks gestation, a plasma concentration of PlGF/sVEGFR-1 of ≤ 0.033 MoM was associated with a shorter interval-to-delivery than those above the cutoff (PlGF/sVEGFR-1 of >0.033); 4) this biomarker was useful to identify patients who required delivery within 2 weeks; 5) plasma concentrations of the PlGF/sVEGFR-1 ratio or the PlGF/sEng ratio may assist clinicians in the management and disposition of patients suspected to have PE who present to the hospital with the suspected diagnosis of PE; and 6) we propose that the ratios reported herein are of clinical value in obstetrical practice.
The clinical challenge of evaluating the patient with suspected PE
Preeclampsia/eclampsia are, generally, retrospective diagnoses which can be confidently made after delivery. Some patients will present with mild gestational hypertension, and it is not possible to ascertain whether they will remain stable or progress to have proteinuria and other signs of multiple organ involvement. This is the rationale for obtaining baseline protein determination and other tests, such as a platelet count, liver function test, etc. Yet, these tests are valuable if an abnormality is detected (e.g. thrombocytopenia, abnormal liver function test, etc.). It is unclear whether such tests (when results are within the normal range) have prognostic value to identify the patient with worsening disease who will require a preterm delivery for maternal or fetal indications.
The plasma concentrations of angiogenic and anti-angiogenic factors have been shown to be altered in patients with preterm PE and the abnormalities in the absolute concentrations of these biomarkers, and their ratios have been shown to precede the clinical diagnosis of PE by several weeks (see below for details). This set of observations was the basis for the hypothesis tested in this study; namely, that the concentration of these biomarkers in peripheral blood would identify the patient in whom the diagnosis of PE is uncertain when she is admitted to the obstetrical triage area. Therefore, we tested whether the biomarkers and their ratios could predict preterm delivery for maternal or fetal indications.
The rationale for the study design
We divided patients into four groups based on clinical severity of PE and gestational age at delivery (term or preterm). Severity was chosen because morbidity/mortality of mothers with PE increases as a function of these criteria [Citation91–94]. Preterm delivery was chosen because neonatal morbidity/mortality is largely dependent on gestational age at delivery [Citation11–13,Citation95]. These assumptions were confirmed because all patients except one (who had severe maternal complications) belonged to Groups III (mild PE who required preterm delivery) and IV (severe PE).
We found that plasma concentrations of angiogenic/anti-angiogenic factors in the obstetrical triage area are of prognostic value for the identification of patients who developed severe PE or those who required delivery within 2 weeks because of worsening PE. Such observations are consistent with results of previous studies reported by our group using a cross-sectional approach [Citation56]. We reported that plasma concentrations of sVEGFR-1 at the time of diagnosis of PE are positively correlated with the clinical severity of PE [Citation56], the degree of proteinuria [Citation56] and abnormalities of uterine artery and umbilical artery Doppler velocimetries [Citation96,Citation97]. Moreover, we have also found a negative correlation between the maternal plasma concentration of sVEGFR-1 and gestational age at diagnosis of PE, platelet count, neonatal birth weight, adjusted neonatal birth weight for gestational age, as well as gestational age at delivery [Citation56]. Similar findings were demonstrated for maternal plasma PlGF [Citation80,Citation98,Citation99], maternal plasma sEng [Citation100,Citation101] and their ratio [Citation54,Citation102–105].
Several investigators have reported that plasma concentrations of angiogenic/anti-angiogenic factors in PE change prior to the clinical manifestation of the disease [Citation55,Citation57,Citation65,Citation68,Citation71,Citation74,Citation75,Citation78,Citation82,Citation83,Citation106–108]. An elevation of plasma sVEGFR-1 concentration began 6–10 weeks prior to the clinical manifestations, and the increase was more pronounced 2–5 weeks before the clinical diagnosis [Citation55,Citation76]. Similarly, a lower plasma PlGF/sEng ratio in PE than women with normal pregnancies was observed 20 weeks before the clinical diagnosis of preterm PE and 10 weeks before the diagnosis of term PE [Citation65].
Patients who developed preterm and term PE had a significantly lower plasma PlGF/sVEGFR-1 ratio 20 and 14 weeks before clinical diagnosis [Citation55,Citation57,Citation65]. Since plasma concentrations of angiogenic/anti-angiogenic factors in PE change several weeks prior to the clinical diagnosis, we reasoned that determination of these biomarkers in the obstetrical triage area could be of value in the assessment of progression of disease (prognosis) or clinical severity, which could be overlooked by traditional clinical criteria. The scientific basis and rationale for the selection of criteria for severity of PE is not clearly established, and the possibility that biomarkers (such as the plasma concentration of angiogenic/anti-angiogenic markers) may be superior to traditional clinical signs or symptoms and other laboratory tests may need to be considered.
