Abstract
Objective: High mobility group box-1 (HMGB1) protein is an alarmin, a normal cell constituent, which is released into the extracellular environment upon cellular stress/damage and capable of activating inflammation and tissue repair. The receptor for advanced glycation end products (RAGE) can bind HMGB1. RAGE, in turn, can induce the production of pro-inflammatory cytokines; this may be modulated by the soluble truncated forms of RAGE, including soluble RAGE (sRAGE) and endogenous secretory RAGE (esRAGE). The objectives of this study were to determine whether: 1) clinical chorioamnionitis at term is associated with changes in amniotic fluid concentrations of HMGB1, sRAGE and esRAGE; and 2) the amniotic fluid concentration of HMGB1 changes with labor or as a function of gestational age. Methods: Amniotic fluid samples were collected from the following groups: 1) mid-trimester (n = 45); 2) term with (n = 48) and without labor (n = 22) without intra-amniotic infection; and 3) term with clinical chorioamnionitis (n = 46). Amniotic fluid concentrations of HMGB1, sRAGE and esRAGE concentrations were determined by ELISA. Results: 1) the median amniotic fluid HMGB1 concentration was higher in patients at term with clinical chorioamnionitis than in those without this condition (clinical chorioamnionitis: median 3.8 ng/mL vs. term in labor: median 1.8 ng/mL, p = 0.007; and vs. term not in labor: median 1.1 ng/mL, p = 0.003); 2) in contrast, patients with clinical chorioamnionitis had a lower median sRAGE concentration than those without this condition (clinical chorioamnionitis: median 9.3 ng/mL vs. term in labor: median 18.6 ng/mL, p = 0.001; and vs. term not in labor median: 28.4 ng/mL, p < 0.001); 3) amniotic fluid concentrations of esRAGE did not significantly change in patients with clinical chorioamnionitis at term (clinical chorioamnionitis: median 5.4 ng/mL vs. term in labor: median 6.1 ng/mL, p = 0.9; and vs. term not in labor: median 9.5 ng/mL, p = 0.06); and 4) there was no significant difference in the median AF HMGB1 concentration between women at term in labor and those not in labor (p = 0.4) and between women in the mid-trimester and those at term not in labor (mid-trimester: median 1.5 ng/mL; p = 0.2). Conclusion: An increase in the amniotic fluid HMGB1 concentration and a decrease in sRAGE were observed in clinical chorioamnionitis at term. This finding provides evidence that an alarmin, HMGB1, and one of its receptors, sRAGE, are engaged in the process of clinical chorioamnionitis at term. These changes are quite different from those observed in cases of intra-amniotic infection/inflammation in preterm gestations.
Introduction
Clinical chorioamnionitis is diagnosed by the presence of maternal fever accompanied by signs and symptoms of intrauterine inflammation (i.e. foul smelling discharge, uterine tenderness, fetal tachycardia, etc) [Citation1–3]. Neonates born to mothers with clinical chorioamnionitis, even at term gestation, are at an increased risk for short- and long- term complications including low Apgar scores, umbilical cord pH <7, delivery room intubation, pneumonia, sepsis [Citation4], neonatal encephalopathy [Citation5,Citation6], long-term cognitive impairment [Citation7] and cerebral palsy [Citation8–12].
Inflammation can be elicited in response to an infection or sterile injury such as trauma or ischemia/reperfusion [Citation13–15]. Indeed, several physiologic processes such as implantation, ovulation [Citation16] and parturition [Citation17,Citation18] utilize cells and mediators involved in the innate immune response. Classically, the innate immune system of multicellular organisms recognizes infection via specific molecular patterns (pathogen-associated molecular patterns or PAMPs) on microbes by specific receptors (pattern recognition receptors or PRRs) on host cells [Citation19–21]. In cases of trauma or sterile injury, cells sense an endogenous “danger signal” through alarmins, normal cell constituents capable of inducing an inflammatory response upon release into the extracellular environment [Citation14,Citation15,Citation22–24]. Currently, several endogenous molecules including S100 proteins [Citation25,Citation26], uric acid [Citation27], interleukin (IL)-1α [Citation28], heat shock protein [Citation29,Citation30], hepatoma-derived growth factor [Citation31], adenosine triphosphate [Citation14] and high mobility group box (HMGB)-1 have been proposed to be alarmins [Citation14].
The specific characteristics of alarmins include the ability to: 1) undergo passive release following necrotic cell death or active secretion through the non-classical pathway; 2) recruit and activate antigen-presenting cells (e.g.: dendritic cells, macrophages) to induce adaptive immune responses; and 3) promote tissue repair [Citation14,Citation23,Citation32–36].
HMGB1, a nuclear protein, is considered to be the only alarmin that meets all the proposed criteria for a danger signal [Citation14]. HMGB1 exerts its biological effects through specific receptors including toll-like receptor (TLR)-2, -4, and -9 [Citation32–36] as well as a receptor for advanced glycation end products (RAGE) [Citation37,Citation38]. Upon binding to RAGE, HMGB1 can induce and sustain the production of pro-inflammatory cytokines which may be modulated by soluble, truncated forms of RAGE including soluble RAGE (sRAGE) and endogenous secretory RAGE (esRAGE) [Citation23,Citation38–45].
