Abstract
Objective: The fetal inflammatory response syndrome (FIRS) is considered the fetal counterpart of the systemic inflammatory response syndrome (SIRS), which can be caused by infection and non-infection-related insults. Although the initial response is mediated by pro-inflammatory signals, the control of this response is achieved by anti-inflammatory mediators which are essential for the successful outcome of the affected individual. Interleukin (IL)-19 is capable of stimulating the production of IL-10, a major anti-inflammatory cytokine, and is a potent inducer of the T-helper 2 (Th2) response. The aim of this study was to determine if there is a change in umbilical cord plasma IL-19 and IL-10 concentrations in preterm neonates with and without acute funisitis, the histologic counterpart of FIRS. Methods: A case-control study was conducted including 80 preterm neonates born after spontaneous labor. Neonates were classified according to the presence (n = 40) or absence of funisitis (n = 40), which is the pathologic hallmark of FIRS. Neonates in each group were also matched for gestational age. Umbilical cord plasma IL-19 and IL-10 concentrations were determined by ELISA. Results: 1) The median umbilical cord plasma IL-19 concentration was 2.5-fold higher in neonates with funisitis than in those without funisitis (median 87 pg/mL; range 20.6–412.6 pg/mL vs. median 37 pg/mL; range 0–101.7 pg/mL; p < 0.001); 2) newborns with funisitis had a significantly higher median umbilical cord plasma IL-10 concentration than those without funisitis (median 4 pg/mL; range 0–33.5 pg/mL vs. median 2 pg/mL; range 0–13.8 pg/mL; p < 0.001); and 3) the results were similar when we included only patients with funisitis who met the definition of FIRS by umbilical cord plasma IL-6 concentrations ≥ 17.5 pg/mL (p < 0.001). Conclusion: IL-19 and IL-10 are parts of the immunologic response of FIRS. A subset of fetuses with FIRS had high umbilical cord plasma IL-19 concentrations. In utero exposure to high systemic concentrations of IL-19 may reprogram the immune response.
Introduction
The fetal inflammatory response syndrome (FIRS) [Citation1–6] can occur in cases of the most advanced stage of microbial invasion of the amniotic cavity. The condition has been operationally defined by an elevation of fetal plasma interleukin (IL)-6 concentration, although derangements in other cytokines (e.g. IL-10, [Citation7,Citation8] granulocyte-colony- stimulating factor, [Citation9] etc.) have been reported. FIRS has been diagnosed in patients with preterm labor and intact membranes, and in preterm prelabor rupture of membranes (PROM). However, FIRS can also be present in the context of congenital viral infections [Citation10–17] and in cases of alloimmune Rh disease. [Citation18] The significance of FIRS is that affected fetuses are at high risk for short- and long-term complications, even after adjusting for gestational age at birth. [Citation19–42] Moreover, FIRS is associated with a short cordocentesis-to-delivery interval, suggesting that the human fetus plays a role in the onset of labor. [Citation1,Citation19] Evidence of multi-systemic involvement in FIRS has been characterized by the detection of high concentrations of cortisol, [Citation43] hematologic disorders (e.g. fetal neutrophilia), [Citation44] decreased volume of amniotic fluid [Citation33] and cardiac dysfunction, [Citation45,Citation46] as well as fetal neuroinflammation. [Citation22–31,Citation42,Citation47–54] These findings have been well-characterized in humans and animal models of intrauterine infection in a wide range of species, including mice, [Citation55–64] rabbits, [Citation65–73] sheep [Citation74–110] and primates. [Citation111–122] Abnormalities in the phenotype of monocytes and granulocytes as well as the production of reactive oxygen radicals in the umbilical cord blood of neonates with funisitis have been documented, [Citation123] suggesting that oxidative stress plays a role in the pathophysiology of the condition. [Citation31,Citation124–128]
FIRS is considered to be the fetal counterpart of the systemic inflammatory response syndrome (SIRS) seen in adults. [Citation129] SIRS is characterized by systemic inflammation after a wide range of insults, including infection-related and non-infection-related injury. [Citation130–132] The initial response is mainly due to inflammatory mediators induced by the innate immune system. [Citation133,Citation134] The timing and the control of this response is crucial for the outcome of the affected individuals because an exaggerated and uncontrolled inflammatory response may be detrimental to the host; on the other hand, if the control mechanisms mediated by anti-inflammatory mediators is pronounced or prolonged, the host may be immunosuppressed and become susceptible to secondary infections. [Citation134–139] Indeed, patients discharged after an episode of sepsis have a high mortality rate that is attributed to this immunosuppressed state. [Citation140]
Suppression of the pro-inflammatory response is accomplished by the anti-inflammatory limb of the immune system. [Citation141–143] Interleukin (IL)-10 is considered to be a major anti-inflammatory mediator since it is mainly produced by monocytes and functions to inhibit the transcription of pro-inflammatory cytokines. [Citation141,Citation144,Citation145] We have previously reported the behavior of IL-10 in the amniotic fluid of patients with preterm labor. [Citation146]
IL-19 is a newly discovered cytokine belonging to the IL-10 family. This protein can induce the production of IL-10 from human peripheral blood mononuclear cells, [Citation147,Citation148] and is considered by some investigators to have an anti-inflammatory effect. [Citation149–152] However, IL-19 is also capable of activating monocytes to release IL-6, tumor necrosis factor (TNF)-α, IL-8 and reactive oxygen species [Citation153,Citation154] and has been implicated in the pathogenesis of sepsis-induced organ injury. [Citation154] Since this cytokine can also up-regulate IL-4 and down-regulate interferon (IFN)-γ on T cells, [Citation155] IL-19 is a potent inducer of the T-helper 2 (Th2) response [Citation148] and has been implicated in a wide variety of allergic (i.e. asthma and atopic dermatitis [Citation156–158]) and non-allergic diseases (i.e. psoriasis, [Citation157,Citation159–161] aging, [Citation162] type-1 diabetes, [Citation163] periodontal disease [Citation164] and cardiovascular disease [Citation151,Citation152]).
The objective of this study was to examine the changes in umbilical cord plasma IL-19 and IL-10 concentrations—both potential members of the anti-inflammatory limb of the immune response—in newborns with and without funisitis (the histologic counterpart of FIRS). [Citation165]
Patients and methods
Study population
A retrospective case-control study was conducted by searching our clinical database and bank of biological samples including 80 pregnant patients with preterm deliveries between 27 and 34 weeks of gestation with (n = 40) and without funisitis (n = 40) and matched for gestational age at delivery within 2 weeks. Umbilical cord blood was collected immediately after birth. Placentas were obtained after delivery and underwent histopathologic examination.
All patients provided written informed consent prior to the collection of samples. The collection and utilization of the samples for research purposes was approved by the Human Investigation Committee of Wayne State University (Detroit, MI) and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies.