The diagnostic and prognostic properties of angiogenic/anti-angiogenic factor concentrations are in contrast to that of a spot urine protein/creatinine ratio or serum uric acid. Although severe proteinuria is often used to diagnose severe PE, many factors such as patient posture, activity and body temperature can affect the amount of protein excreted in the urine [Citation109]. Moreover, once the diagnosis of proteinuria is made, the magnitude of changes in the degree of proteinuria does not appear to alter solid clinical endpoints, although it may change the classification of the disease to mild or severe [Citation110–112].
A spot urine protein/creatinine ratio has a high sensitivity to exclude patients with PE at the time of clinical presentation, but not to make a positive diagnosis [Citation113,Citation114]. This is the reason why most clinicians prefer to quantitate protein excretion with a 24-hour urine collection. To complicate matters, different cut-off values for urine protein/creatinine ratio have been proposed, and this may reflect a high variability of protein excretion in a clinical setting [Citation109,Citation114,Citation115]. Similarly, although the mean serum uric acid concentration is higher in women with PE than in normal pregnant women, an elevation of uric acid is a poor predictor of the development of PE as well as the subsequent development of maternal and fetal complications [Citation43].
A quantile regression model was used in the current study because the distribution of angiogenic/anti-angiogenic factor concentrations, especially in the 3rd trimester, is close to, but not normally distributed even if logarithmic transformation is employed. Moreover, the standard deviation tends to increase with advancing gestational age. These findings are similar to those reported in Japanese population by Ohkuchi et al. [Citation116]. The median is a better measure of central tendency than the mean if the distribution of the data is asymmetric (i.e. not normally distributed) [Citation90]. In the current study, we used MoM cut-off instead of a particular percentile cut-off (derived from median regression analysis) because the purpose of the study was not to differentiate patients with PE from those without this disorder, but rather to identify those who were at risk for worsening disease or who would require preterm delivery. Indeed, the majority of patients with PE (who eventually had a term or preterm delivery) had plasma MoM concentrations of angiogenic/anti-angiogenic factors below the reference range of normal pregnant women.
The clinical significance of the findings of the study
In the current study, a PlGF/sVEGFR-1 ratio of ≤ 0.05 MoM and a PlGF/sEng ratio of ≤ 0.07 MoM were associated with PE requiring preterm delivery with a likelihood ratio for a positive test of 8.3 and 8.6, respectively. This association remained significant [adjusted odds ratio (OR) = 27 (95% CI 6.4–109) and adjusted OR 30 (95% CI 6.9–126), respectively] after adjustment for gestational age at presentation, average systolic and diastolic blood pressure in the obstetrical triage area and a history of chronic hypertension, parameters which are associated with the development of PE among patients with gestational hypertension [Citation43].
The soluble form of VEGFR-1 is able to bind VEGF in the circulation and decrease the amount of free VEGF, which functions to stabilize endothelial cells in mature blood vessels [Citation117–119]. sVEGFR-1 is also essential in maintaining the integrity of the basement membrane, especially in the fenestrated endothelium of the kidney, liver and brain [Citation73,Citation120–124]. In addition, the soluble form of Eng can bind to TGF-β and interfere with its nitric oxide-mediated vasodilation effect [Citation54]. Thus, it is biologically plausible that plasma concentrations of angiogenic/anti-angiogenic factors reflect the severity of the pathophysiologic state of PE and its multiple organ involvement [Citation2,Citation41,Citation42].
Although plasma concentrations of the PlGF/sVEGFR-1 ratio of ≤0.05 MoM or the PlGF/sEng ratio of ≤0.07 MoM had a high likelihood ratio for a positive test for the identification of patients in Groups III and IV, 14 false negative cases were noted and 5 of these were presented after 34 weeks of gestation. Among the other 9 patients, only 2 had plasma concentrations of these biomarkers above the 5th centile. The interval from blood sampling to delivery, however, was 64 and 74 days, respectively. Therefore, it seems that the concentrations of these angiogenic/anti-angiogenic factors are likely to perform better if the outcome was evaluated in a shorter period of time (i.e. within 4–5 weeks from first assessment) or if repeated or sequential testing was performed. Alternatively, other mechanisms of diseases may be operative in these patients [Citation125–159]. However, this requires further study.