We have examined the changes of amniotic fluid concentrations of pro-inflammatory cytokines [Citation46–54], anti-inflammatory cytokines [Citation55], chemokines [Citation56–62], proteases/anti-proteases [Citation63], matrix metalloproteinase [Citation64–71], pro- and anti-angiogenic factors [Citation72–74], coagulation factors [Citation75,Citation76], adipocytokines [Citation77–79], anti-microbial peptides [Citation80] and prostaglandins [Citation81–84] in patients with intra-amniotic infection/inflammation (IAI) both at term and preterm gestations. Changes in amniotic fluid concentrations of sRAGE and esRAGE have been reported in patients with IAI in preterm gestations and in women in labor at term [Citation85], whereas the amniotic fluid concentration of HMGB1 in patients at term with clinical chorioamnionitis or in women in labor at term has not yet been examined.
The objectives of this study were to determine if: 1) clinical chorioamnionitis at term is associated with changes in amniotic fluid concentrations of HMGB1, sRAGE and esRAGE; and 2) amniotic fluid concentration of HMGB1 changes as a function of gestational age or labor at term, a condition generally considered as a sterile inflammatory response [Citation17].
Materials and methods
Study design and population
A retrospective cross-sectional study was conducted by searching our clinical database and bank of biologic samples. Women with singleton pregnancies who had amniotic fluid samples obtained by transabdominal amniocentesis from the following groups were included: 1) those in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered at term (n = 45); 2) women at term gestation and without intra-amniotic fluid infection with (n = 22) and without labor (n = 48); and 3) those at term with clinical chorioamnionitis (n = 46).
All women provided written informed consent before the collection of amniotic fluid samples. The collection and utilization of the samples was approved by the Human Investigation Committee of the participating institutions and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies of sRAGE and esRAGE in intra-amniotic infection.
Clinical definition
Clinical chorioamnionitis was diagnosed by the presence of a temperature elevation to 37.8°C or higher and two or more of the following criteria: uterine tenderness, malodorous vaginal discharge, fetal tachycardia (heart rate >160 beats/min), maternal tachycardia (heart rate >100 beats/min) and maternal leukocytosis (leukocyte count >15,000 cells/mm3) [Citation1,Citation3]. Spontaneous term labor was defined as the presence of regular uterine contractions with a frequency of at least one every 10 min and cervical changes after 37 weeks of gestation. Intra-amniotic infection was defined as a positive microbiological culture in amniotic fluid, and intra-amniotic inflammation as an amniotic fluid IL-6 concentration of 2.6 ng/mL or more [Citation86].
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for genetic indications, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women with clinical chorioamnionitis underwent amniocentesis to evaluate infection and inflammatory status of the amniotic cavity. This information was used by obstetricians and neonatologists in the management of mothers and neonates in terms of treatment with antibiotics. Women at term in labor consisted of those who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering whether these patients were at term in labor was derived retrospectively, and were: 1) spontaneous labor; 2) delivery within 24 hours of amniocentesis; 3) analysis of amniotic fluid was consistent with fetal lung maturity; 4) birthweight >2500 g; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by a pediatrician was consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell count [Citation87], glucose concentration [Citation88] and Gram stain [Citation89] were also performed shortly after collection as previously described [Citation87,Citation88]. The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were obtained in some patients and used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged at 1300g for 10 min at 4°C and the supernatant was stored at−70°C.
Determination of HMGB1, sRAGE and esRAGE in amniotic fluid
Concentrations of HMGB1, sRAGE, and esRAGE in amniotic fluid were determined by sensitive and specific enzyme immunoassays obtained from IBL International (Toronto, Canada); and IL-6 immunoassay from R&D Systems (Minneapolis, MN). The initial assay validation was performed in our laboratory prior to the conduction of this study. Briefly, the immunoassay utilized the quantitative sandwich enzyme immunoassay technique and the concentrations were determined by interpolation from the standard curves. The inter-assay coefficients of variations for HMGB1, sRAGE, esRAGE and IL-6 were 3.1%, 3.2%, 4.8%, and 8.7%, respectively. Intra-assay coefficients of variations for HMGB1, sRAGE, esRAGE and IL-6 were 4.4%, 4.2%, 2.1% and 4.6%, respectively. The sensitivities of the assays for HMGB1, sRAGE, esRAGE and IL-6 were 0.2 ng/mL, 33 pg/mL, 28 pg/mL and 0.09 pg/mL, respectively. The results of plasma sRAGE and esRAGE concentrations in patients in the mid-trimester and at term with and without labor have been previously reported [Citation85], but were included in this article in order to provide a comprehensive picture of HMGB1 and its soluble receptors.
Statistical analysis
The Kolmogorov–Smirnov test and Shapiro–Wilk test were used to determine if the data were normally distributed. Kruskal–Wallis and ‘post hoc’ Mann–Whitney U tests were used to compare continuous nonparametric variables among and between groups. Comparisons between proportions were performed using Chi-square or Fisher’s exact tests. A p value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 15 (SPSS Inc., Chicago, IL).
