Abstract
Objectives: Angiogenic/anti-angiogenic factors have emerged as one of the promising biomarkers for the prediction of preeclampsia. Since not all patients with preeclampsia can be identified by these analytes, the search for additional biomarkers continues. The soluble form of ST2 (sST2), a protein capable of binding to interleukin (IL)-33 and thus contributing to a Th1-biased immune response, has been reported to be elevated in maternal plasma of women with preeclampsia. The aims of this study were to examine: (1) differences in maternal plasma concentrations of sST2 and IL-33 between women diagnosed with preeclampsia and those having uncomplicated pregnancies; (2) the relationship between sST2, umbilical and uterine artery Doppler velocimetry, and the severity of preeclampsia; and (3) the performance of sST2 and angiogenic/anti-angiogenic factors in identifying patients with preeclampsia at the time of diagnosis.
Methods: This cross-sectional study included women with preeclampsia (n = 106) and women with an uncomplicated pregnancy (n = 131). Plasma concentrations of sST2, IL-33, soluble vascular endothelial growth factor receptor (sVEGFR)-1, soluble endoglin (sEng) and placental growth factor (PlGF) were determined by enzyme linked immune sorbent assay. Area under the receiver operating characteristic curve (AUC) for the identification of preeclampsia was examined for each analyte.
Results: (1) Patients with preeclampsia had a higher mean plasma concentrations of sST2 than those with an uncomplicated pregnancy (p < 0.0001), while no significant difference in the mean plasma concentration of IL-33 between the two groups was observed; (2) the magnitude of this difference was greater in early-onset, compared to late-onset disease, and in severe compared to mild preeclampsia; (3) sST2 plasma concentrations did not correlate with the results of uterine or umbilical artery Doppler velocimetry (p = 0.7 and p = 1, respectively) among women with preeclampsia; (4) sST2 correlated positively with plasma concentrations of sVEGFR1-1 and sEng (Spearman’s Rho = 0.72 and 0.63; each p < 0.0001), and negatively with PlGF (Spearman’s Rho = −0.56, p < 0.0001); and (5) while the AUC achieved by sST2 and angiogenic/anti-angiogenic factors in identifying women with preeclampsia at the time of diagnosis were non-significantly different prior to term (<37 weeks of gestation), thereafter the AUC achieved by sST2 was significantly less than that achieved by angiogenic/anti-angiogenic factors.
Conclusions: Preeclampsia is associated with increased maternal plasma concentrations of sST2. The findings that sST2 concentrations do not correlate with uterine or umbilical artery Doppler velocimetry in women with preeclampsia suggest that elevated maternal plasma sST2 concentrations in preeclampsia are not related to the increased impedance to flow in the utero-placental circulation. The performance of sST2 in identifying preeclampsia at the time of diagnosis prior to 37 weeks of gestation was comparable to that of angiogenic/anti-angiogenic factors. It remains to be elucidated if an elevation of maternal plasma sST2 concentrations in pregnancy is specific to preeclampsia.
Introduction
Preeclampsia, one of the “great obstetrical syndromes” [Citation1,Citation2], remains a leading cause of maternal and neonatal morbidity [Citation3–8]. A defect of deep placentation [Citation9–13] is proposed to generate utero-placental ischemia [Citation14–16], placental endoplasmic reticulum and oxidative stress [Citation17–22], and subsequently systemic intravascular inflammation [Citation23–25] and endothelial dysfunction [Citation17,Citation26–34]. The circulating concentrations of pro-inflammatory cytokines, such as interleukin (IL)-1-β and tumor necrosis factor (TNF)-α [Citation35–39], as well as other inflammatory mediators (such as IL-6) [Citation36,Citation40–42] are typically elevated in patients with preeclampsia, although this is not a universal feature [Citation43,Citation44]. The placenta is essential for the development of preeclampsia, while the fetus is not [Citation45–47]. Preeclampsia has been referred to as “toxemia of pregnancy” because it is believed that “toxins” released from a damaged placenta are responsible for the clinical manifestations of the disease [Citation3,Citation48–50].
It has become increasingly clear that the magnitude of derangements underlying preeclampsia is greater when it is diagnosed prior to 34 weeks of gestation [Citation51]. The frequency of placental lesions [Citation15,Citation52,Citation53], as is the frequency of multisystemic involvement [Citation54–57], is higher in these patients than those who are diagnosed later. For example, hemolysis, elevated liver enzymes, low platelet (HELLP) syndrome is more common in patients with early-onset disease than in those diagnosed at term [Citation58–60]. The increased perinatal morbidity associated with preeclampsia is also closely linked to early-onset disease because indicated preterm delivery is associated with an increased risk of neonatal complications [Citation61]. Late-onset disease is, however, much more common than early-onset preeclampsia [Citation56,Citation57,Citation62].