It is noteworthy that all three false positive cases (a plasma PlGF/sVEGFR-1 ratio below 0.05 MoM in Groups I and II) were delivered spontaneously shortly after blood sampling. We have demonstrated that an imbalance of angiogenic/anti-angiogenic factor concentrations is not a unique characteristic of patients with PE since this perturbation can occur in isolated fetal growth restriction with abnormal uterine artery Doppler velocimetry [Citation96], unexplained fetal death [Citation160–163], twin-to-twin transfusion syndrome [Citation164] and viral-induced hydrops fetalis [Citation165,Citation166] or Ballantynes syndrome [Citation167]. Moreover, a subset of patients with spontaneous preterm labor with intact membranes also had an abnormal angiogenic/anti-angiogenic profile in maternal circulation prior to the diagnosis of preterm labor/delivery [Citation168]. However, the magnitude of the changes of these angiogenic/anti-angiogenic factor concentrations in patients destined to have a spontaneous preterm labor are different from those who subsequently will have a normal delivery at term; yet, the magnitude of the abnormality is milder and is detected prior to the diagnosis of preterm labor/delivery (<5 weeks for PlGF, sVEGFR-1, sVEGFR-2 and > 5 weeks for sEng) than that observed in patients with PE [Citation168]. It is possible that when the maternal angiogenic/anti-angiogenic factor ratio is very low (which reflects the condition of the placenta), pregnant women would need to deploy adaptive mechanisms which could be increasing maternal blood pressure to maintain perfusion to the fetus and placenta, or alternatively, resort to the onset of labor to allow the fetus to exit a hostile intrauterine environment. However, the precise molecular mechanisms employed under each of these circumstances remains to be elucidated.
It is noteworthy that the likelihood ratios of a positive test were higher when the ratios of angiogenic/anti-angiogenic factor concentrations were used as biomarkers for the identification of severe PE requiring preterm delivery than those of individual concentrations of angiogenic/anti-angiogenic factors. In contrast, individual angiogenic or anti-angiogenic factor concentrations seem to have a lower likelihood ratio of a negative test for the prediction of preterm delivery than the use of ratios of these factors (see for a detailed comparison).
Since plasma concentrations of a PlGF/sVEGFR-1 ratio of ≤0.05 MoM and a PlGF/sEng ratio of ≤0.07 MoM performed better in diagnosing rather than excluding patients who would develop severe PE (higher specificity than sensitivity), the decision was made to establish another cut-off point of the PlGF/sVEGFR-1 ratio of ≥0.35 MoM and the PlGF/sEng ratio of ≥0.30 MoM, which had a high specificity for the identification of patients with and without PE before 34 weeks of gestation who remained stable until term (or to exclude severe PE).
We have demonstrated that plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in identifying patients with severe PE requiring preterm delivery. Recently, automated assay systems for PlGF and sVEGFR-1 have been developed and the results could be obtained within 18 minutes [Citation169]. Moreover, studies have recently demonstrated a very high correlation between these automated assay systems and the conventional ELISA assay method used in our study [Citation116,Citation169]. Plasma sVEGFR-1, PlGF and their ratio determined by an electrochemiluminescence immunoassay correlated with clinical severity, gestational age at onset and at delivery of PE in nested case-controlled studies [Citation170,Citation171]. Such results are consistent with those found using ELISA methods. The concentrations of angiogenic/anti-angiogenic factors in maternal blood may be useful in assisting physicians in the clinical assessment of patients presenting to the obstetrical triage area before 34 weeks with a diagnosis of “suspected preeclampsia” if the results of these assays are available for clinical decision-making in the obstetrical triage area.
In our study, the rate of preterm delivery (before 34 weeks or delivery within 7 or 14 days) was correlated with the proposed 3 zone criteria of plasma MoM concentrations of the PlGF/sVEGFR-1 ratio and the PlGF/sEng ratio. When the PlGF/sVEGFR-1 ratio was ≥35% of the median (Zone 1), the rate of preterm delivery before 34 weeks was 6.3%. When this ratio was 5–35% (Zone 2) and ≤5% (Zone 3) of the median, the rate of preterm delivery was 30 and 79%, respectively.
