Abstract
Objective: To assess the diagnostic performance of transabdominal sonographic measurement of cervical length in identifying patients with a short cervix. Methods: Cervical length was measured in 220 pregnant women using transabdominal and transvaginal ultrasound (US). Reproducibility and agreement between and within both methods were assessed. The diagnostic accuracy of transabdominal US for identifying cases with a cervical length <25 mm was evaluated. Results: Twenty-one out of 220 cases (9.5%) had a cervical length <25 mm by transvaginal US. Only 43% (n = 9) of patients with a short cervix were correctly identified by transabdominal US. In patients with a cervical length of <25 mm by transvaginal US, transabdominal measurement of the cervix overestimated this parameter by an average of 8 mm (95% LOAs, −26.4 to 10.5 mm). Among women without a short cervix, transabdominal US underestimated cervical length on average (LOA) by 1.1 mm (95% LOAs, −11.0 to 13.2 mm). Transvaginal US was also more reproducible (intraclass correlation coefficient: (ICC) (0.96; 95% CI, 0.94 to 0.97) based on comparisons between 2D images and immediately acquired 3D volume datasets relative to transabdominal US (ICC: 0.71; 95% CI, 0.57 to 0.84). Transvaginal US detected 13 cases with funneling and six cases with sludge whereas only three cases of funneling and one of sludge were detected by transabdominal US. Conclusion: Transabdominal measurement overestimated cervical LOA by 8 mm among women with a short cervix and resulted in the underdiagnosis of 57% of cases.
Introduction
Sonographic cervical length is a powerful method to identify patients at risk for preterm delivery [Citation1–9]. The shorter the cervix, the greater the risk for preterm delivery [Citation10–18]. Patients with a cervical length < 15 mm have an approximate 50% likelihood of preterm delivery < 32 weeks, regardless of risk factors [Citation19,Citation20]. Moreover, it is possible to calculate the individual risk for preterm delivery based on cervical length and other patient characteristics [Citation21].
A short uterine cervix during pregnancy is syndromic in nature and can be the consequence of congenital factors [Citation22–25], prior surgery of the uterine cervix [Citation26–28], subclinical intra-amniotic infection/inflammation [Citation29–36], an entity clinically referred to as cervical insufficiency [Citation37–39], or due to a suspension of progesterone action [Citation40]. Therapeutic interventions for patients with a short cervix include cervical cerclage [Citation41,Citation42], cervical pessary [Citation43,Citation44], and the administration of vaginal progesterone [Citation45–54]. Vaginal progesterone has emerged as an effective therapy to prevent preterm delivery in women with a short cervix in the midtrimester of pregnancy. This intervention also reduces admission to the newborn intensive care unit, respiratory distress syndrome, the requirement for mechanical ventilation, and composite neonatal morbidity and mortality score [Citation54].
While transvaginal ultrasound (US) is considered the “gold standard” for the diagnosis of a short cervix during pregnancy, and its advantages in terms of accuracy and acceptability for patients have been previously described [Citation55–66], several investigators continue to propose that transabdominal cervical length assessment can be used to identify patients with a short cervix. For example, Saul et al. [Citation67] reported that transabdominal sonography had a 100% sensitivity in detecting a short cervix defined by transvaginal sonography (<25 mm). The cut-off used for transabdominal US was a cervical length <30 mm. Stone et al. [Citation68] also proposed that transabdominal US measurement of cervical length could be the primary method for identifying patients with a short cervix.
To address the relative accuracy of transabdominal sonography, as compared with transvaginal ultrasound in the detection of a short cervix during pregnancy, we compared endocervical length obtained by both methods.
Materials and methods
Patients
Two hundred and twenty consecutive pregnant women with a singleton gestation were evaluated at Hutzel Women’s Hospital of the Detroit Medical Center from May to August 2011. All patients provided written informed consent before the US examination. The collection of data for research purposes was approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Sonographic examination
All sonographic examinations were performed using Voluson E8 (GE Healthcare, Milwaukee, WI, USA), Acuson Sequoia (Siemens Medical Systems, Malvern, PA, USA), or Philips iU22 (Philips Healthcare, Bothell, WA, USA) equipment. Three-dimensional (3D) US volumes were obtained only using Voluson E8, and volumetric evaluations performed using 4D View software (GE Healthcare, Milwaukee, WI, USA).