Clinical definition
Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly, according to criteria previously published. [Citation165] FIRS was diagnosed by umbilical cord blood IL-6 concentrations ≥ 17.5 pg/mL. [Citation40,Citation54] The diagnosis of preterm labor (PTL) was made in the presence of regular uterine contractions (at least 3 in 30 min) and a documented cervical change in patients with a gestational age between 20 and 36 6/7 weeks. Preterm PROM was diagnosed with sterile speculum examination with a combination of vaginal pooling, nitrazine and ferning tests.
Sample collection and determination of IL-19, IL-10 and IL-6 in umbilical cord plasma
Umbilical cord blood was collected after birth into tubes containing EDTA. Blood was centrifuged at 1300g for 10 min at 4°C. The plasma obtained was stored at -70°C until analysis. Concentrations of IL-19, IL-10 and IL-6 in umbilical cord plasma were determined by sensitive and specific enzyme immunoassays from R&D Systems (Minneapolis, MN). The initial assay validation was performed in our laboratory prior to the conduction of this study. Briefly, the immunoassay utilized the quantitative sandwich enzyme immunoassay technique and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation were 4.6% and 4.3%, respectively, for IL-19; 6.9% and 4.4%, respectively, for IL-10; and 8.7% and 4.6%, respectively, for IL-6. The sensitivities of the assays for IL-19, IL-10 and IL-6 were 17.4 pg/mL, 0.65 pg/mL, and 0.09 pg/mL, respectively.
Determination of funisitis
Tissue sections for histopathologic evaluation included one chorioamniotic membrane roll, two full-thickness sections from the placental disc and one section of the umbilical cord. The tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hemotoxylin and eosin. Histopathologic examination was performed by a perinatal pathologist (CJK) who was blinded to the clinical information. Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly.
Statistical analysis
Shapiro–Wilk tests were used to determine if the data was normally distributed. A two-tailed Mann–Whitney U test was used to compare continuous nonparametric variables. The Wilcoxon rank sum test was also performed for matched-case analysis. Comparisons between proportions were performed using Chi-square or Fisher’s exact tests. Spearman rank correlation was utilized to assess correlations between two continuous variables. A p value < 0.05 was considered statistically significant. The analysis was performed with SPSS, version 15 (SPSS Inc., Chicago, IL).
Results
Demographics and clinical characteristics of the study population
presents the demographic and clinical characteristics of patients included in the study. The median gestational age at delivery was not significantly different between patients with and without funisitis. Similarly, the frequency of patients presenting with spontaneous PTL and intact membranes [without funisitis: 67.5% (27/40) vs. with funisitis: 65% (26/40); p > 0.05] or preterm PROM [without funisitis: 32.5% (13/38) vs. with funisitis: 35% (14/40); p > 0.05] was not significantly different between the 2 groups. One patient without funisitis had umbilical cord plasma IL-19 below the detection limit of the assay. Two and five patients did not have plasma samples available for IL-6 and IL-10 determination, respectively.
Table I. Clinical characteristics of the study population with and without funisitis.
Funisitis was associated with elevation of cord plasma IL-19 and IL-10 concentrations
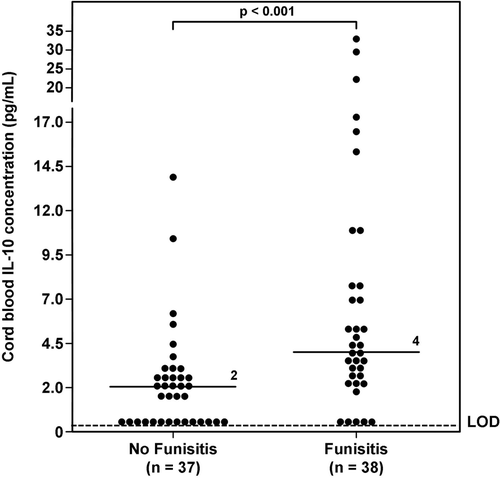
The median umbilical cord plasma IL-19 concentration was significantly higher in neonates with funisitis than in those without funisitis (median 87 pg/mL; range 20.6–412.6 pg/mL vs. median 37 pg/mL; range 0–101.7 pg/mL; p < 0.001; ). Newborns with funisitis had a significantly higher median umbilical cord plasma IL-10 concentration than those without funisitis (median 4 pg/mL; range 0–33.5 pg/mL vs. median 2 pg/mL; range 0–13.8 pg/mL; p < 0.001; ). Similar results were obtained using the Wilcoxon rank sum test for both comparisons (p < 0.001 for IL-19 and 0.001 for IL-10).
Figure 1. Umbilical cord plasma interleukin (IL)-19 concentrations in neonates with and without funisitis. The median umbilical cord plasma IL-19 concentration was significantly higher in neonates with funisitis than that of those without funisitis (median 87 pg/mL; range 20.6–412.6 pg/mL vs. median 37 pg/mL; range 0–101.7 pg/mL; p < 0.001). LOD = limit of detection.
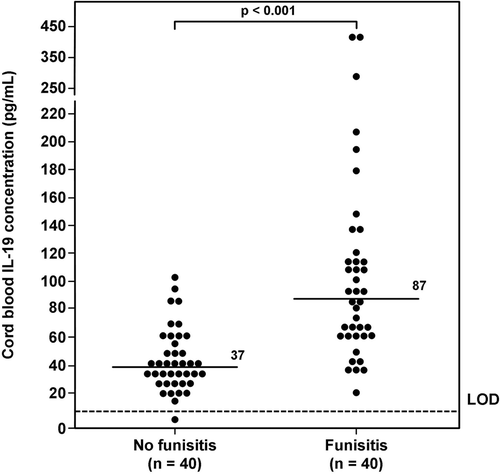
Figure 2. Umbilical cord plasma interleukin (IL)-10 concentrations in neonates with and without funisitis. Newborns with funisitis had a significantly higher median umbilical cord plasma IL-10 concentration than those without funisitis (median 4 pg/mL; range 0–33.5 pg/mL vs. median 2 pg/mL; range 0–13.8 pg/mL; p < 0.001). LOD = limit of detection.
FIRS was associated with elevation of cord plasma IL-19 and IL-10 concentrations
Demographic and clinical characteristics of patients who were diagnosed with and without FIRS are displayed in . Two patients did not have plasma IL-6 available. Eight newborns with funisitis had cord plasma IL-6 concentrations < 17.5 pg/mL, whereas none from the group without funisitis had cord plasma IL-6 concentrations above this cut-off. Similarly, the frequency of PTL, intact membranes and preterm PROM were not significantly different between the 2 groups (see ). The median gestational age at delivery was not significantly different between patients with and without FIRS.
Table II. Clinical characteristics of the study population with and without FIRS.