When examining the disposition of patients with “suspected PE” before 34 weeks of gestation from the obstetrical triage area according to the proposed 3 zone criteria, these biomarkers could assist physicians or mid-level providers in triaging these patients. For example, this approach may reduce the number of hospital admissions of patients suspected to have PE when the plasma angiogenic/anti-angiogenic factor concentrations are in Zone 1. Alternatively, hospitalization may be limited to patients who are in Zone 3. The management of patients in Zone 2 remains uncertain at this point because patients in this zone may have disease progression or remain stable. It is possible that additional biomarkers will be required to assist in the assessment of these patients, or that serial tests may prove to be informative. This was not performed in this study because of its retrospective nature.
Strengths and limitations of the study
This is the first study to demonstrate an association between plasma concentrations of angiogenic/anti-angiogenic factors and the prognosis of PE in terms of progression to severe PE or preterm delivery in patients who presented to the obstetrical triage area before 34 weeks for suspected PE. Moreover, a new approach to integrate these biomarkers into clinical practice has been proposed. We believe that this simple approach may assist clinicians in managing the uncertainty presented by patients with the clinical diagnosis “rule out PE”.
All proposed cut-off values have been standardized and reported as multiples of the median. This allows independent replication of these results in other populations that may have a different median or distribution of the concentration of angiogenic/anti-angiogenic factors [Citation172,Citation173]. Limitations of the study are: 1) a high number of patients with a diagnosis of severe PE and adverse outcomes were included in this study—this may reflect a selection bias toward preferential enrollment of patients with severe disease in this retrospective cohort study; and 2) the management for PE was based on clinical findings, and most patients who were diagnosed to have severe PE, either stable or not stable, were induced at 34 weeks of gestation. This practice precludes the assessment of the natural history between the behavior of biomarkers and clinical progression or preterm delivery.
Conclusion
We propose that maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in the obstetrical triage area. These observations strengthen the clinical value of these biomarkers in obstetrics. Prospective studies are desirable to confirm the observations reported herein and to evaluate other outcomes, such as maternal and neonatal morbid events. Importantly, our findings suggest that determination of PlGF, sVEGFR-1 and sEng can have clinical value, even if there is no treatment to reverse PE at this time. Their potential value would be to improve the efficiency with which healthcare providers can manage patients at risk. This is a clinical challenge which presents itself on a daily basis in busy obstetrical units.
Acknowledgment
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Declaration of interest: Tinnakorn Chaiworapongsa is a consultant in the preeclampsia advisory board of Roche Diagnostics. This study was conducted without any support from Roche Diagnostics. The immunoassays used in this study were not acquired from Roche Diagnostics.
References
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: A systematic review. Lancet 2006;367:1066–1074.
- Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: New concepts in an old disease. Semin Perinatol 1988;12:302–323.
- Sibai BM. Hypertensive disorders of pregnancy: The United States perspective. Curr Opin Obstet Gynecol 2008;20:102–106.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113:1299–1306.
- Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol 2009;113:1075–1081.
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011 Feb 16. E-pub ahead of print PMID 21333604.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–1594.
- Roberts JM, Gammill HS. Preeclampsia: Recent insights. Hypertension 2005;46:1243–1249.
- von Dadelszen P, Menzies J, Magee LA. The complications of hypertension in pregnancy. Minerva Med 2005;96:287–302.
- Ganzevoort W, Rep A, de Vries JI, Bonsel GJ, Wolf H; PETRA-investigators. Prediction of maternal complications and adverse infant outcome at admission for temporizing management of early-onset severe hypertensive disorders of pregnancy. Am J Obstet Gynecol 2006;195:495–503.
- Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol 2006;30:16–19.
- Chappell LC, Enye S, Seed P, Briley AL, Poston L, Shennan AH. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: A prospective study. Hypertension 2008;51:1002–1009.
- Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol 2000;182:607–611.
- Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, Heydanus R, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension 2008;51:1034–1041.
- Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, van der Post JA, Porath M, Ponjee G, et al. 10-Year cardiovascular event risks for women who experienced hypertensive disorders in late pregnancy: The HyRAS study. BMC Pregnancy Childbirth 2010;10:28.
- Barden A. Pre-eclampsia: Contribution of maternal constitutional factors and the consequences for cardiovascular health. Clin Exp Pharmacol Physiol 2006;33:826–830.
- Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology 2005;16:206–215.
- Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001;323:1213–1217.
- Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–951.
- Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am J Obstet Gynecol 2011 Apr 27. E-pub ahead of print PMID 21529757.
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS; ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med 2006;354:1796–1806.