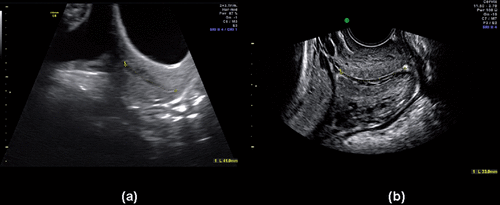
Cervical length was first measured by transabdominal US, with the patient in the supine position and with a full bladder. If the bladder was not sufficiently full to provide an acoustic window, the examination was delayed until visualization of the cervix was achieved. The cervix was identified in the mid-sagittal plane and cervical length was measured by placing the calipers at each end of the endocervical canal (). In 75 patients, an additional transabdominal 3D sonographic volume of the cervix was obtained using an angle of 95°, a fast acquisition, and adjusting the sample box to include the entire cervix. The acquisition plane was a mid-sagittal scan.
A transvaginal US was performed by a different operator blinded to the results of the transabdominal measurement. Patients had an empty bladder for this examination. Cervical length was measured in a mid-sagittal plane from the internal to the external os using methods previously described (). In 100 cases, a 3D US cervical volume was recorded using the sagittal plane as the starting point of acquisition. The presence or absence of funneling and sludge was documented during each examination. The criteria proposed by Burger et al. [Citation69] was used to standardize endocervical canal biometry and examination of the uterine cervix.
Each cervical volume dataset was evaluated by an individual who had not performed the US examination. Multi-planar reformatting was used to select the plane for measurement. In volumes obtained transabdominally and transvaginally, the cervix was displayed in the sagittal plane in quadrant A, the transverse plane in quadrant B and the coronal plane in quadrant C. The optimal plane of measurement was identified by scrolling in quadrants A and C. The image was rotated on the X, Y or Z axis and the size was adjusted to obtain the best image of the cervix which displayed the entire endocervical canal.
Statistical methods
The interclass correlation coefficient and the 95% limits of agreement (LOA) were calculated when comparing transabdominal to transvaginal cervical length measurements [Citation70]. The sensitivity of transabdominal US to detect a short cervix (<25 mm by transvaginal US) was calculated. Measurements of the endocervical canal obtained by transvaginal US were considered the “gold standard”. The frequency with which each method identified sludge and funneling was recorded.
Reproducibility was measured via a modified intraclass correlation coefficient, calculated among 2D and 3D measurements; this is a ‘modified’ indicator because two separate measurements were not obtained. Rather, a 3D volume dataset was acquired immediately following the 2D measurement, meaning that the US probe was not removed and re-inserted to obtain a “true” second measurement. This was done for pragmatic reasons and to decrease any additional discomfort for patients.
Normality of the data distributions was assessed using the Kolmogorov–Smirnov test and inspection of histograms and quantile-quantile (Q-Q) plots. Mean and median differences in cervical length measurements were assessed by parametric (paired t-test) and non-parametric (Wilcoxon Rank Sum) tests. A linear mixed model with random effects was used to account for the paired repeated measures design and, additionally, to adjust for gestational age at time of measurement when comparing transvaginal and transabdominal cervical length measurements. An effect modification term was used to determine whether transvaginal measurements differed from transabdominal by whether the patient had a short cervix as determined by transvaginal US; stratified models were used to illustrate the effect modification. Measurements obtained using 2D relative to 3D US acquired volume datasets were similarly evaluated as an indicator of reproducibility. A p value of < 0.05 was considered significant. Statistical analyses were conducted using SAS statistical analysis software version 9.2 (SAS Institute Inc., Cary, NC).
Results
The median maternal age at the time of the study was 23 years (range: 16–41 years); median gestational age: 24 3/7 weeks (range: 6 2/7 – 39 weeks); and median number of previous pregnancies: 1 (range: 1–4). The prevalence of a short cervix (length <25 mm) was 9.5% (21/220), as determined by transvaginal sonography.
Accuracy of transabdominal versus transvaginal ultrasound
Descriptive statistics of cervical length measurements obtained by the two methods are summarized in . While transabdominal cervical length measurements were normally distributed, cervical length measurements obtained transvaginally departed from normality (Kolmogorov–Smirnov Test p < 0.01). Inspection of histograms and Q-Q plots suggested transvaginal cervical measurements departed only slightly from normality; accordingly, we conducted both parametric and non-parametric tests in assessing differences in cervical length measurements by method.
Table I. Descriptive statistics of the cervical length measurement (mm) performed by transabdominal and transvaginal ultrasound.
Overall, there was moderate correlation between transabdominal and transvaginal cervical length measurements (r = 0.49; 95% CI = 0.39–0.56). The 95% LOAs between transvaginal and transabdominal cervical length measurements ranged from −13.5 to 14 mm (mean difference 0.2 mm). Both parametric and non-parametric tests indicated that overall mean/median cervical length measurements were similar when examined as continuous variables and determined with either method (p = 0.62, p = 0.30, respectively). Adjustment for the paired design, and further, for gestational age at time of examination, did not alter this association (p = 0.63); however, cervical length measurements differed significantly by method with respect to the diagnosis of a short cervix (p =< 0.001).