Results
Demographic and clinical characteristics of the study population
and display the demographic and clinical characteristics of patients in the mid-trimester, term not in labor, term in labor and term with clinical chorioamnionitis. Among patients with the clinical diagnosis of chorioamnionitis, 41% (19/46) had a positive microbial culture in amniotic fluid and 76% (35/46) had evidence of intra-amniotic inflammation (defined as an amniotic fluid concentration of IL-6 of 2.6 ng/mL or more). The most common organisms were Ureaplasma urealyticum (n = 7), Mycoplasma hominis (n = 3) and Streptococcus agalactiae (n = 3). All but one patient had a placenta available for histologic examination. Approximately half of these patients [48.9% (22/45)] had histologic chorioamnionitis and/or funisitis. Collectively, only 17.3% (8/46) did not have either intra-amniotic inflammation or histologic evidence of inflammation in the placenta.
Table I. Clinical and obstetrical characteristics of women in the mid-trimester and those at term no labor.
Table II. Clinical and obstetrical characteristics of women at term with and without labor and term with clinical chorioamnionitis.
Clinical chorioamnionitis is associated with high HMGB1 concentrations
Patients with clinical chorioamnionitis at term had a significantly higher median amniotic fluid HMGB1 concentration than women at term (clinical chorioamnionitis: median 3.8 ng/mL; range: 0–37.4 ng/mL vs. term without labor: median 1.1 ng/mL; range: 0–8.8 ng/mL; p = 0.007; vs. term with labor: median 1.8 ng/mL; range: 0–21.5 ng/mL; p = 0.003; ). Similar results were obtained when the analysis was restricted to patients with clinical chorioamnionitis with evidence of intra-amniotic infection/inflammation (clinical chorioamnionitis with IAI (n = 35): median 5.1 ng/mL; range: 0–37.4 ng/mL vs. term without labor; p = 0.001; vs. term with labor: p = 0.001).
Figure 1. Amniotic fluid concentrations of high-mobility group box-1 (HMGB1) in women at term with and without labor and patients with clinical chorioamnionitis. Patients with clinical chorioamnionitis at term had a significantly higher median amniotic fluid HMGB1 concentration than women at term with and without labor (clinical chorioamnionitis: median 3.8 ng/mL; range: 0–37.4 ng/mL vs. term without labor: median 1.1 ng/mL; range: 0–8.8 ng/mL; p = 0.007; vs. term with labor: median 1.8 ng/mL; range: 0–21.5 ng/mL; p = 0.003).
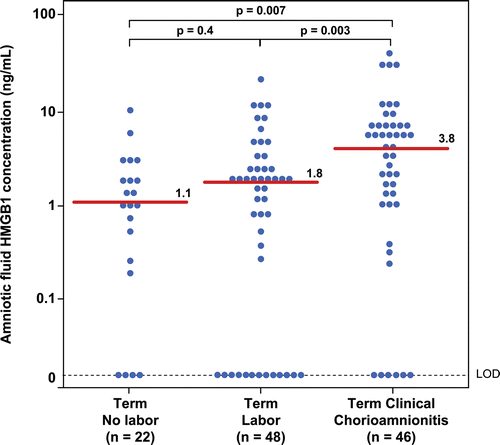
Amniotic fluid concentrations of sRAGE decreased in patients with clinical chorioamnionitis at term
Patients with clinical chorioamnionitis at term had a lower median sRAGE concentration than those without chorioamnionitis (clinical chorioamnionitis: median 9.3 ng/mL; range: 0–29.3 ng/mL vs. term not in labor: median 28.4 ng/mL; range: 1.1–56.8 ng/mL; p < 0.001; vs. term in labor: median 18.6 ng/mL; range: 0–79.8 ng/mL; p = 0.001; ). In contrast, there were no significant differences in the median amniotic fluid concentration of esRAGE between patients with clinical chorioamnionitis at term and patients at term without this condition (clinical chorioamnionitis: median: 5.4 ng/mL; range: 0–18.1 ng/mL vs. term not in labor median: 9.5 ng/mL; range: 0–22.6 ng/mL; p = 0.06; vs. term in labor median: 6.1 ng/mL; range: 0–15.1 ng/mL; p = 0.9; ).
Figure 2. Amniotic fluid concentrations of soluble receptor for advanced glycation end products (sRAGE) in women at term with and without labor and patients with clinical chorioamnionitis. Patients with clinical chorioamnionitis at term had a lower median sRAGE concentration than those without chorioamnionitis regardless of labor status (clinical chorioamnionitis: median 9.3 ng/mL; range: 0–29.3 ng/mL vs. term not in labor: median 28.4 ng/mL; range: 1.1–56.8 ng/mL; p < 0.001; vs. term in labor: median 18.6 ng/mL; range: 0–79.8 ng/mL; p = 0.001).