- Rumbold A, Duley L, Crowther C, Haslam R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev 2008 Jan; 23(1):CD004227.
- Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, et al.; WHO Vitamin C and Vitamin E trial group. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG 2009;116:780–788.
- Spinnato JA 2nd, Freire S, Pinto E Silva JL, Cunha Rudge MV, Martins-Costa S, Koch MA, Goco N, et al. Antioxidant therapy to prevent preeclampsia: A randomized controlled trial. Obstet Gynecol 2007;110:1311–1318.
- Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: A double-blind, placebo-controlled trial. Am J Obstet Gynecol 2005;192:520–521.
- Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, Smith G, et al.; INTAPP study group. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am J Obstet Gynecol 2010;202:239.e1–239.e10.
- Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, Paul RH, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1993;329:1213–1218.
- Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, VanDorsten P, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1998;338:701–705.
- Rotchell YE, Cruickshank JK, Gay MP, Griffiths J, Stewart A, Farrell B, Ayers S, et al. Barbados Low Dose Aspirin Study in Pregnancy (BLASP): A randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol 1998;105:286–292.
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguère Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet Gynecol 2010;116:402–414.
- Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, Barkai G, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med 1989;321:351–356.
- Imperiale TF, Petrulis AS. A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA 1991;266:260–264.
- Brennecke SP, Brown MA, Crowther CA, Hague WM, King J, McCowan L, Morris J, et al. Aspirin and prevention of preeclampsia. Position statement of the use of low-dose aspirin in pregnancy by the Australasian Society for the Study of Hypertension in Pregnancy. Aust N Z J Obstet Gynaecol 1995;35:38–41.
- Kyle PM, Buckley D, Kissane J, de Swiet M, Redman CW. The angiotensin sensitivity test and low-dose aspirin are ineffective methods to predict and prevent hypertensive disorders in nulliparous pregnancy. Am J Obstet Gynecol 1995;173:865–872.
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, DerSimonian R, et al. Trial of calcium to prevent preeclampsia. N Engl J Med 1997;337:69–76.
- Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, Robinson JS. Calcium supplementation in nulliparous women for the prevention of pregnancy-induced hypertension, preeclampsia and preterm birth: An Australian randomized trial. FRACOG and the ACT Study Group. Aust N Z J Obstet Gynaecol 1999;39:12–18.
- Bailey DJ, Walton SM. Routine investigations might be useful in pre-eclampsia, but not in gestational hypertension. Aust N Z J Obstet Gynaecol 2005;45:144–147.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998;105:1177–1184.
- Milne F, Redman C, Walker J, Baker P, Black R, Blincowe J, Cooper C, et al.; PRECOG II Group. Assessing the onset of pre-eclampsia in the hospital day unit: Summary of the pre-eclampsia guideline (PRECOG II). BMJ 2009;339:b3129.
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;77:67–75.
- Romero R, Mazor M, Lockwood CJ, Emamian M, Belanger KP, Hobbins JC, Duffy T. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am J Perinatol 1989;6:32–38.
- Romero R, Vizoso J, Emamian M, Duffy T, Riely C, Halford T, Oyarzun E, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol 1988;5:146–151.
- Anumba DO, Lincoln K, Robson SC. Predictive value of clinical and laboratory indices at first assessment in women referred with suspected gestational hypertension. Hypertens Pregnancy 2010;29:163–179.
- Bell SC, Halligan AW, Martin A, Ashmore J, Shennan AH, Lambert PC, Taylor DJ. The role of observer error in antenatal dipstick proteinuria analysis. Br J Obstet Gynaecol 1999;106:1177–1180.
- Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS; Tests in Prediction of Pre-eclampsia Severity review group. Accuracy of serum uric acid in predicting complications of pre-eclampsia: A systematic review. BJOG 2006;113:369–378.
- Menzies J, Magee LA, Macnab YC, Ansermino JM, Li J, Douglas MJ, Gruslin A, et al. Current CHS and NHBPEP criteria for severe preeclampsia do not uniformly predict adverse maternal or perinatal outcomes. Hypertens Pregnancy 2007;26:447–462.
- Gazmararian JA, Petersen R, Jamieson DJ, Schild L, Adams MM, Deshpande AD, Franks AL. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol 2002;100:94–100.
- Bacak SJ, Callaghan WM, Dietz PM, Crouse C. Pregnancy-associated hospitalizations in the United States, 1999-2000. Am J Obstet Gynecol 2005;192:592–597.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol 2008;199:133.e1–133.e8.