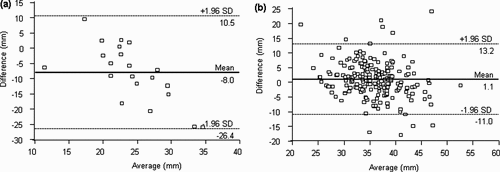
Among patients with a cervical length <25 mm by transvaginal US, a systematic overestimation of cervical length by transabdominal US was observed (mean difference, 8 mm; 95% LOAs, −26.4 to 10.5 mm). Among women without a short cervix, transabdominal US underestimated cervical length on average by 1.1 mm (95% LOAs, −11.0 to 13.2 mm) (). Both parametric and non-parametric tests indicated that the mean/median cervical length measurements obtained via transvaginal US were significantly lower than those obtained by transabdominal US among women with a short cervix (p < 0.001 for both); adjustment for the paired design, and further, for gestational age at time of examination, did not alter this association (p <0.001). Among women without a short cervix, both parametric and non-parametric tests revealed significant mean/median differences in cervical length (p = 0.002 and p = 0.01, respectively). However, as indicated in , the direction of effect was reversed among patients with transvaginally determined cervical lengths > 25 mm, meaning transabdominal measurements were systematically shorter than transvaginal measurements (p = 0.01), although the mean difference did not appear to be clinically significant.
Table II. Differences in cervical length by ultrasound method in the complete study group and stratified by short cervix as determined transvaginally.
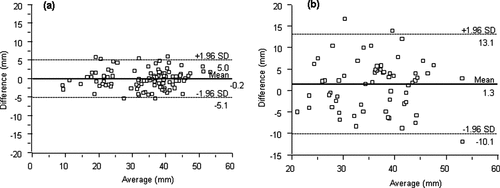
Figure 2. Agreement of cervical measurements < 25 mm (a), or ≥ 25 mm (b) by transvaginal ultrasound with those obtained by transabdominal ultrasound.
Transabdominal cervical length was able to identify only 43% (9 of 21) of patients with a short cervix (cervical length < 25 mm by transvaginal US); in the remaining 12 patients, transabdominal US overestimated the cervical length on average by 14 mm (range 5.6–26 mm). If a 30 mm cut-off had been used to screen women for a short cervix transabdominally in our study, only 3 of 12 missed cases would have been detected. Further, while transvaginal US detected 13 cases with funneling and six cases with sludge, only three cases of funneling and one of sludge were detected by transabdominal US. There were no cases in which funneling or sludge were observed by transabdominal US, but not by transvaginal US.
Reproducibility: 2D versus 3D measurements
Transvaginal measurements were more reproducible based on comparisons between 2D images and immediately acquired 3D volume datasets (intraclass correlation coefficient: 0.96; 95% CI, 0.94–0.97) compared to transabdominal measurements (intraclass correlation coefficient: 0.71; 95% CI, 0.57–0.84). Greater agreement was also observed among measurements obtained transvaginally (95% LOAs, −5.1 to 5.0 mm; mean difference 0.2 mm) than transabdominally (95% LOAs, −10.1 to 13.1 mm; mean difference 1.3 mm) (). The mean/median differences between 2D and 3D volume measurements were marginally statistically significant (p = 0.17 and p = 0.08, respectively); differences among transabdominal measurements were greater than those among transvaginal measurements.
Discussion
Principal findings of this study
This study shows that:
Transabdominal US measurement of cervical length was unable to identify 57% of cases with a short cervix (<25 mm) as determined by transvaginal US;
Transabdominal US systematically overestimated cervical length relative to transvaginal US among women with a short cervix (mean difference, 8 mm; 95% LOAs, −10.5 to 26.4 mm);
Among women with a normal cervical length, transabdominal US underestimated cervical length relative to transvaginal US;
Transvaginal US is more reproducible based on comparisons between 2D images and immediately acquired 3D volume datasets relative to transabdominal US;
Transabdominal US did not detect funneling and sludge in all cases;
The accuracy of transabdominal US differed significantly according to whether a patient had a short cervix or a normal cervical length.
Potential implications for identification of women at risk for preterm delivery
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide [Citation71–77]. In the United States, the cost of preterm birth has been estimated to be $26 billion annually (2005 values), and survivors are at a significantly greater risk of complications including asthma or reactive airway disease, cerebral palsy, developmental delays, autism and behavioral/emotional disorders than infants born at term [Citation78–94].