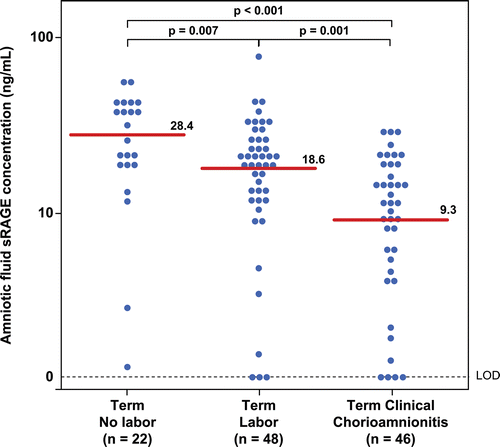
Figure 3. Amniotic fluid concentrations of endogenous secretory RAGE (esRAGE) in women at term with and without labor and patients with clinical chorioamnionitis. There were no significant difference in the median amniotic fluid concentration of esRAGE between patients with clinical chorioamnionitis at term and patients at term with and without labor (clinical chorioamnionitis: median: 5.4 ng/mL; range: 0–18.1 ng/mL vs. term not in labor median: 9.5 ng/mL; range: 0–22.6 ng/mL; p = 0.06; vs. term in labor median: 6.1 ng/mL; range: 0–15.1 ng/mL; p = 0.9).
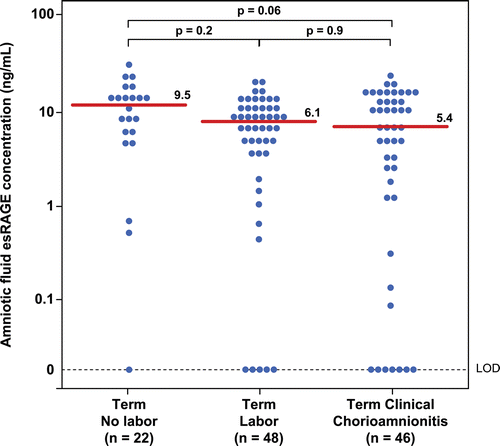
When the analysis was restricted to patients with clinical chorioamnionitis with evidence of intra-amniotic infection/inflammation, similar findings were observed (clinical chorioamnionitis with IAI: sRAGE median 9.2 ng/mL; range: 0–29.3 ng/mL vs. term without labor; p < 0.001; vs. term with labor: p = 0.001 and clinical chorioamnionitis with IAI: esRAGE median 5.1 ng/mL; range: 0–18.1 ng/mL vs. term without labor; p = 0.06; vs. term with labor: p = 1.0).
Among patients with clinical chorioamnionitis, there was a significant correlation between amniotic fluid concentrations of HMGB1 and the soluble forms of its receptor (sRAGE: Spearman’s Rho 0.53; p < 0.001 and esRAGE Spearman’s Rho 0.46; p < 0.001) and between HMGB1 and markers of inflammation (WBC: Spearman’s Rho 0.4; p = 0.005 and IL-6: Spearman’s Rho 0.49; p = 0.001).
Amniotic fluid concentration of HMGB1 did not change with spontaneous labor at term
There was no significant difference in the median amniotic fluid HMGB1 concentration between women at term with and without labor (p = 0.4; ). In contrast, similar to results previously reported [Citation85], spontaneous labor at term was associated with a decrease in the median amniotic fluid concentration of sRAGE and esRAGE (p = 0.007 and p = 0.02, respectively; and ).
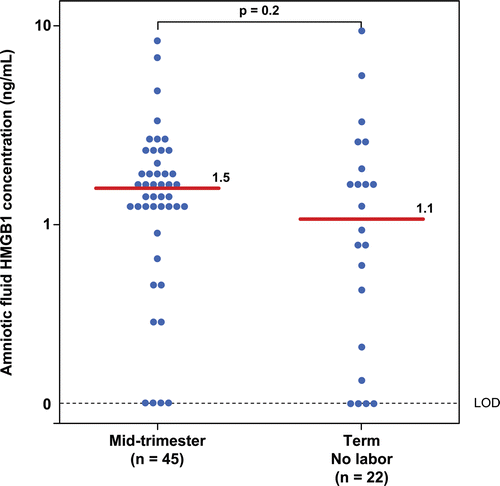
Amniotic fluid concentrations of HMGB1 did not change as a function of gestational age
There was no significant difference in the median amniotic fluid concentration of HMGB1 between women in the mid-trimester and those at term not in labor (mid-trimester median: 1.5 ng/mL; range: 0–8 ng/mL vs. term not in labor median: 1.1 ng/mL; range: 0–8.8 ng/mL; p = 0.2; ).
Figure 4. Amniotic fluid concentrations of high-mobility group box-1 (HMGB1) in women in the mid-trimester and those at term not in labor. There was no significant difference in the median amniotic fluid concentration of HMGB1 between patients in the mid-trimester and those at term not in labor (mid-trimester median: 1.5 ng/mL; range: 0–8 ng/mL vs. term not in labor median: 1.1 ng/mL; range: 0–8.8 ng/mL; p = 0.2).