- Liu S, Heaman M, Sauve R, Liston R, Reyes F, Bartholomew S, Young D, Kramer MS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. An analysis of antenatal hospitalization in Canada, 1991-2003. Matern Child Health J 2007;11:181–187.
- Brooten D, Kaye J, Poutasse SM, Nixon-Jensen A, McLean H, Brooks LM, Groden S, et al. Frequency, timing, and diagnoses of antenatal hospitalizations in women with high-risk pregnancies. J Perinatol 1998;18:372–376.
- Hadker N, Garg S, Costanzo C, Miller JD, Foster T, van der Helm W, Creeden J. Financial impact of a novel pre-eclampsia diagnostic test versus standard practice: A decision-analytic modeling analysis from a UK healthcare payer perspective. J Med Econ 2010;13:728–737.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–649.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Gonçalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–7; discussion 1547.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, et al.; CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol 2000;183:1554–1557.
- Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:17–31.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–1544.
- Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998;59:1540–1548.
- Charnock-Jones DS, Sharkey AM, Boocock CA, Ahmed A, Plevin R, Ferrara N, Smith SK. Vascular endothelial growth factor receptor localization and activation in human trophoblast and choriocarcinoma cells. Biol Reprod 1994;51:524–530.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: Pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Ahmad S, Ahmed A. Antiangiogenic effect of soluble vascular endothelial growth factor receptor-1 in placental angiogenesis. Endothelium 2005;12:89–95.
- Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Gonçalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16.
- Chaiworapongsa T, Romero R, Tarca AL, Kusanovic JP, Gotsch F, Mittal P, Kim SK, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol 2010;202:550.e1–550.10.
- Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension 2007;50:35–36.
- Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol 2006;194:551–556.
- McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol 2004;191:1240–1246.
- Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004;145:4838–4845.
- Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 2003;278:12605–12608.
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004;89:770–775.
- Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–1038.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18.
- Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303–309.
- Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn 2011;31:66–74.
- Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, Hodges-Savola C. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: A prospective clinical study. Am J Obstet Gynecol 2010;202:40.e1–40.e7.
- Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, et al. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res 2007;30:151–159.
- Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol 2007;197:244.e1–244.e8.
- Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol 2008;32:732–739.
- Stepan H, Geipel A, Schwarz F, Krämer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol 2008;198:175.e1–175.e6.
- Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, Connor JM, Dobbie R. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol 2007;109:1316–1324.
- Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, Bartz J, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: Are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol 2008;199:268.e1–268.e9.
- Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, Lindheimer MD, et al.; Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS ONE 2010;5:e13263.
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997;177:1003–1010.
- Oggè G, Romero R, Kusanovic JP, Chaiworapongsa T, Dong Z, Mittal P, Vaisbuch E, et al. Serum and plasma determination of angiogenic and anti-angiogenic factors yield different results: The need for standardization in clinical practice. J Matern Fetal Neonatal Med 2010;23:820–827.
- He X, Fu B, Fung WK. Median regression for longitudinal data. Stat Med 2003;22:3655–3669.
- Yücesoy G, Ozkan S, Bodur H, Tan T, Caliskan E, Vural B, Corakçi A. Maternal and perinatal outcome in pregnancies complicated with hypertensive disorder of pregnancy: A seven year experience of a tertiary care center. Arch Gynecol Obstet 2005;273:43–49.
- von DP, Payne B, Li J, Ansermino JM, Broughton PF, Cote AM, et al. Prediction of adverse maternal outcomes in pre-eclampsia: Development and validation of the fullPIERS model. Lancet 2011;377:219–227.
- Fatemeh T, Marziyeh G, Nayereh G, Anahita G, Samira T. Maternal and perinatal outcome in nulliparious women complicated with pregnancy hypertension. J Pak Med Assoc 2010;60:707–710.
- Erdemoglu M, Kuyumcuoglu U, Kale A, Akdeniz N. Factors affecting maternal and perinatal outcomes in HELLP syndrome: Evaluation of 126 cases. Clin Exp Obstet Gynecol 2010;37:213–216.
- Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, Klebanoff M, et al.; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol 2002;186:66–71.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Than NG, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol 2010;35:155–162.
- Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mörtl M, Beckmann MW, Lang U. Angiogenic growth factor levels in maternal and fetal blood: Correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2007;29:407–413.
- Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255–259.
- Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat Diagn 2009;29:464–470.