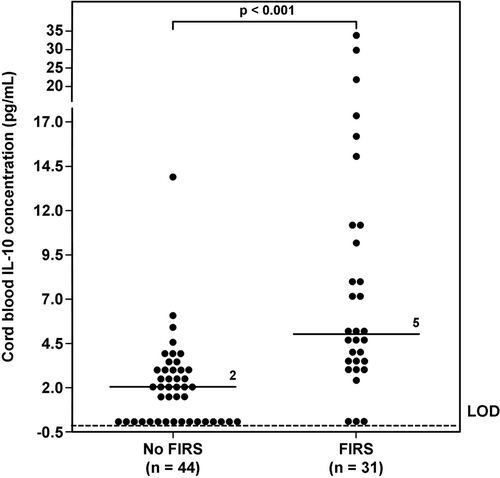
Newborns who were diagnosed with FIRS had a significantly higher median umbilical cord plasma IL-19 concentration than those without FIRS (with FIRS median: 90 pg/mL; range 13.6–412.6 pg/mL vs. without FIRS median: 43 pg/mL; range 17.9–198 pg/mL; p < 0.001; ). The median umbilical cord plasma IL-10 concentration in neonates with FIRS was higher than that of those without FIRS (with FIRS median: 5 pg/mL; range 0–33.5 pg/mL vs. without FIRS median: 2 pg/mL; range 0–13.8 pg/mL; p < 0.001; ).
Figure 3. Umbilical cord plasma interleukin (IL)-19 concentrations in neonates with and without Fetal Inflammatory Response Syndrome (FIRS). Newborns who were diagnosed with FIRS had a significantly higher median umbilical cord plasma IL-19 concentration than those who did not have FIRS (FIRS median: 90 pg/mL; range 13.6–412.6 pg/mL vs. No FIRS median: 43 pg/mL; range 17.9–198 pg/mL; p < 0.001).
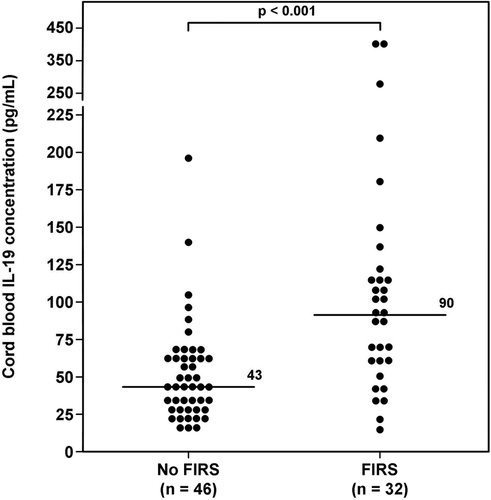
Figure 4. Umbilical cord plasma interleukin (IL)-10 concentrations in neonates with and without Fetal Inflammatory Response Syndrome (FIRS). The median umbilical cord plasma IL-10 concentration in neonates with FIRS was higher than that of those without FIRS (FIRS median: 5 pg/mL; range 0–33.5 pg/mL vs. No FIRS median: 2 pg/mL; range 0–13.8 pg/mL; p < 0.001). LOD = limit of detection.
When patients were stratified according to membrane status (PTL with intact membranes or preterm PROM), neonates with FIRS still had higher median umbilical cord plasma concentrations of IL-19 and IL-10 than those without FIRS (p < 0.05 for each).
Umbilical cord plasma concentration of IL-19 was correlated with IL-10 in funisitis and FIRS
Among neonates with funisitis, there was a significant positive correlation between umbilical cord plasma concentrations of IL-19 and IL-10 (Spearman Rho = 0.5, p = 0.003; , but not between IL-19 and IL-6 (p = 0.3). In contrast, among patients who did not have funisitis, there were no correlations in any of the cytokines (p > 0.05; .
Figure 5. Correlation between umbilical cord plasma concentrations of interleukin (IL)-19 and IL-10 in neonates with (5a) and without funisitis (5b). Among neonates with funisitis, there was a significant positive correlation between umbilical cord plasma concentrations of IL-19 and IL-10 (Spearman Rho = 0.5, p = 0.003; . In contrast, among neonates without funisitis, there was no correlation between umbilical cord plasma concentrations of IL-19 and IL-10 (p>0.05; .
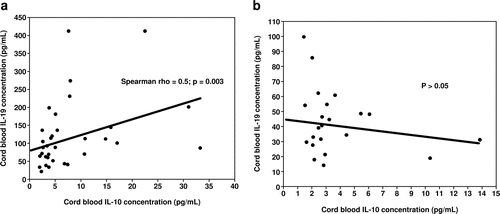
Similarly, among neonates who were diagnosed with FIRS, there was a significant positive correlation between umbilical cord plasma concentrations of IL-19 and IL-10 (Spearman Rho = 0.4, p = 0.01), but not between IL-19 and IL-6 (p = 0.4). In contrast, among neonates without FIRS, there was no significant correlation among the plasma concentration of any of the cytokines (p > 0.05).
Discussion
Principal findings of this study
1) FIRS, diagnosed by either neonatal cord plasma IL-6 concentrations ≥ 17.5 pg/mL or the presence of funisitis, is associated with a higher concentration of umbilical cord plasma IL-19 and IL-10 than neonates without FIRS and funisitis; 2) there was a significant correlation between the umbilical cord plasma concentrations of IL-19 and IL-10 in neonates with FIRS; and 3) these observations represent the first evidence that changes in IL-19 occur during fetal life, and that this cytokine participates in the host response associated with preterm delivery and acute inflammation.
The original description of FIRS
FIRS was originally described in patients with spontaneous PTL and preterm PROM and defined by fetal plasma IL-6 concentrations ≥11 pg/mL obtained by cordocentesis. [Citation1] Subsequently, umbilical cord plasma IL-6 concentrations ≥17.5 pg/mL [Citation54] were shown to be associated with increased neonatal complications, [Citation1,Citation23,Citation26,Citation41,Citation42,Citation52,Citation54] and therefore, this definition was accepted as evidence of FIRS. [Citation166] Indeed, funisitis is associated with an elevation of umbilical cord concentrations of IL-6 as well as short- and long-term complications in preterm neonates. [Citation1,Citation8,Citation19,Citation22–30,Citation33,Citation35–37,Citation41–43,Citation45,Citation47,Citation49,Citation51,Citation54,Citation165–184]
FIRS is frequently found in patients with intra-amniotic infection/inflammation. While several studies observed an elevation of IL-6, [Citation19,Citation23,Citation37,Citation40,Citation41,Citation52–54,Citation168,Citation174,Citation179,Citation183,Citation185–191] IL-1β, [Citation37,Citation52,Citation174,Citation179,Citation188,Citation191–196] tumor necrosis factor-α and its receptor [Citation37,Citation52,Citation53,Citation188,Citation196] IL-8, [Citation188,Citation197] matrix degrading enzymes (i.e. MMP-8, MMP-9, etc) [Citation173,Citation198–210] and C-reactive protein [Citation37,Citation173,Citation198,Citation211–214] in the amniotic fluid and umbilical cord blood of patients with intra-amniotic infection/inflammation, only few studies examined IL-10, the anti-inflammatory limb of the immune response, in the amniotic fluid [Citation146] and cord blood [Citation7,Citation8,Citation215] of these patients.