Discussion
Principal findings of the study: 1) Clinical chorioamnionitis at term was associated with an increase in amniotic fluid concentration of HMGB1, but a decrease in its soluble receptor (sRAGE); 2) amniotic fluid concentration of HMGB1 did not change with spontaneous labor at term, a condition in which amniotic fluid concentrations of sRAGE and esRAGE decreased [Citation85]; and 3) amniotic fluid concentration of HMGB1 did not change as a function of gestational age.
The biology of HMGB1
HMGB1, a non-histone, chromatin-associated protein, was originally characterized to be involved in DNA organization and the regulation of transcription [Citation24,Citation39,Citation40,Citation44,Citation90]. Subsequently, this protein has been proposed to be a late mediator of sepsis [Citation42] and to play an important role in infection-induced lung injury and lethality [Citation91,Citation92].
HMGB1 is constitutively expressed in almost every cell type that has a nucleus [Citation24]. This alarmin can be released actively or passively into the extracellular environment. The active release of HMGB1 outside the cell is accomplished through a nontraditional “leaderless” pathway (i.e. not through endoplasmic reticulum or Golgi apparatus) [Citation14,Citation93] upon stress (ischemia, oxidative stress) [Citation94–96] or stimulation with bacterial products [Citation97–100] or cytokines such as tumor necrosis factor (TNF)-α, IL-1, or interferon-γ [Citation100–102]. The passive release of HMGB1 out of the cells was observed during cellular necrosis [Citation103,Citation104]. Extracellular HMGB1 can potentially be associated with DNA, RNA, endotoxin, nucleosomes [Citation24], thrombospondin [Citation105–107] or CD24 [Citation108,Citation109] to augment or decrease the activity of HMGB1 itself when they bind to its receptors [Citation110].
HMGB1 exerts a wide variety of biological activities, including induction of the maturation and migration of dendritic cells, neutrophils and monocytes [Citation43,Citation111–114], stimulation of the production of reactive oxygen species [Citation115], chemotaxis of neutrophils [Citation116], secretion of inflammatory cytokines from immune cells [Citation23,Citation40,Citation117–119], proliferation of T cells [Citation43,Citation120] and migration of stem cells for tissue repair [Citation121–128]. These effects are accomplished through the binding of HMGB1 to its receptors, which include TLR-2, -4, and -9 [Citation32–35] as well as RAGE [Citation37,Citation38,Citation43,Citation111,Citation129,Citation130].
The biology of soluble RAGE and endogenous secretory RAGE
RAGE was first described as a transmembrane receptor for advanced glycation products (AGE), the product of nonenzymatic glycation and oxidation of proteins and lipids that accumulate under the condition of oxidative stress and hyperglycemia. AGE induces the expression of pro-inflammatory mediators through mitogen-activated protein kinase and nuclear factor (NF)-κB [Citation131]. Other ligands of RAGE are amyloid-β peptides (accumulating in Alzheimer’s disease) [Citation132–135], amyloid A (accumulating in systemic amyloidosis) [Citation136], S100/calgranulins [Citation137], surface molecules on bacteria [Citation138], prions [Citation139], leukocytes [Citation140] and HMGB1 [Citation130]. Engagement of RAGE and its ligand also results in a rapid and sustained activation of NF-κB with a positive feedback loop, in which ligand interaction increases expression of the receptor itself [Citation141]. Thus, activation of NF-κB results in increased RAGE expression and the numbers of ligand-binding sites, thereby prolonging NF-κB activation [Citation37].
RAGE is expressed in vascular smooth muscle and endothelial cells, cardiac myocytes, neural tissues, macrophages [Citation142], human pregnant myometrium [Citation143], first trimester human chorionic villi [Citation144] and human term placenta [Citation145,Citation146]. Animal experiments and studies in humans indicate that RAGE is involved in the pathophysiologic processes of neurogenerative disorders [Citation147], rheumatoid arthritis [Citation148,Citation149], chronic renal disease [Citation150], inflammatory bowel disease and chronic vascular disorders which include diabetic complications (i.e. neuropathy, nephropathy) and atherosclerosis [Citation37,132,136,Citation151–153]. Moreover, intense immunostaining for RAGE in both myometrium and omental blood vessels of patients with preeclampsia [Citation143] has been observed.
The soluble form of RAGE is composed of the extracellular ligand-binding domain without transmembrane and cytosolic regions. This protein is originally thought to function as a decoy receptor abrogating cellular activation [Citation140,Citation154–157]. Subsequently, sRAGE has also been found to have pro-inflammatory activity, depending on the cell types and conditions of target cells [Citation158,Citation159]. Recently, a novel splice variant of RAGE mRNA has been identified as esRAGE and reported to be released from human microvascular endothelial cells and pericytes [Citation160,Citation161].
Amniotic fluid concentrations of HMGB1 increased in term clinical chorioamnionitis
The finding that clinical chorioamnionitis at term was associated with an increase in the amniotic fluid concentration of HMGB1 suggests that this alarmin participates in the inflammatory response. Moreover, a relationship between amniotic fluid concentrations of HMGB1 and IL-6 was observed. Bacterial endotoxin is capable of stimulating macrophages to release HMGB1 partly through CD14 and TNF-dependent mechanisms, since either genetic disruption of CD14 expression or neutralization of TNF activity blocks endotoxin-induced TNF production, but only partially attenuates HMGB1 release from macrophages [Citation98]. However, either endotoxin or pro-inflammatory cytokines, individually, are capable of inducing HMGB1 release into the extracellular environment [Citation39]. These observations are consistent with our findings in preterm gestations in which the amniotic fluid concentration of HMGB1 was elevated in patients with preterm labor or preterm PROM with IAI [Citation162].