- Maynard SE, Moore Simas TA, Bur L, Crawford SL, Solitro MJ, Meyer BA. Soluble endoglin for the prediction of preeclampsia in a high risk cohort. Hypertens Pregnancy 2010;29:330–341.
- Staff AC, Harsem NK, Braekke K, Hyer M, Hoover RN, Troisi R. Maternal, gestational and neonatal characteristics and maternal angiogenic factors in normotensive pregnancies. Eur J Obstet Gynecol Reprod Biol 2009;143:29–33.
- Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol 2008;111:1403–1409.
- Troisi R, Braekke K, Harsem NK, Hyer M, Hoover RN, Staff AC. Blood pressure augmentation and maternal circulating concentrations of angiogenic factors at delivery in preeclamptic and uncomplicated pregnancies. Am J Obstet Gynecol 2008;199:653.e1–653.10.
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol 2006;108:338–344.
- Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–287.
- Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007;49:818–824.
- Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol 2007;196:239.e1–239.e6.
- Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: The need for a more pathophysiological approach. Obstet Gynecol 2010;115:365–375.
- Newman MG, Robichaux AG, Stedman CM, Jaekle RK, Fontenot MT, Dotson T, Lewis DF. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria. Am J Obstet Gynecol 2003;188:264–268.
- Thangaratinam S, Coomarasamy A, O’Mahony F, Sharp S, Zamora J, Khan KS, Ismail KM. Estimation of proteinuria as a predictor of complications of pre-eclampsia: A systematic review. BMC Med 2009;7:10.
- Schiff E, Friedman SA, Kao L, Sibai BM. The importance of urinary protein excretion during conservative management of severe preeclampsia. Am J Obstet Gynecol 1996;175:1313–1316.
- Price CP, Newall RG, Boyd JC. Use of protein: Creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin Chem 2005;51:1577–1586.
- Côté AM, Brown MA, Lam E, von Dadelszen P, Firoz T, Liston RM, Magee LA. Diagnostic accuracy of urinary spot protein: Creatinine ratio for proteinuria in hypertensive pregnant women: Systematic review. BMJ 2008;336:1003–1006.
- Lindow SW, Davey DA. The variability of urinary protein and creatinine excretion in patients with gestational proteinuric hypertension. Br J Obstet Gynaecol 1992;99:869–872.
- Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, Suzuki M. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res 2010;33:422–427.
- Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 2010;14:1857–1867.
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111:707–716.
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336–30343.
- Wang A, Rana S, Karumanchi SA. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158.
- Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, et al. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 2006;17:724–735.
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 1998;140:947–959.
- Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol 2001;159:599–608.
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 2007;49:186–193.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201.
- DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Seok KS, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–513.
- Mazaki-Tovi S, Vaisbuch E, Romero R, Kusanovic JP, Chaiworapongsa T, Kim SK, Nhan-Chang CL, et al. Maternal and neonatal circulating visfatin concentrations in patients with pre-eclampsia and a small-for-gestational age neonate. J Matern Fetal Neonatal Med 2010;23:1119–1128.
- Mazaki-Tovi S, Romero R, Kim SK, Vaisbuch E, Kusanovic JP, Erez O, Chaiworapongsa T, et al. Could alterations in maternal plasma visfatin concentration participate in the phenotype definition of preeclampsia and SGA? J Matern Fetal Neonatal Med 2010;23:857–868.
- Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med 2010;23:646–657.
- Illanes S, Parra M, Serra R, Pino K, Figueroa-Diesel H, Romero C, Arraztoa JA, et al. Increased free fetal DNA levels in early pregnancy plasma of women who subsequently develop preeclampsia and intrauterine growth restriction. Prenat Diagn 2009;29:1118–1122.
- Erez O, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Kim CJ, Chaiworapongsa T, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med 2009;22:672–687.
- Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiworapongsa T, Gotsch F, et al. Retinol binding protein 4–a novel association with early-onset preeclampsia. J Perinat Med 2010;38:129–139.
- Erez O, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Chaiworapongsa T, Than NG, et al. Maternal anti-protein Z antibodies in pregnancies complicated by pre-eclampsia, SGA and fetal death. J Matern Fetal Neonatal Med 2009;22:662–671.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat Med 2009;37:349–363.
- Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch 2008;453:387–400.
- Than NG, Romero R, Erez O, Kusanovic JP, Tarca AL, Edwin SS, Kim JS, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol 2008;60:333–345.
- Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, Than NG, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: Soluble Tie-2. J Matern Fetal Neonatal Med 2008;21:389–402.
- Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J, Edwin S, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol 2008;199:122.e1–122.e11.
- Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, et al. Adiponectin in severe preeclampsia. J Perinat Med 2007;35:503–512.
- Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, et al. CXCL10/IP-10: A missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med 2007;20:777–792.
- Kusanovic JP, Romero R, Hassan SS, Gotsch F, Edwin S, Chaiworapongsa T, Erez O, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med 2007;20:867–878.
- Erez O, Hoppensteadt D, Romero R, Espinoza J, Goncalves L, Nien JK, Kusanovic JP, et al. Preeclampsia is associated with low concentrations of protein Z. J Matern Fetal Neonatal Med 2007;20:661–667.
- Mittal P, Espinoza J, Hassan S, Kusanovic JP, Edwin SS, Nien JK, Gotsch F, et al. Placental growth hormone is increased in the maternal and fetal serum of patients with preeclampsia. J Matern Fetal Neonatal Med 2007;20:651–659.
- Ho JT, Lewis JG, O’Loughlin P, Bagley CJ, Romero R, Dekker GA, Torpy DJ. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol (Oxf) 2007;66:869–877.
- Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 2007;196:261.e1–261.e6.
- Goddard KA, Tromp G, Romero R, Olson JM, Lu Q, Xu Z, Parimi N, et al. Candidate-gene association study of mothers with pre-eclampsia, and their infants, analyzing 775 SNPs in 190 genes. Hum Hered 2007;63:1–16.
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 2006;133:751–759.
- Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, et al. Toll-like receptor 4: A potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol 2005;193:921–927.
- Bianchi DW, Romero R. Biological implications of bi-directional fetomaternal cell traffic: A summary of a National Institute of Child Health and Human Development-sponsored conference. J Matern Fetal Neonatal Med 2003;14:123–129.
- Neale D, Demasio K, Illuzi J, Chaiworapongsa T, Romero R, Mor G. Maternal serum of women with pre-eclampsia reduces trophoblast cell viability: Evidence for an increased sensitivity to Fas-mediated apoptosis. J Matern Fetal Neonatal Med 2003;13:39–44.
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002;11:362–367.
- Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol 2002;187:889–893.
- Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: A mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med 1998;7:277–286.
- Huppertz B, Frank HG, Reister F, Kingdom J, Korr H, Kaufmann P. Apoptosis cascade progresses during turnover of human trophoblast: Analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest 1999;79:1687–1702.
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet 2002;360:1215–1219.
- Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 2010;62:105–120.
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506.
- Sagol S, Ozkinay E, Ozsener S. Impaired antioxidant activity in women with pre-eclampsia. Int J Gynaecol Obstet 1999;64:121–127.
- Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–797.
- Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, Mittal P, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: Results of a longitudinal study. J Matern Fetal Neonatal Med 2010;23:1384–1399.
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, et al. Unexplained fetal death: Another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507.
- Chaiworapongsa T, Romero R, Kusanovic JP, Savasan ZA, Kim SK, Mazaki-Tovi S, Vaisbuch E, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern Fetal Neonatal Med 2010;23:794–805.
- Chaiworapongsa T, Kusanovic JP, Savasan ZA, Mazaki-Tovi S, Kim SK, Vaisbuch E, Tarca AL, et al. Fetal death: A condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med 2010;23:960–972.
- Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, et al. Twin-to-twin transfusion syndrome: An antiangiogenic state? Am J Obstet Gynecol 2008;198:382.e1–382.e8.
- Stepan H, Faber R. Cytomegalovirus-induced mirror syndrome associated with elevated levels of angiogenic factors. Obstet Gynecol 2007;109:1205–6; author reply 1206.
- Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol 2007;109:549–552.
- Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome). J Matern Fetal Neonatal Med 2006;19:607–613.
- Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, Gotsch F, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: Evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med 2009;22:1122–1139.
- Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, Fiedler GM, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 2010;43:768–770.
- Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigó J Jr. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res 2010;33:892–898.
- Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010;202:161.e1–161.e11.
- Wolf M, Shah A, Lam C, Martinez A, Smirnakis KV, Epstein FH, Taylor RN, et al. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol 2005;193:16–22.
- Mijal RS, Holzman CB, Rana S, Karumanchi SA, Wang J, Sikorskii A. Midpregnancy levels of angiogenic markers in relation to maternal characteristics. Am J Obstet Gynecol 2011;204:244.e1–244.12.