Cahill et al. [Citation95] evaluated different strategies to reduce the rate of preterm delivery, including identifying patients at risk according to a previous history, by sonographic examination of the cervix, and treatment modalities, including cervical cerclage, 17-α-hydroxyprogesterone caproate and vaginal progesterone. Among different strategies, the authors concluded that universal assessment of cervical length with transvaginal sonography followed by vaginal progesterone administration was the most cost-effective approach [Citation95]. Similarly, Werner et al. [Citation96] concluded that universal cervical screening and vaginal progesterone administration to women with a short cervix will lead to a cost savings of $19 million per 100,000 pregnant women or more than $500 million per year in the United States alone [Citation96].
How should cervical examination be performed to assess the risk of preterm delivery?
Historically, cervical examination was first performed with transabdominal sonography (and subsequently with transperineal sonography), but eventually transvaginal US became the gold standard [Citation97–101]. Visualization of the cervix with transabdominal sonography requires a full maternal bladder to provide an acoustic window to visualize the endocervical canal, internal cervical os and external os [Citation100]. Despite a full bladder, clear definition of the anatomical landmarks is not always possible [Citation102,Citation103]. Therefore, Robinson et al. [Citation104], subsequently proposed the placement of a saline solution into the vagina to improve the definition of the ectocervix; however, this approach is not optimal for patients.
Bladder size also contributes to the variability of measurements obtained transabdominally: while a full bladder allows better visualization of the cervix [Citation105], it can also affect the identification of the landmarks for measurements, and artificially increase the cervical length due to overdistension [Citation100,Citation106]. To et al. [Citation107] reported that the size of the bladder can affect the visualization of the cervix; when the urine volume was <50 ml, the cervix was visualized in only 42% of women. There is also evidence that transabdominal US may be associated with greater discomfort than transvaginal examination. Braithwaite et al. [Citation108] reported that while 3.8% of women reported marked discomfort with transabdominal US, only 0.7% of women reported the same when examined by transvaginal US.
Transvaginal US measurements are affected by the degree of pressure applied with the US transducer to the cervix which can slightly change the orientation and measurements. Maternal age, uterine contractions and cervical dynamic changes can also affect the measurement [Citation109–112]. Londero et al. [Citation113] reported that women younger than 20 years of age had longer cervices than older women, and Meijer-Hoogeveen et al. [Citation114] reported that uterine contractility and bowel peristalsis can modify cervical length by up to 5 mm.
We undertook this study because we were surprised that some investigators continue to propose that transabdominal sonography can be used to screen patients to detect those with a short cervix [Citation67,Citation68]. This strategy had been used in the past in certain units to reduce the number of transvaginal examinations performed. However, this was at the expense of accuracy in cervical length determination, patient comfort (i.e. transabdominal sonography requires a full maternal bladder for optimal visualization, which is uncomfortable and represents a challenge in managing the waiting room of an US unit), and time management efficiency for patients and health care personnel. The current study clearly indicates that screening with transabdominal US for a short cervix would underdiagnose this condition and deny patients the opportunity to benefit from vaginal progesterone administration.
An interesting observation of this study is that when the cervix was short (<25 mm), transabdominal sonography failed to detect 57% of the cases, and the cervical length was overestimated with the transabdominal approach. This was not the case when the cervix was long. Why? One explanation for this is when cervical ripening occurs, the cervix is more compliant [Citation106,Citation115,Citation116]. Consequently, a distended maternal bladder (required for transabdominal sonography) could lengthen the cervix to a greater extent than when the cervix is not ripe (or longer). This interpretation is consistent with an observation that there is not a substantial difference in transabdominal and transvaginal US in which cervical ripening does not occur [Citation117].
Limitations of this study
Reproducibility was not assessed using truly separate measurements, although our finding of greater reproducibility using transvaginal US is consistent with previous reports [Citation55,Citation56,Citation118,Citation119]. Our results may also be specific to our population; however, it is unlikely that demographic patient characteristics have an effect on the results of cervical length using different approaches. One of the limitations of this study is that the number of patients with sludge was small (n = 6), and therefore, the sample size is inadequate to test the sensitivity of transabdominal sonography in the detection of sludge. This important sonographic sign was initially described with transvaginal sonography [Citation120,Citation121], and there is no evidence that transabdominal sonography has a comparable diagnostic value. Indeed, the high frequency transducers used for transvaginal sonography produces images of the cervix and the amniotic fluid in direct proximity to the endocervical canal that are consistently superior to those of transabdominal transducers.