Interleukin-19
IL-19 was discovered as a member of IL-10 family. [Citation147] This protein is produced by activated monocytes and, to a lesser extent, B-cells, [Citation216] but it is also identified in non-immune cells such as keratinocytes, [Citation161] bronchial epithelial cells [Citation158,Citation217] and the chorioamniotic membranes. [Citation218] The expression of IL-19 on monocytes can be induced by LPS, IL-4 and granulocyte macrophage-colony stimulating factor (GM-CSF). [Citation147]
IL-19 exerts its biological activities through a heterodimer complex, IL-20 RI and IL-20 RII. [Citation219,Citation220] However, IL-19 only binds to IL-20RII and requires a conformational change before it binds to IL-20RI. The receptors are present in various tissues including skin, lung, heart, muscle, placenta, adrenal gland, small intestine, salivary gland and reproductive organs such as uterus, ovary and testis. [Citation220]
IL-19 activates monocytes in an autocrine and paracrine fashion to release IL-6, TNF-α, IL-8 and reactive oxygen species. [Citation147,Citation154,Citation155] However, this cytokine can also dose-dependently induce IL-10, a major anti-inflammatory cytokine, in human peripheral blood mononuclear cell cultures. [Citation148] IL-19 can amplify its own expression during the course of the immune response. [Citation148]
IL-19 can also participate in the activation of the adaptive immune response, since this cytokine is a potent inducer of the Th2 responses. [Citation148,Citation155] The treatment of T cells with IL-19 up-regulates IL-4 and down-regulates IFN-γ. [Citation155] Similarly, the treatment of maturing antigen presenting cells (eg: dendritic cells) with IL-19 induces IL-10 production, which in turn, promotes the Th2/regulatory T response. [Citation148,Citation220] However, IL-19 expression seems to be controlled by IL-10 since IL-10 inhibits IL-19 expression in monocyte-derived dendritic cells and the addition of anti-IL-10 monoclonal antibodies causes an increase in IL-19 transcription. [Citation148]
FIRS is associated with an elevation of IL-19
The finding that neonates with FIRS had higher plasma concentrations of IL-19 than those without FIRS is consistent with the observation in adults that IL-19 is involved in the pathophysiology of sepsis. Serum IL-19 concentrations were elevated in adult patients with sepsis. [Citation154] In an animal model of endotoxemia, intraperitoneal administration of endotoxin up-regulated mRNA expression of IL-19 and its receptors (IL-20RI and RII) in several organs (heart, liver, lung and kidneys). [Citation154] Moreover, IL-19 was shown to induce the production of reactive oxygen species, TNF-α and COX-2 mRNA expression, as well as apoptosis in lung epithelial cells and hepatocytes. [Citation154] The treatment of mice with an IL-19 blocker (soluble receptor plasmid DNA) before the administration of endotoxin reduced neutrophil infiltration in the lung and liver, as well as the serum concentrations of liver enzymes. [Citation154] Therefore, IL-19 is a part of the immunological response in SIRS. It remains to be determined, however, if systemic elevation of IL-19 is associated with fetal injury or end-organ damage in fetuses with FIRS.
FIRS was associated with an elevation of IL-10 concentration
The observation that umbilical cord plasma IL-10 concentrations were elevated in preterm neonates with FIRS is consistent with the findings from previous studies. [Citation7,Citation8] Preterm infants who had funisitis in the umbilical cord had a higher gene expression of IL-10 in cord blood mononuclear cells. [Citation7] Additionally, histologic chorioamnionitis was associated with elevated cord blood IL-10 concentrations in very preterm infants. [Citation8] The risks of bronchopulmonary dysplasia and severity of respiratory distress in these infants were associated with elevated cord blood IL-10 concentrations. [Citation8] One interpretation of these findings is that IL-10 is elevated in FIRS to counteract the effect of several pro-inflammatory cytokines observed in FIRS including IL-19, since IL-10 is capable of inhibiting IL-19 expression. Consistent with the hypothesis, we observed a relationship between cord plasma concentrations of IL-19 and IL-10 in neonates with FIRS.
The protective effect of IL-10 in obstetrical diseases has been demonstrated in several studies. [Citation215,Citation221,Citation222] The administration of recombinant IL-10 prevented preterm delivery and miscarriage in IL-10 knockout mice that had a high rate of pregnancy loss after exposure to endotoxin. [Citation215] Similarly, the treatment of IL-10 significantly reduced lipopolysaccharide (LPS)-induced IL-6 production in human chorioamniotic membranes. [Citation223] Moreover, IL-10 was shown to be protective in infection/inflammation-induced fetal brain injury. [Citation224–227]
Potential long-term consequences of IL-19 elevation in human fetuses
Asthma, a disease characterized by airway hyperreactivity, has been proposed to be a consequence of excessive production of IL-4, IL-5 and IL-13 by Th2 cells. [Citation228–231] IL-19 is a potent inducer of the Th2 response, and the concentration of this cytokine is elevated in systemic circulation of patients with asthma. [Citation155,Citation156,Citation158] Moreover, IL-19 expression has been shown to be up-regulated in the airway epithelia of these patients. [Citation156] Accumulating evidence suggests that in utero exposure to immunomodulatory factors (i.e. endotoxin, allergens from farming environments, and tobacco smoke) may play a role in airway development, inflammation and remodeling, which act in concert with postnatal factors to predispose to the development of asthma later in life. [Citation232–235] Although, in animal experiments, antenatal exposure of endotoxin to the mothers has been proposed to promote the Th1 immune environment which suppresses the development of allergic airway disease in offspring later in life, [Citation233,Citation236] it is possible that a subset of preterm neonates with FIRS who are exposed to high systemic concentrations of IL-19 in utero may have an increased risk of childhood asthma. Indeed, clinical chorioamnionitis at preterm gestation, but not at term gestation, is a risk factor for childhood asthma (<8 years old). [Citation232] Evidence in support of this hypothesis are: 1) FIRS is more frequently observed in patients with early spontaneous preterm delivery [Citation1,Citation174,Citation237] rather than in term delivery; [Citation49] 2) amniotic fluid concentrations of IL-19 were elevated in patients with intra-amniotic infection / inflammation in preterm gestation, but not at term; [Citation238] and 3) neonates with FIRS had systemic elevation of IL-19 concentrations as demonstrated in this study.