Amniotic fluid concentrations of sRAGE decreased in term clinical chorioamnionitis
Patients with clinical chorioamnionitis or those with IAI at term had lower amniotic fluid concentrations of sRAGE than those without clinical chorioamnionitis. These findings differ from our observation in preterm gestations, in which amniotic fluid concentrations of sRAGE and esRAGE were elevated in patients with intra-amniotic inflammation [Citation85].
It is possible that a lower amniotic concentration of sRAGE observed herein reflects a relationship between an inflammatory response in amniotic fluid and sRAGE in women at term. A similar observation in sRAGE in synovial fluid and blood has been reported in patients with rheumatoid arthritis [Citation149]. The increased HMGB1 concentration in these patients may be responsible, in part, for the concentration of sRAGE in the amniotic fluid of patients with clinical chorioamnionitis at term by stimulating RAGE receptor production. Consistent with this hypothesis, amniotic fluid concentrations of HMGB1 were correlated with sRAGE. This relationship was observed in IAI only at term, but not in preterm gestation. Similarly, amniotic fluid concentrations of HMGB1 also correlated with IL-6 in intra-amniotic inflammation only at term, but not in preterm gestations. Thus, HMGB1 and sRAGE may modulate the inflammatory response differently in term and preterm gestations. Future studies are required to elucidate the factors responsible for the differential response to intra-amniotic inflammation at different gestational ages. Alternatively, a decreased sRAGE concentration in the amniotic fluid of patients with clinical chorioamnionitis at term reflects an increase in susceptibility to intra-amniotic infection of these patients [Citation149].
Advancing gestational age or parturition at term did not change amniotic fluid concentrations of HMGB1
Amniotic fluid concentrations of HMGB1 did not change with advancing gestational age or with the presence of spontaneous labor at term without intra-amniotic infection. In contrast, in our previous study, sRAGE increased with advancing gestational age and decreased with parturition at term [Citation85]. The difference in behavior of HMGB1 and sRAGE could be explained, in part, by the multi-ligand nature of both proteins [Citation37,Citation39]. sRAGE may modulate the physiologic inflammatory response of labor at term through different ligands other than HMGB1.
Strengths and limitations
This is the first study to evaluate amniotic fluid concentrations of HMGB1, an alarmin, in patients with clinical chorioamnionitis at term. Moreover, amniotic fluid concentrations of its soluble receptors, sRAGE and esRAGE, were also determined. However, due to the cross-sectional nature of the study, a temporal relationship of this alarmin as well as its soluble receptors and clinical chorioamnionitis at term could not be established. In addition, we have not utilized molecular techniques to define microbial invasion of amniotic fluid cavity (either bacteria or viruses) in this study. Based on studies in patients with preterm labor [Citation163] and preterm prelabor rupture of membranes [Citation164], it is likely that the prevalence of intra-amniotic infection would have increased if such molecular techniques were applied in this study.
Conclusion
A substantial increase in the amniotic fluid concentration of HMGB1 and a decrease in sRAGE were observed in clinical chorioamnionitis at term. This is evidence that an alarmin (a “danger signal”), HMGB1, and one of its receptors, sRAGE, are altered in clinical chorioamnionitis at term. These observations are different from those made in preterm gestations with intra-amniotic infection/inflammation. It is possible that HMGB1 and sRAGE may play roles in the regulation of the inflammatory responses in both term and preterm gestations.
Acknowledgement
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Presented at the 56th annual meeting of the Society for Gynecologic Investigation. March 16–19, 2011, Miami, FL.
Declaration of interest: The authors declare no conflicts of interest exist.
References
- Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol 1988;72:823–828.
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–326.
- Hauth JC, Gilstrap LC 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol 1985;66:59–62.
- Alexander JM, McIntire DM, Leveno KJ. Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 1999;94:274–278.
- Locatelli A, Incerti M, Paterlini G, Doria V, Consonni S, Provero C, Ghidini A. Antepartum and intrapartum risk factors for neonatal encephalopathy at term. Am J Perinatol 2010;27:649–654.
- Shalak LF, Perlman JM. Infection markers and early signs of neonatal encephalopathy in the term infant. Ment Retard Dev Disabil Res Rev 2002;8:14–19.
- Versland LB, Sommerfelt K, Elgen I. Maternal signs of chorioamnionitis: persistent cognitive impairment in low-birthweight children. Acta Paediatr 2006;95:231–235.
- Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 2000;284:1417–1424.
- Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 2003;290:2677–2684.
- Hagberg H, Wennerholm UB, Sävman K. Sequelae of chorioamnionitis. Curr Opin Infect Dis 2002;15:301–306.
- Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics 2002;110:673–680.
- Willoughby RE Jr, Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol 2002;29:603–621.