Conclusion
The study indicates that transabdominal US systematically overestimates endocervical length among women with a short cervix. This approach missed 57% of short cervices in the study. Therefore, we conclude that the use of transabdominal US is not appropriate to identify the patients who should have a subsequent transvaginal US to diagnose a short cervix. Our observations suggest that if one of the goals of US examination is to identify patients with a short cervix, transvaginal US should be used as the primary method for measuring the endocervical canal. Otherwise, clinicians are at risk for underdiagnosing not only a short cervix, but also the presence of cervical changes, such as sludge, which is less well-visualized with transabdominal US. The presence of sludge has prognostic value above that provided by cervical length alone. This sign cannot be reliably identified with transabdominal sonography. The need to identify sludge has clinical implications because patients with sludge are at greater risk for intra-amniotic infection/inflammation [Citation121]. The under-diagnosis of a short cervix would result in denying effective and safe therapy for the prevention of preterm birth for a substantial number of patients.
Acknowledgment
This research was supported, in part, by Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Declaration of Interest: The authors report no conflicts of interest.
References
- Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev 2009;(3): CD007235.
- Daskalakis G, Thomakos N, Hatziioannou L, Mesogitis S, Papantoniou N, Antsaklis A. Cervical assessment in women with threatened preterm labor. J Matern Fetal Neonatal Med 2005;17:309–312.
- Hasegawa I, Tanaka K, Takahashi K, Tanaka T, Aoki K, Torii Y, Okai Tet al. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med 1996;5:305–309.
- Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol 2003;22:305–322.
- Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol 2003;101:402–412.
- Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol 2007;30:675–686.
- Rozenberg P, Gillet A, Ville Y. Transvaginal sonographic examination of the cervix in asymptomatic pregnant women: review of the literature. Ultrasound Obstet Gynecol 2002;19:302–311.
- To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol 2006;27:362–367.
- Welsh A, Nicolaides K. Cervical screening for preterm delivery. Curr Opin Obstet Gynecol 2002;14:195–202.
- Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol 2008;31:579–587.
- Crane JM, Hutchens D. Transvaginal ultrasonographic measurement of cervical length in asymptomatic high-risk women with a short cervical length in the previous pregnancy. Ultrasound Obstet Gynecol 2011;38:38–43.
- Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol 2005;193:1170–1174.
- Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 1998;12:312–317.
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996;334:567–572.
- Owen J, Yost N, Berghella V, MacPherson C, Swain M, Dildy GA 3rd, Miodovnik M, et al.; Maternal-Fetal Medicine Units Network. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol 2004;191:298–303.
- Owen J, Szychowski JM, Hankins G, Iams JD, Sheffield JS, Perez-Delboy A, Berghella V, et al.; Vaginal Ultrasound Trial Consortium. Does midtrimester cervical length =25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol 2010;203:393.e1–393.e5.
- Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18-22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol 1998;92:902–907.
- Di Renzo GC, Roura LC, Facchinetti F, Antsaklis A, Breborowicz G, Gratacos E, Husslein P, et al. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Matern Fetal Neonatal Med 2011;24:659–667.
- Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000;182:1458–1467.
- Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, Romero Vet al. Clinical significance of early (< 20 weeks) vs. late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol 2010;36:471–481.
- Celik E, To M, Gajewska K, Smith GC, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol 2008;31:549–554.
- Hovsepian DM, Auyeung A, Ratts VS. A combined surgical and radiologic technique for creating a functional neo-endocervical canal in a case of partial congenital cervical atresia. Fertil Steril 1999;71:158–162.
- Rock JA, Roberts CP, Jones HW. Congenital anomalies of the uterine cervix: lessons from 30 cases managed clinically by a common protocol. Fertil Steril 2010;94:1858–1863.
- Acién P, Acién MI, Quereda F, Santoyo T. Cervicovaginal agenesis: spontaneous gestation at term after previous reimplantation of the uterine corpus in a neovagina: Case Report. Hum Reprod 2008;23:548–553.
- Anum EA, Hill LD, Pandya A, Strauss JF 3rd. Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta 2009;30:207–215.
- Ortoft G, Henriksen T, Hansen E, Petersen L. After conisation of the cervix, the perinatal mortality as a result of preterm delivery increases in subsequent pregnancy. BJOG 2010;117:258–267.
- Roberts CP, Rock JA. Surgical methods in the treatment of congenital anomalies of the uterine cervix. Curr Opin Obstet Gynecol 2011;23:251–257.
- Fischer RL, Sveinbjornsson G, Hansen C. Cervical sonography in pregnant women with a prior cone biopsy or loop electrosurgical excision procedure. Ultrasound Obstet Gynecol 2010;36:613–617.
- Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–19.