Strengths and limitations of the study
This is the first study to report the changes of umbilical cord plasma concentrations of IL-19 and IL-10 in neonates with FIRS. The diagnosis of FIRS was defined stringently by cord plasma IL-6 concentrations and by histopathologic examination of the umbilical cord. Patients with funisitis were matched for gestational age at delivery with those without funisitis. Moreover, umbilical cord plasma IL-10 concentrations, a major anti-inflammatory cytokine, were examined in these patients. The correlation between cord plasma IL-19 and IL-10 concentrations observed in patients with FIRS indicates a close relationship between these two cytokines. A limitation of this study is that all patients were enrolled from a single medical center and that most patients were African-American; thus, it would be desirable for this study to be replicated in other populations.
Conclusion
We conclude that IL-19 and IL-10 are part of the immunologic response of FIRS. In utero exposure to high systemic IL-19 concentrations may be a link between clinical chorioamnionitis in preterm gestation and the subsequent reprogramming of the immune system. This may include the development of a sustained immunosuppressive state, or a shift to a Th2 response which may predispose to the development of childhood asthma. It is important to stress that sudden infant death syndrome (SIDS) remains unexplained in a majority of cases, [Citation239–241] and that preterm birth is a risk factor for SIDS. [Citation242] It is possible that the activation of the anti-inflammatory limb of the immune response after in utero exposure to microorganisms or microbial products may predispose to SIDS, [Citation243,Citation244] just as sepsis predisposes to death in adult patients. This hypothesis has substantial implications for the prenatal diagnosis of infection and the assessment of the immunological state of the neonate and the infant after birth. Some immune responses may be short-lived, while others may develop a chronic phase and confer different risks for the individual by reprogramming the immune system.
Acknowledgement
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Presented at the 56th Annual Meeting of the Society for Gynecologic Investigation, March 16–19, 2011, Miami, FL
Declaration of Interest: The authors declare no conflicts of interest exist.
References
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202.
- Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–683.
- Andrews WW, Cliver SP, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt AI, Faye-Petersen O, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol 2008;198:466.e1–466.e11.
- Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J Nutr 2003;133:1668S–1673S.
- Arad I, Ergaz Z. The fetal inflammatory response syndrome and associated infant morbidity. Isr Med Assoc J 2004;6:766–769.
- Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol 2007;21:479–489.
- Wirbelauer J, Seidenspinner S, Thomas W, Kunzmann S, Speer CP. Funisitis is associated with increased interleukin-10 gene expression in cord blood mononuclear cells in preterm infants = 32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol 2011;155:31–35.
- Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr 2009;154:39–43.e3.
- Berry SM, Gomez R, Athayde N, Ghezzi F, Mazor M, Yoon BH, Edwin SS et al. The role of granulocyte colony stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;178:S202.
- Heifetz SA, Bauman M. Necrotizing funisitis and herpes simplex infection of placental and decidual tissues: study of four cases. Hum Pathol 1994;25:715–722.
- Hyde SR, Giacoia GP. Congenital herpes infection: placental and umbilical cord findings. Obstet Gynecol 1993;81:852–855.
- Von Herzen JL, Benirschke K. Unexpected disseminated herpes simplex infection in a newborn. Obstet Gynecol 1977;50:728–730.
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry 2002;7:633–640.
- McCarthy M, Auger D, Whittemore SR. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J Hum Virol 2000;3:215–228.
- Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 2010;185:1248–1257.
- Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol 2011;65:110–117.
- Gabrielli L, Bonasoni MP, Lazzarotto T, Lega S, Santini D, Foschini MP, Guerra B, et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol 2009;46 Suppl 4:S16–S21.
- Vaisbuch E, Romero R, Gomez R, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, Hassan SS. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2011;24:391–396.
- Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–193.
- Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 2011;53:170–177.
- Mittendorf R, Covert R, Montag AG, elMasri W, Muraskas J, Lee KS, Pryde PG. Special relationships between fetal inflammatory response syndrome and bronchopulmonary dysplasia in neonates. J Perinat Med 2005;33:428–434.
- Mittendorf R, Montag AG, MacMillan W, Janeczek S, Pryde PG, Besinger RE, Gianopoulos JG, Roizen N. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol 2003;188:1438–4; discussion 1444.
- Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–1440.
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8.
- Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 1999;46:566–575.
- Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr 2000;12:99–104.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–681.
- Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J Obstet Gynaecol Can 2002;24:705–709.
- Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112 Suppl 1:16–18.
- Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med 2006;34:5–12.
- Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, Bianchi DW. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995;173:1315–1320.
- Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, Brozanski BS. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51:310–316.
- Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, Park JS. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1999;181:784–788.
- Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, Loverro G. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol 2006;194:153–159.
- Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology 2006;49:506–514.
- Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, Chi JG. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol 2001;185:496–500.
- Santana C, Guindeo MC, González G, García-Muñoz F, Saavedra P, Doménech E. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr 2001;90:1176–1181.
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–808.
- Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10.
- Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–970.
- Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–779.
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110 Suppl 20:124–127.
- Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, Park JS. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998;179:1107–1114.
- Gomez R, Berry SM, Yoon BH, Mazor M, Athayde N, Ghezzi F, Romero R. The hematologic profile of the fetus with systemic inflammatory response syndrome. Am J Obstet Gynecol 1998;179:S202.
- Romero R, Espinoza J, Gonçalves LF, Gomez R, Medina L, Silva M, Chaiworapongsa T, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–157.
- Letti Müller AL, Barrios Pde M, Kliemann LM, Valério EG, Gasnier R, Magalhães JA. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet Gynecol 2010;36:26–31.
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci 2011 March 4 [e-pup ahead of publication] PMID 21382466.
- Kannan S, Balakrishnan B, Muzik O, Romero R, Chugani D. Positron emission tomography imaging of neuroinflammation. J Child Neurol 2009;24:1190–1199.
- Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol 2001;32:623–629.
- Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, Kim M, Shim SS. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–306.
- Sergeeva VA, Nesterenko SN, Shabalov NP, Aleksandrovich IuS. [Fetal inflammatory response in the development of multiple organ dysfunction in newborn]. Anesteziol Reanimatol 2010;1:30–34.
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26.
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 1997;177:406–411.
- Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–1129.
- Bry K, Hogmalm A, Bäckström E. Mechanisms of inflammatory lung injury in the neonate: lessons from a transgenic mouse model of bronchopulmonary dysplasia. Semin Perinatol 2010;34:211–221.
- Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thébaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res 2009;65:430–436.
- Londhe VA, Nguyen HT, Jeng JM, Li X, Li C, Tiozzo C, Zhu N, Minoo P. NF-kB induces lung maturation during mouse lung morphogenesis. Dev Dyn 2008;237:328–338.
- Han YM, Romero R, Kim YM, Kim JS, Richani K, Friel LA, Kusanovic JP, et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol 2007;211:489–496.
- Prince LS, Okoh VO, Moninger TO, Matalon S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2004;287:L999–1006.