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010;140:771–776.
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1–5.
- Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol 2007;601:185–194.
- Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction 2009;138:903–919.
- Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 1996;3:328–335.
- Houben ML, Nikkels PG, van Bleek GM, Visser GH, Rovers MM, Kessel H, de Waal WJ, et al. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PLoS ONE 2009;4:e6572.
- Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 1997;91:295–298.
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145.
- Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002;296:298–300.
- Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science 2009;323:1683–1684.
- Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 2004;34:1503–1512.
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331–342.
- Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol 2007;81:108–118.
- Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–393.
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003;425:516–521.
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–123.
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 2007;81:15–27.
- Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–461.
- Zhou Z, Yamamoto Y, Sugai F, Yoshida K, Kishima Y, Sumi H, Nakamura H, Sakoda S. Hepatoma-derived growth factor is a neurotrophic factor harbored in the nucleus. J Biol Chem 2004;279:27320–27326.
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004;279:7370–7377.
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol, Cell Physiol 2006;290:C917–C924.
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007;8:487–496.
- Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 2007;110:1970–1981.
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006;26:174–179.
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 2005;83:876–886.
- Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005;61:1–9.
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med 2008;14:476–484.
- Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res 2011;343:189–199.
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 2009;31:280–284.
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251.
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 2005;174:7506–7515.
- Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol 2005;26:381–387.
- Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 2007;220:35–46.
- Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–341.
- Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–1123.
- Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol 2001;184:1399–405; discussion 1405.
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–1130.
- Kusanovic JP, Romero R, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Vaisbuch E, Erez O, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:34–47.
- Cruciani L, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Mazaki-Tovi S, Mittal P, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med 2010;38:161–171.
- Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999;181:1142–1148.
- Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, Insunza A, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med 1993;38:543–548.
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–1400.
- Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–547.
- Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–373.
- Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10.
- Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–820.
- Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, Mittal P, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2008;21:763–775.
- Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, Espinoza J, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 2008;60:246–257.
- Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, Than NG, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008;36:217–227.
- Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005;18:405–416.
- Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002;12:237–246.
- Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–1253.
- Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, Yoon BH, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med 1999;8:213–219.
- Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, Seubert DE. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–1553.
- Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000;183:887–894.
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–920.
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–99.
- Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, Edwin S. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:1143–1148.
- Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–316.
- Savasan ZA, Romero R, Chaiworapongsa T, Kusanovic JP, Kim SK, Mazaki-Tovi S, Vaisbuch E, et al. Evidence in support of a role for anti-angiogenic factors in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2010;23:828–841.
- Seubert DE, Maymon E, Pacora P, Gervasi MT, Berry SM, Torry DS, Romero R. A study of the relationship between placenta growth factor and gestational age, parturition, rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1633–1637.
- Pacora P, Romero R, Chaiworapongsa T, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med 2009;37:503–511.
- Erez O, Romer R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Gotsch F, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med 2009;22:971–982.
- Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, Gotsch F, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Matern Fetal Neonatal Med 2010;23:23–33.
- Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–496.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, Gotsch F, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:120–130.
- Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than NG, Kim SK, Dong Z, et al. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:111–119.
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21.
- Romero R, Wu YK, Sirtori M, Oyarzun E, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–161.
- Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454–1460.
- Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–1467.
- Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990;26:425–428.
- Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, Erez O, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008;36:388–398.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–830.
- Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–974.
- Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–119.
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem 1973;38:14–19.
- Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med 2001;164:1768–1773.
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 2004;101:296–301.
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002;3:995–1001.
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 2008;28:927–938.
- Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 2007;204:2913–2923.
- Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 2007;103:590–603.
- Jiang W, Li J, Gallowitsch-Puerta M, Tracey KJ, Pisetsky DS. The effects of CpG DNA on HMGB1 release by murine macrophage cell lines. J Leukoc Biol 2005;78:930–936.
- Chen G, Li J, Ochani M, Rendon-Mitchell B, Qiang X, Susarla S, Ulloa L, et al. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol 2004;76:994–1001.
- Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, et al. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand J Immunol 2004;60:566–573.
- Wähämaa H, Vallerskog T, Qin S, Lunderius C, LaRosa G, Andersson U, Harris HE. HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J Leukoc Biol 2007;81:129–136.
- Jiang W, Pisetsky DS. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol 2006;177:3337–3343.
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol 2003;170:3890–3897.
- Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004;5:825–830.
- Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity 2007;40:285–289.
- Crikis S, Zhang XM, Dezfouli S, Dwyer KM, Murray-Segal LM, Salvaris E, Selan C, et al. Anti-inflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant 2010;10:242–250.
- Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med 2009;37:2181–2186.
- Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 2008;28:1825–1830.
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009;323:1722–1725.
- Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol 2009;30:557–561.
- Branco-Madeira F, Lambrecht BN. High mobility group box-1 recognition: the beginning of a RAGEless era? EMBO Mol Med 2010;2:193–195.
- Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol 2005;35:2184–2190.
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol, Cell Physiol 2003;284:C870–C879.
- Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 2007;81:59–66.
- Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepäntalo M, et al. Regulation of monocyte migration by amphoterin (HMGB1). Blood 2004;104:1174–1182.
- Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol 2007;178:6573–6580.
- Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J 2007;26:1129–1139.
- Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-?B signaling by RAGE and increases the production of TNF-a in human umbilical vein endothelial cells. Immunobiology 2010;215:956–962.
- Qin YH, Dai SM, Tang GS, Zhang J, Ren D, Wang ZW, Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol 2009;183:6244–6250.
- Rauvala H, Rouhiainen A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta 2010;1799:164–170.
- Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 2004;173:307–313.
- De Mori R, Straino S, Di Carlo A, Mangoni A, Pompilio G, Palumbo R, Bianchi ME, et al. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol 2007;27:2377–2383.
- Palumbo R, Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol 2004;68:1165–1170.
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 2004;164:441–449.
- Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 2006;176:12–15.
- Sorci G, Riuzzi F, Arcuri C, Giambanco I, Donato R. Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol Cell Biol 2004;24:4880–4894.
- Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 2001;152:1197–1206.
- Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res 2005;97:e73–e83.
- Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res 2007;100:204–212.
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010;28:367–388.
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 1995;270:25752–25761.
- Lappas M, Permezel M, Rice GE. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-kappaB and extracellular signal-regulated kinase ½. J Endocrinol 2007;193:269–277.
- Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, et al. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol, Cell Physiol 2000;279:C1772–C1781.
- Leclerc E, Sturchler E, Vetter SW, Heizmann CW. Crosstalk between calcium, amyloid beta and the receptor for advanced glycation endproducts in Alzheimer’s disease. Rev Neurosci 2009;20:95–110.
- Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA 1997;94:5296–5301.
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996;382:685–691.
- Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med 2000;6:643–651.
- Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res 2010;106:93–101.
- Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002;295:851–855.
- Sasaki N, Takeuchi M, Chowei H, Kikuchi S, Hayashi Y, Nakano N, Ikeda H, et al. Advanced glycation end products (AGE) and their receptor (RAGE) in the brain of patients with Creutzfeldt-Jakob disease with prion plaques. Neurosci Lett 2002;326:117–120.
- Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 2003;198:1507–1515.
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001;50:2792–2808.
- Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 1993;143:1699–1712.
- Cooke CL, Brockelsby JC, Baker PN, Davidge ST. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens Pregnancy 2003;22:173–184.
- Konishi H, Nakatsuka M, Chekir C, Noguchi S, Kamada Y, Sasaki A, Hiramatsu Y. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum Reprod 2004;19:2156–2162.
- Holmlund U, Wähämaa H, Bachmayer N, Bremme K, Sverremark-Ekström E, Palmblad K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology 2007;122:430–437.
- Chekir C, Nakatsuka M, Noguchi S, Konishi H, Kamada Y, Sasaki A, Hao L, Hiramatsu Y. Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta 2006;27:225–233.
- Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol 2001;171:29–45.
- Drinda S, Franke S, Eidner T, Schmidt C, Rüster C, Bondeva T, Hein G, Wolf G. Decreased RAGE expression in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Clin Exp Rheumatol 2009;27:483–490.
- Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther 2005;7:R817–R824.
- Hou FF, Ren H, Owen WF Jr, Guo ZJ, Chen PY, Schmidt AM, Miyata T, Zhang X. Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol 2004;15:1889–1896.
- Maillard-Lefebvre H, Boulanger E, Daroux M, Gaxatte C, Hudson BI, Lambert M. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology (Oxford) 2009;48:1190–1196.
- Chen Y, Yan SS, Colgan J, Zhang HP, Luban J, Schmidt AM, Stern D, Herold KC. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol 2004;173:1399–1405.
- Hudson BI, Bucciarelli LG, Wendt T, Sakaguchi T, Lalla E, Qu W, Lu Y, et al. Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys 2003;419:80–88.
- Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep 2002;4:228–237.
- Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, et al. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest 2004;114:1741–1751.
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 2004;113:1641–1650.
- Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 2003;111:959–972.
- Pullerits R, Brisslert M, Jonsson IM, Tarkowski A. Soluble receptor for advanced glycation end products triggers a proinflammatory cytokine cascade via beta2 integrin Mac-1. Arthritis Rheum 2006;54:3898–3907.
- Wang Y, Wang H, Piper MG, McMaken S, Mo X, Opalek J, Schmidt AM, Marsh CB. sRAGE induces human monocyte survival and differentiation. J Immunol 2010;185:1822–1835.
- Sakurai S, Yonekura H, Yamamoto Y, Watanabe T, Tanaka N, Li H, Rahman AK, et al. The AGE-RAGE system and diabetic nephropathy. J Am Soc Nephrol 2003;14:S259–S263.
- Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 2003;370:1097–1109.
- Romero R, Chaiworapongsa T, Alpay S, Madan I, Dong Z, Kusanovic J, Kim C et al. HMGB1, A Late Mediator of Sepsis and Death, is Involved in the Host Response to Intra-amniotic Infection/Inflammation in Preterm Gestation. Reproductive Science 2011;18:245 A.
- DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE 2008;3:e3056.
- DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, Gotsch F, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57.