- Park KH, Hong JS, Kang WS, Shin DM. Transvaginal ultrasonographic measurement of cervical length in predicting intra-amniotic infection and impending preterm delivery in preterm labor: a comparison with amniotic fluid white blood cell count. J Perinat Med 2008;36:479–484.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113 Suppl 3:17–42.
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39.
- Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol 2010;202:433.e1–433.e8.
- Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kusanovic JP, Mittal P, Gotsch F, et al. The risk of impending preterm delivery in asymptomatic patients with a nonmeasurable cervical length in the second trimester. Am J Obstet Gynecol 2010;203:446.e1–446.e9.
- Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci 2010;1205:118–122.
- Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol 2009;200:374.e1–374.e5.
- Schlembach D, Mackay L, Shi L, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: from biochemical and molecular studies to in vivo clinical examination. Eur J Obstet Gynecol Reprod Biol 2009;144 Suppl 1:S70–S76.
- Ventolini G, Neiger R. Management of painless mid-trimester cervical dilatation: Prophylactic vs emergency placement of cervical cerclage. J Obstet Gynaecol 2008;28:24–27.
- Feingold M, Brook I, Zakut H. Detection of cervical incompetence by ultrasound. Acta Obstet Gynecol Scand 1984;63:407–410.
- Haluska GJ, Cook MJ, Novy MJ. Inhibition and augmentation of progesterone production during pregnancy: effects on parturition in rhesus monkeys. Am J Obstet Gynecol 1997;176:682–691.
- Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol 2006;194:1–9.
- Berghella V, Mackeen AD.. Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis Obstet Gynecol 2011;118:148–155.
- Sieroszewski P, Jasinski A, Perenc M, Banach R, Oszukowski P. The Arabin pessary for the treatment of threatened mid-trimester miscarriage or premature labour and miscarriage: a case series. J Matern Fetal Neonatal Med 2009;22:469–472.
- Kimber-Trojnar Z, Patro-Malysza J, Leszczynska-Gorzelak B, Marciniak B, Oleszczuk J. Pessary use for the treatment of cervical incompetence and prevention of preterm labour. J Matern Fetal Neonatal Med 2010;23:1493–1499.
- da Fonseca EB, Bittar RE, Damião R, Zugaib M.. Prematurity prevention: the role of progesterone. Curr Opin Obstet Gynecol 2009;21:142–147.
- Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol 2011;38:1–9.
- DeFranco EA, O’Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, Soma-Pillay P, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30:697–705.
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;357:462–469.
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, et al.; PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2011;38:18–31.
- Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet 2011;283:423–429.
- Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, Tabor A. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstet Gynecol Scand 2009;88:1180–1189.
- Lim AC, Goossens A, Ravelli AC, Boer K, Bruinse HW, Mol BW. Use of progesterone treatment for the prevention of recurrent preterm birth: identification of obstacles to change. Am J Perinatol 2010;27:241–249.
- Dodd JM, Flenady VJ, Cincotta R, Crowther CA.. Progesterone for the prevention of preterm birth: a systematic review Obstet Gynecol 2008;112:127–134.
- Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien J, Cetingoz Eet al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data Am J Obstet Gynecol 2012;206:124.e1–124.e19.
- Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound 1991;19:77–83.
- Heath VC, Southall TR, Souka AP, Novakov A, Nicolaides KH. Cervical length at 23 weeks of gestation: relation to demographic characteristics and previous obstetric history. Ultrasound Obstet Gynecol 1998;12:304–311.
- Palacio M, Cobo T, Bosch J, Filella X, Navarro-Sastre A, Ribes A, Gratacós E. Cervical length and gestational age at admission as predictors of intra-amniotic inflammation in preterm labor with intact membranes. Ultrasound Obstet Gynecol 2009;34:441–447.
- Rane SM, Guirgis RR, Higgins B, Nicolaides KH. The value of ultrasound in the prediction of successful induction of labor. Ultrasound Obstet Gynecol 2004;24:538–549.
- Hertzberg BS, Livingston E, DeLong DM, McNally PJ, Fazekas CK, Kliewer MA. Ultrasonographic evaluation of the cervix: transperineal versus endovaginal imaging. J Ultrasound Med 2001;20:1071–8; quiz 1080.
- Rizzo G, Capponi A, Angelini E, Vlachopoulou A, Grassi C, Romanini C. The value of transvaginal ultrasonographic examination of the uterine cervix in predicting preterm delivery in patients with preterm premature rupture of membranes. Ultrasound Obstet Gynecol 1998;11:23–29.
- Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol 1992;2:402–409.
- Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1990;163:859–867.
- Hibbard JU, Tart M, Moawad AH. Cervical length at 16-22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol 2000;96:972–978.