- Sakai M, Tanebe K, Sasaki Y, Momma K, Yoneda S, Saito S. Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol Hum Reprod 2001;7:595–602.
- Fidel PI Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med 1998;7:222–226.
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res 2010;88:1872–1881.
- Ernst LM, Gonzalez J, Ofori E, Elovitz M. Inflammation-induced preterm birth in a murine model is associated with increases in fetal macrophages and circulating erythroid precursors. Pediatr Dev Pathol 2010;13:273–281.
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–1045.
- Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177:797–802.
- Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J, Wolf N, Gomez R. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003;14:57–64.
- Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, Chugani D. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol 2009;24:1179–1189.
- Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol 2008;199:651.e1–651.e7.
- Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-®-PK11195 and small-animal PET. J Nucl Med 2007;48:946–954.
- Gibbs RS, McDuffie RS Jr, Kunze M, Barr JM, Wolf DM, Sze CI, Shikes R, Sherman MP. Experimental intrauterine infection with Prevotella bivia in New Zealand White rabbits. Am J Obstet Gynecol 2004;190:1082–1086.
- Vayrynen O, Glumoff V, Hallman M. Inflammatory and anti-inflammatory responsiveness of surfactant proteins in fetal and neonatal rabbit lung. Pediatr Res 2004;55:55–60.
- Gras-Le Guen C, Debillon T, Toquet C, Jarry A, Winer N, Jacqueline C, Kergueris MF, et al. Persistent bacteremia in rabbit fetuses despite maternal antibiotic therapy in a novel intrauterine-infection model. Antimicrob Agents Chemother 2003;47:2125–2130.
- Debillon T, Gras-Leguen C, Leroy S, Caillon J, Rozé JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Brain Res Dev Brain Res 2003;145:39–48.
- Berry CA, Nitsos I, Hillman NH, Pillow JJ, Polglase GR, Kramer BW, Kemp MW, et al. Interleukin 1 in Lipopolysaccharide Induced Chorioamnionitis in the Fetal Sheep. Reprod Sci 2011 April 14 [e-pup ahead of publication] PMID 21493953.
- Nitsos I, Newnham JP, Rees SM, Harding R, Moss TJ. The Impact of Chronic Intrauterine Inflammation on the Physiologic and Neurodevelopmental Consequences of Intermittent Umbilical Cord Occlusion in Fetal Sheep. Reprod Sci 2011 March 18 [e-pup ahead of publication] PMID 21421894.
- Lee AJ, Lambermont VA, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, Beilharz MW, et al. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am J Obstet Gynecol 2011;204:364.e17–364.e24.
- Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, Kuypers E, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 2010;299:L852–L860.
- Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, Jobe AH, Kallapur SG. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res 2010;68:210–215.
- Polglase GR, Dalton RG, Nitsos I, Knox CL, Pillow JJ, Jobe AH, Moss TJ, et al. Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 2010;299:L232–L241.
- Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, Speer CP, et al. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol 2010;202:476.e1–476.e9.
- Polglase GR, Hooper SB, Gill AW, Allison BJ, Crossley KJ, Moss TJ, Nitsos I, et al. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. J Appl Physiol 2010;108:1757–1765.
- Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Polglase GP, Newnham JP, Jobe AH. Modulation of fetal inflammatory response on exposure to lipopolysaccharide by chorioamnion, lung, or gut in sheep. Am J Obstet Gynecol 2010;202:77.e1–77.e9.
- Kallapur SG, Moss TJ, Auten RL Jr, Nitsos I, Pillow JJ, Kramer BW, Maeda DY, et al. IL-8 signaling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. Am J Physiol Lung Cell Mol Physiol 2009;297:L512–L519.
- Saito M, Matsuda T, Okuyama K, Kobayashi Y, Kitanishi R, Hanita T, Okamura K. Effect of intrauterine inflammation on fetal cerebral hemodynamics and white-matter injury in chronically instrumented fetal sheep. Am J Obstet Gynecol 2009;200:663.e1–663.11.
- Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun 2009;15:101–107.
- Gavilanes AW, Strackx E, Kramer BW, Gantert M, Van den Hove D, Steinbusch H, Garnier Y, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol 2009;200:437.e1–437.e8.
- Cheah FC, Pillow JJ, Kramer BW, Polglase GR, Nitsos I, Newnham JP, Jobe AH, Kallapur SG. Airway inflammatory cell responses to intra-amniotic lipopolysaccharide in a sheep model of chorioamnionitis. Am J Physiol Lung Cell Mol Physiol 2009;296:L384–L393.
- Kramer BW, Albertine KH, Moss TJ, Nitsos I, Ladenburger A, Speer CP, Newnham JP, Jobe AH. All-trans retinoic acid and intra-amniotic endotoxin-mediated effects on fetal sheep lung. Anat Rec (Hoboken) 2008;291:1271–1277.
- Sweet DG, Huggett MT, Warner JA, Moss TJ, Kloosterboer N, Halliday HL, Newnham JP, et al. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep. Neonatology 2008;94:79–86.
- Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol 2008;198:122.e1–122.e8.
- Cheah FC, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr Res 2008;63:274–279.
- Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, Newnham JP, Kramer BW. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol 2007;179:8491–8499.
- Watanabe T, Matsuda T, Hanita T, Okuyama K, Cho K, Kobayashi K, Kobayashi Y. Induction of necrotizing funisitis by fetal administration of intravenous granulocyte-colony stimulating factor and intra-amniotic endotoxin in premature fetal sheep. Pediatr Res 2007;62:670–673.
- Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 2007;293:L345–L353.
- Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, Jobe AH. IL-1 alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res 2006;60:294–298.
- Kallapur SG, Moss TJ, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med 2005;172:1315–1321.
- Kallapur SG, Nitsos I, Moss TJ, Kramer BW, Newnham JP, Ikegami M, Jobe AH. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol 2005;288:L966–L974.
- Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med 2005;171:73–77.
- Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 2004;287:L1178–L1185.
- Mulrooney N, Jobe AH, Ikegami M. Lung inflammatory responses to intratracheal interleukin-1alpha in ventilated preterm lambs. Pediatr Res 2004;55:682–687.
- Moss TJ, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol 2003;189:1771–1776.
- Newnham JP, Kallapur SG, Kramer BW, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol 2003;189:1458–1466.
- Ikegami M, Kallapur SG, Jobe AH. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am J Physiol Lung Cell Mol Physiol 2004;286:L573–L579.
- Ikegami M, Moss TJ, Kallapur SG, Mulrooney N, Kramer BW, Nitsos I, Bachurski CJ, et al. Minimal lung and systemic responses to TNF-alpha in preterm sheep. Am J Physiol Lung Cell Mol Physiol 2003;285:L121–L129.
- Ikegami M, Jobe AH. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to Escherichia coli endotoxin. Pediatr Res 2002;52:356–362.
- Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 2002;283:L452–L459.
- Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol 2002;186:1062–1068.
- Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002;165:805–811.
- Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 2001;164:982–988.
- Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2001;280:L527–L536.
- Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG 2011;118:136–144.
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70.
- Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci 2008;15:121–127.
- Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, Novy MJ. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol 2007;197:518.e1–518.e8.
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006;195:1578–1589.
- Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol 2003;188:252–263.
- Macias AE, Wong SW, Sadowsky DW, Luetjens CM, Axthelm MK, Gravett MG, Haluska GJ, Novy MJ. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol 2001;55:159–170.
- Gravett MG, Novy MJ. Endocrine-immune interactions in pregnant non-human primates with intrauterine infection. Infect Dis Obstet Gynecol 1997;5:142–153.
- Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol 1996;174:1725–31; discussion 1731.
- Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig 1996;3:121–126.
- Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol 1994;171:1668–1672.
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–1667.
- Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, Erez O, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med 2009;37:543–552.
- Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med 2002;33:1173–1185.
- von Dessauer B, Bongain J, Molina V, Quilodrán J, Castillo R, Rodrigo R. Oxidative stress as a novel target in pediatric sepsis management. J Crit Care 2011;26:103.e1–103.e7.
- Brenner T, Hofer S, Rosenhagen C, Steppan J, Lichtenstern C, Weitz J, Bruckner T, et al. Macrophage migration inhibitory factor (MIF) and manganese superoxide dismutase (MnSOD) as early predictors for survival in patients with severe sepsis or septic shock. J Surg Res 2010;164:e163–e171.
- Huet O, Dupic L, Harrois A, Duranteau J. Oxidative stress and endothelial dysfunction during sepsis. Front Biosci 2011;16:1986–1995.
- Mishra V. Oxidative stress and role of antioxidant supplementation in critical illness. Clin Lab 2007;53:199–209.
- Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92.
- Bhatia M, He M, Zhang H, Moochhala S. Sepsis as a model of SIRS. Front Biosci 2009;14:4703–4711.
- Werdan K, Schmidt H, Ebelt H, Zorn-Pauly K, Koidl B, Hoke RS, Heinroth K, Müller-Werdan U. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol 2009;87:266–274.
- Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007;38:1336–1345.
- Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol 2009;69:479–491.
- Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 2001;16:83–96.
- Payen D, Faivre V, Lukaszewicz AC, Losser MR. Assessment of immunological status in the critically ill. Minerva Anestesiol 2000;66:757–763.
- Goris RJ. MODS/SIRS: result of an overwhelming inflammatory response? World J Surg 1996;20:418–421.
- Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost 2009;101:36–47.
- Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med 2008;29:617–25, viii.
- Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr Pharm Des 2008;14:1870–1881.
- Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res 2001;24:273–287.
- Keel M, Ungethüm U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood 1997;90:3356–3363.
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109.
- Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med 2005;33:S468–S471.
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–3822.
- Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol 1993;11:165–190.
- Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–547.
- Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun 2000;1:442–450.
- Jordan WJ, Eskdale J, Boniotto M, Lennon GP, Peat J, Campbell JD, Gallagher G. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol 2005;35:1576–1582.
- Azuma YT, Matsuo Y, Nakajima H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Takeuchi T. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci 2011;115:105–111.
- Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, et al. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis 2010;16:1017–1028.
- Cuneo AA, Herrick D, Autieri MV. Il-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol 2010;49:647–654.
- Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol 2011;31:167–175.
- Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J Immunol 2002;169:4288–4297.
- Hsing CH, Chiu CJ, Chang LY, Hsu CC, Chang MS. IL-19 is involved in the pathogenesis of endotoxic shock. Shock 2008;29:7–15.
- Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol 2004;4:615–626.
- Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, Wu R. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J Allergy Clin Immunol 2008;121:1415–21, 1421.e1.
- Li HH, Lin YC, Chen PJ, Hsiao CH, Lee JY, Chen WC, Tzung TY, et al. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol 2005;153:591–595.
- Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, et al. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol 2004;173:6712–6718.
- Kingo K, Mössner R, Rätsep R, Raud K, Krüger U, Silm H, Vasar E, et al. Association analysis of IL20RA and IL20RB genes in psoriasis. Genes Immun 2008;9:445–451.
- Kõks S, Kingo K, Vabrit K, Rätsep R, Karelson M, Silm H, Vasar E. Possible relations between the polymorphisms of the cytokines IL-19, IL-20 and IL-24 and plaque-type psoriasis. Genes Immun 2005;6:407–415.
- Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, et al. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 2006;15:991–1004.
- Okayama N, Suehiro Y, Hamanaka Y, Nakamura J, Hinoda Y. Association of interleukin-19 gene polymorphisms with age. J Gerontol A Biol Sci Med Sci 2007;62:507–511.
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, et al.; Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707.
- Pinkerton MN, Wescott DC, Gaffey BJ, Beggs KT, Milne TJ, Meikle MC. Cultured human periodontal ligament cells constitutively express multiple osteotropic cytokines and growth factors, several of which are responsive to mechanical deformation. J Periodont Res 2008;43:343–351.
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25.
- Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, Gomez R. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 2002;186:1178–1182.
- Liechty KW, Koenig JM, Mitchell MD, Romero R, Christensen RD. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin-1. Pediatr Res 1991;29:1–4.
- Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 220.
- Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol 2001;6:153–163.
- Schendel DE. Infection in pregnancy and cerebral palsy. J Am Med Womens Assoc 2001;56:105–108.
- Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13.
- Baud O, Fontaine RH, Olivier P, Maury L, El Moussawi F, Bauvin I, Arsac M, et al. [Premature rupture of membranes: pathophysiology of neurological impact]. Arch Pediatr 2007;14 Suppl 1:S49–S53.
- Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294.e1–294.e6.
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39.
- Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, Achiron R. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol 2007;29:639–643.
- El-Haieg DO, Zidan AA, El-Nemr MM. The relationship between sonographic fetal thymus size and the components of the systemic fetal inflammatory response syndrome in women with preterm prelabour rupture of membranes. BJOG 2008;115:836–841.
- Kramer BW. Antenatal inflammation and lung injury: prenatal origin of neonatal disease. J Perinatol 2008;28 Suppl 1:S21–S27.
- Ledger WJ. Perinatal infections and fetal/neonatal brain injury. Curr Opin Obstet Gynecol 2008;20:120–124.
- Skogstrand K, Hougaard DM, Schendel DE, Bent NP, Svaerke C, Thorsen P. Association of preterm birth with sustained postnatal inflammatory response. Obstet Gynecol 2008;111:1118–1128.
- Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med 2009;14:2–7.
- Lee J, Seong HS, Kim BJ, Jun JK, Romero R, Yoon BH. Evidence to support that spontaneous preterm labor is adaptive in nature: neonatal RDS is more common in “indicated” than in “spontaneous” preterm birth. J Perinat Med 2009;37:53–58.
- Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–923.
- Nishimaki S, Sato M, An H, Shima Y, Akaike T, Yokoyama U, Yokota S. Comparison of markers for fetal inflammatory response syndrome: fetal blood interleukin-6 and neonatal urinary beta(2)-microglobulin. J Obstet Gynaecol Res 2009;35:472–476.
- Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol 2010;34:408–415.
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–1400.
- Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–183.
- Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–816.
- Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–830.
- Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, Edwin S, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med 2003;13:32–38.
- Volante E, Moretti S, Pisani F, Bevilacqua G. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med 2004;16 Suppl 2:13–16.
- Fukuda H, Masuzaki H, Ishimaru T. Interleukin-6 and interleukin-1 receptor antagonist in amniotic fluid and cord blood in patients with pre-term, premature rupture of the membranes. Int J Gynaecol Obstet 2002;77:123–129.
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–123.
- Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, Mitchell MD. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol 1992;167:863–872.
- Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, Cotton DB. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol 1994;171:912–921.
- Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–1123.
- Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, Mansour JM, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod 2006;74:29–34.
- Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–820.
- Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med 2010;38:39–44.
- Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med 1999;27:362–368.
- Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol 2001;184:1399–405; discussion 1405.
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–1130.
- Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–1253.
- Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, Yoon BH, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med 1999;8:213–219.
- Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000;183:887–894.
- Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, Seubert DE. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–1553.
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–99.
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–920.
- Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, Edwin S. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:1143–1148.
- Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, Edwin S. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1149–1155.
- Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–316.
- Borna S, Mirzaie F, Abdollahi A. Mid-trimester amniotic fluid C-reactive protein, ferritin and lactate dehydrogenase concentrations and subsequent risk of spontaneous preterm labour. Aust N Z J Obstet Gynaecol 2009;49:400–403.
- Mathai E, Christopher U, Mathai M, Jana AK, Rose D, Bergstrom S. Is C-reactive protein level useful in differentiating infected from uninfected neonates among those at risk of infection? Indian Pediatr 2004;41:895–900.
- Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90.
- Mazor M, Kassis A, Horowitz S, Wiznitzer A, Kuperman O, Meril C, Glezerman M. Relationship between C-reactive protein levels and intraamniotic infection in women with preterm labor. J Reprod Med 1993;38:799–803.
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol 2006;177:4888–4896.
- Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 2002;168:5397–5402.
- Zhong H, Wu Y, Belardinelli L, Zeng D. A2B adenosine receptors induce IL-19 from bronchial epithelial cells, resulting in TNF-alpha increase. Am J Respir Cell Mol Biol 2006;35:587–592.
- Menon R, Ismail L, Ismail D, Merialdi M, Lombardi SJ, Fortunato SJ. Human fetal membrane expression of IL-19 and IL-20 and its differential effect on inflammatory cytokine production. J Matern Fetal Neonatal Med 2006;19:209–214.
- Leng RX, Pan HF, Tao JH, Ye DQ. IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets 2011;15:119–126.
- Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem 2002;277:47517–47523.
- Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol 2009;200:308.e1–308.e9.
- Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–480.
- Fortunato SJ, Menon R, Swan KF, Lombardi SJ. Interleukin-10 inhibition of interleukin-6 in human amniochorionic membrane: transcriptional regulation. Am J Obstet Gynecol 1996;175:1057–1065.
- Frøen JF, Munkeby BH, Stray-Pedersen B, Saugstad OD. Interleukin-10 reverses acute detrimental effects of endotoxin-induced inflammation on perinatal cerebral hypoxia-ischemia. Brain Res 2002;942:87–94.
- Pang Y, Rodts-Palenik S, Cai Z, Bennett WA, Rhodes PG. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli induced neonatal white matter injury. Brain Res Dev Brain Res 2005;157:141–149.
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry 2008;13:208–221.
- Wallace KL, Lopez J, Shaffery JP, Wells A, Paul IA, Bennett WA. Interleukin-10/Ceftriaxone prevents E. coli-induced delays in sensorimotor task learning and spatial memory in neonatal and adult Sprague-Dawley rats. Brain Res Bull 2010;81:141–148.
- Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, Sopori ML. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol 2009;183:2115–2121.
- Tournoy KG, Kips JC, Pauwels RA. The allergen-induced airway hyperresponsiveness in a human-mouse chimera model of asthma is T cell and IL-4 and IL-5 dependent. J Immunol 2001;166:6982–6991.
- van Rijt LS, Lambrecht BN. Role of dendritic cells and Th2 lymphocytes in asthma: lessons from eosinophilic airway inflammation in the mouse. Microsc Res Tech 2001;53:256–272.
- Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 2001;167:4668–4675.
- Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, Jacobsen SJ. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med 2010;164:187–192.
- Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr 2008;20:682–687.
- Abe K, Shapiro-Mendoza CK, Hall LR, Satten GA. Late preterm birth and risk of developing asthma. J Pediatr 2010;157:74–78.
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med 2010;182:25–33.
- Cao L, Wang J, Zhu Y, Tseu I, Post M. Maternal endotoxin exposure attenuates allergic airway disease in infant rats. Am J Physiol Lung Cell Mol Physiol 2010;298:L670–L677.
- Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65:S194–S202.
- Alpay Savasan Z, Chaiwaropongsa T, Kusanovic JP, Dong Z, Kim CJ, Hassan SS, Romero R. Studies of the Novel Cytokine, Interleukin-19, in Pregnancy, Parturition (Term and Preterm) and Intra-Amniotic Infection/Inflammation. Reprod Sci 2011; 18(3 Supplement), 264A.
- Mitchell EA, Tuohy PG, Brunt JM, Thompson JM, Clements MS, Stewart AW, Ford RP, Taylor BJ. Risk factors for sudden infant death syndrome following the prevention campaign in New Zealand: a prospective study. Pediatrics 1997;100:835–840.
- Stewart AJ, Williams SM, Mitchell EA, Taylor BJ, Ford RP, Allen EM. Antenatal and intrapartum factors associated with sudden infant death syndrome in the New Zealand Cot Death Study. J Paediatr Child Health 1995;31:473–478.
- Hunt CE, Hauck FR. Sudden infant death syndrome. CMAJ 2006;174:1861–1869.
- Thompson JM, Mitchell EA; New Zealand Cot Death Study Group. Are the risk factors for SIDS different for preterm and term infants? Arch Dis Child 2006;91:107–111.
- Naeye RL. Placental abnormalities in victims of the sudden infant death syndrome. Biol Neonate 1977;32:189–192.
- Friebe-Hoffmann U, Bender DP, Sims CJ, Rauk PN. Candida albicans chorioamnionitis associated with preterm labor and sudden intrauterine demise of one twin. A case report. J Reprod Med 2000;45:354–356.