- Rocco BP, Garrone C. Can examination of the cervix provide useful information for prediction of cervical incompetence and following preterm labour? Aust N Z J Obstet Gynaecol 1999;39:296–300.
- Yu H, Li W, Yang T. [Sonographic measurement of uterine cervix in pregnancy]. Hua Xi Yi Ke Da Xue Xue Bao 1999;30:208–9, 213.
- Brown JE, Thieme GA, Shah DM, Fleischer AC, Boehm FH. Transabdominal and transvaginal endosonography: evaluation of the cervix and lower uterine segment in pregnancy. Am J Obstet Gynecol 1986;155:721–726.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med 2008;27:1305–1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Transabdominal scanning of the cervix at the 20-week morphology scan: comparison with transvaginal cervical measurements in a healthy nulliparous population. Aust N Z J Obstet Gynaecol 2010;50:523–527.
- Burger M, Weber-Rössler T, Willmann M. Measurement of the pregnant cervix by transvaginal sonography: an interobserver study and new standards to improve the interobserver variability. Ultrasound Obstet Gynecol 1997;9:188–193.
- Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol 2003;22:85–93.
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–38.
- Korvenranta E, Lehtonen L, Rautava L, Häkkinen U, Andersson S, Gissler M, Hallman M, et al.; PERFECT Preterm Infant Study Group. Impact of very preterm birth on health care costs at five years of age. Pediatrics 2010;125:e1109–e1114.
- Berghella V. Every 30 seconds a baby dies of preterm birth. What are you doing about it? Am J Obstet Gynecol 2010;203:416–417.
- Chandler JC, Hebra A. Necrotizing enterocolitis in infants with very low birth weight. Semin Pediatr Surg 2000;9:63–72.
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–456.
- Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health 2009;12:1124–1134.
- Mistry H, Dowie R, Franklin RC, Jani BR. Costs of neonatal care for low-birthweight babies in English hospitals. Acta Paediatr 2009;98:1123–1129.
- Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 2010;126:115–128.
- Farooqi A, Hägglöf B, Sedin G, Serenius F. Impact at age 11 years of major neonatal morbidities in children born extremely preterm. Pediatrics 2011;127:e1247–e1257.
- Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, Merkatz IR, et al.; March Of Dimes Scientific Advisory Committee On Prematurity. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 2005;193:626–635.
- Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008;359:262–273.
- Patrianakos-Hoobler AI, Msall ME, Huo D, Marks JD, Plesha-Troyke S, Schreiber MD. Predicting school readiness from neurodevelopmental assessments at age 2 years after respiratory distress syndrome in infants born preterm. Dev Med Child Neurol 2010;52:379–385.
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 2009;141:79–87.
- Sommer C, Urlesberger B, Maurer-Fellbaum U, Kutschera J, Müller W. Neurodevelopmental outcome at 2 years in 23 to 26 weeks old gestation infants. Klin Padiatr 2007;219:23–29.
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. Preterm Birth Causes, Consequences, and Prevention, Behrman RE, Butler AS (eds). Institute of Medicine of the National Academies. The National Academies Press: Washington D.C., 2007.
- Wilson-Costello D. Risk factors for neurologic impairment among very low-birth-weight infants. Semin Pediatr Neurol 2001;8:120–126.
- Hou C, Norcia AM, Madan A, Tith S, Agarwal R, Good WV. Visual cortical function in very low birth weight infants without retinal or cerebral pathology. Invest Ophthalmol Vis Sci 2011;52:9091–9098.
- Ecsedy M, Varsányi B, Szigeti A, Szrnka G, Németh J, Récsán Z. Cone function in children with a history of preterm birth. Doc Ophthalmol 2011;122:141–148.
- Quinn GE, Gilbert C, Darlow BA, Zin A. Retinopathy of prematurity: an epidemic in the making. Chin Med J 2010;123:2929–2937.
- Kamholz KL, Cole CH, Gray JE, Zupancic JA. Cost-effectiveness of early treatment for retinopathy of prematurity. Pediatrics 2009;123:262–269.
- Pau H. Retinopathy of prematurity: clinic and pathogenesis. Disproportion between apoptosis of vitreal and proliferation of retinal vascularization. Ophthalmologica 2008;222:220–224.
- Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010;125:1020–1030.
- Farstad T, Bratlid D, Medbø S, Markestad T; Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia - prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr 2011;100:53–58.
- Doyle LW, Anderson PJ. Pulmonary and neurological follow-up of extremely preterm infants. Neonatology 2010;97:388–394.
- Cahill AG, Odibo AO, Caughey AB, Stamilio DM, Hassan SS, Macones GA, Romero R. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol 2010;202:548.e1–548.e8.
- Werner EF, Han CS, Pettker CM, Buhimschi CS, Copel JA, Funai EF, Thung SF. Universal cervical-length screening to prevent preterm birth: a cost-effectiveness analysis. Ultrasound Obstet Gynecol 2011;38:32–37.
- Jeanty P, d’Alton M, Romero R, Hobbins JC. Perineal scanning. Am J Perinatol 1986;3:289–295.
- Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol 1990;162:991–993.
- Meijer-Hoogeveen M, Stoutenbeek P, Visser GH. Methods of sonographic cervical length measurement in pregnancy: a review of the literature. J Matern Fetal Neonatal Med 2006;19:755–762.
- Podobnik M, Bulic M, Smiljanic N, Bistricki J. Ultrasonography in the detection of cervical incompetency. J Clin Ultrasound 1988;16:383–391.
- Sonek JD, Iams JD, Blumenfeld M, Johnson F, Landon M, Gabbe S. Measurement of cervical length in pregnancy: comparison between vaginal ultrasonography and digital examination. Obstet Gynecol 1990;76:172–175.
- Bernstine RL, Lee SH, Crawford WL, Shimek MP. Sonographic evaluation of the incompetent cervix. J Clin Ultrasound 1981;9:417–420.
- Zemlyn S. The length of the uterine cervix and its significance. J Clin Ultrasound 1981;9:267–269.
- Robinson JN, Economy KE, Feinberg BR, Norwitz ER. Cervical hydrosonography in pregnancy to assess cervical length by transabdominal ultrasound. Obstet Gynecol 2000;96:1023–1025.
- van Dongen L. Ultrasonography of the cervix. S Afr Med J 1984; 65:82–85
- Romero R, Mazor M, Gomez R, Gonzalez R, Galasso M, Cotton D. Cervix, incompetence and premature labor. The Fetus 1993;3:1–10.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15:292–296.
- Braithwaite JM, Economides DL. Acceptability by patients of transvaginal sonography in the elective assessment of the first-trimester fetus. Ultrasound Obstet Gynecol 1997;9:91–93.
- Erasmus I, Nicolaou E, van Gelderen CJ, Nicolaides KH. Cervical length at 23 weeks’ gestation–relation to demographic characteristics and previous obstetric history in South African women. S Afr Med J 2005;95:691–695.
- Lewis D, Pelham JJ, Done E, Sawhney H, Talucci M, Berghella V. Uterine contractions in asymptomatic pregnant women with a short cervix on ultrasound. J Matern Fetal Neonatal Med 2005;18:325–328.
- Berghella V, Iams JD, Newman RB, Macpherson C, Goldenberg RL, Mueller-Heubach E, Caritis SN, Dombrowski MP; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Frequency of uterine contractions in asymptomatic pregnant women with or without a short cervix on transvaginal ultrasound scan. Am J Obstet Gynecol 2004;191:1253–1256.
- Sonek J, Blumenfeld M, Foley M, Johnson F, Iams J. Cervical length may change during ultrasonographic examination. Am J Obstet Gynecol 1990;162:1355–1357.
- Londero AP, Bertozzi S, Fruscalzo A, Driul L, Marchesoni D. Ultrasonographic assessment of cervix size and its correlation with female characteristics, pregnancy, BMI, and other anthropometric features. Arch Gynecol Obstet 2011;283:545–550.
- Meijer-Hoogeveen M, Stoutenbeek P, Visser GH. Dynamic cervical length changes: preliminary observations from 30-minute transvaginal ultrasound recordings. J Matern Fetal Neonatal Med 2007;20:481–486.
- Leppert P.. Cervical softening,effacement and dilatation: A complex biochemical cascade J Matern Fetal Med 2011;1:213–23.
- Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol 1978;130:414–418.
- Jackson GM, Ludmir J, Bader TJ. The accuracy of digital examination and ultrasound in the evaluation of cervical length. Obstet Gynecol 1992;79:214–218.
- Valentin L, Bergelin I. Intra- and interobserver reproducibility of ultrasound measurements of cervical length and width in the second and third trimesters of pregnancy. Ultrasound Obstet Gynecol 2002;20:256–262.
- Farrell T, Cairns M, Leslie J. Reliability and validity of two methods of three-dimensional cervical volume measurement. Ultrasound Obstet Gynecol 2003;22:49–52.
- Espinoza J, Gonçalves LF, Romero R, Nien JK, Stites S, Kim YM, Hassan S, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol 2005;25:346–352.
- Kusanovic JP, Espinoza J, Romero R, Gonçalves LF, Nien JK, Soto E, Khalek Net al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol 2007;30:706–714.