Considerable effort has been made to identify biomarkers that could predict women who will develop preeclampsia [Citation63–78]. Although markers of systemic inflammation [Citation79,Citation80] and endothelial dysfunction [Citation81–88] in maternal blood have been associated with preeclampsia, their prognostic performance for the identification of patients with preeclampsia prior to diagnosis has been disappointing [Citation79–81,Citation83,Citation89–91]. This indicates that either inflammation occurs late (even during the subclinical phase of the disease), or that the current methods available to detect intravascular inflammation are not sensitive enough.
Preeclampsia [Citation63–67,Citation69,Citation70,Citation72,Citation92–103], as well as other obstetrical syndromes [Citation46,Citation104–118], is characterized by an anti-angiogenic state. Plasma concentrations of the angiogenic factor, placental growth factor (PlGF), are decreased [Citation69,Citation70,Citation92,Citation93,Citation96,Citation100–102], and the anti-angiogenic factors (soluble vascular endothelial growth factor receptor-1 [sVEGFR-1] and soluble endoglin [sEng]) are elevated both prior to [Citation67,Citation72,Citation119–125] and at the time of diagnosis [Citation94,Citation95,Citation97–99]. Moreover, the concentrations of these factors can be used to predict the outcome of the disease in patients presented to the obstetrical triage area [Citation73,Citation75,Citation126,Citation127]. However, the prognostic performance of these biomarkers evaluated in the mid-trimester for late-onset preeclampsia has been suboptimal [Citation105,Citation128–131]. Recently, an elevated plasma concentration of sST2 was observed prior to and at the time of the diagnosis of preeclampsia in a case–control study [Citation132]. Therefore, sST2 was proposed as a novel candidate biomarker for the prediction of preeclampsia [Citation132].
ST2 is a member of the IL-1 receptor family gene, and the ST2 protein has four isoforms, of which the best characterized are a membrane-bound receptor form (ST2 receptor or ST2L) and a soluble form (sST2). ST2L expresses on mast cells, macrophages and exclusively on Type 2 T-helper cells [Citation133–140]. Upon binding to ST2L, IL-33 is capable of stimulating the Th2 immune response and cytokine production [Citation141–144]. In contrast, sST2 acts as a decoy receptor for IL-33 and is thought to inhibit IL-33 function, thus favoring a shift toward the Th1 immune response [Citation145]. Moreover, sST2 can be secreted in response to inflammatory signals by endothelial cells [Citation146,Citation147]. The involvement of sST2 has been observed in a variety of pathological conditions characterized by: (1) intravascular inflammation [Citation145,Citation148–151] (i.e. myocardial infarction [Citation152–154], atherosclerotic vascular disease [Citation155], sepsis and trauma [Citation149,Citation150]); and (2) an imbalance of Th1/Th2 immune response [Citation141,Citation156,Citation157] (i.e. asthma and other allergic conditions [Citation145,Citation148,Citation158,Citation159]), both of which are also observed in patients with preeclampsia [Citation23,Citation25,Citation132,Citation160,Citation161].
The objectives of this study were to: (1) determine whether plasma concentrations of sST2 and IL-33 in patients with preeclampsia at the time of diagnosis differ from those in women with uncomplicated pregnancies; (2) examine the relationships between plasma concentrations of sST2 and Doppler velocimetry in the uterine and umbilical arteries, gestational age at diagnosis and severity of preeclampsia; and (3) compare the performance of sST2 and angiogenic/anti-angiogenic factors at different gestational ages in identifying preeclampsia at the time of diagnosis.
Material and methods
Study design and population
A retrospective cross-sectional study was conducted by searching the clinical database and bank of biologic samples of Wayne State University/Detroit Medical Center/Perinatology Research Branch. All patients were enrolled at Hutzel Women’s Hospital in Detroit, MI, USA. The following groups were included: (1) women with an uncomplicated pregnancy; and (2) patients with preeclampsia. Chronic hypertension, multiple pregnancies, or fetuses with chromosomal or structural anomalies were excluded. Women with an uncomplicated pregnancy between 20 and 42 weeks of gestation were enrolled from either the labor and delivery unit (in case of scheduled cesarean section) or from the antenatal clinic, and followed until delivery. Patients with preeclampsia had a venipuncture upon diagnosis. Doppler velocimetry examination of the uterine and umbilical arteries was performed in women with preeclampsia within 48 hours of venipuncture.
All women provided written informed consent prior to the collection of plasma samples. The collection and utilization of the samples for research purposes was approved by the Institutional Review Board of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples were used previously in studies of intravascular inflammation, angiogenesis and cytokine biology in normal and complicated pregnancies.
Clinical definition
Patients were considered to have an uncomplicated pregnancy outcome if they did not experience any obstetrical, medical, or surgical complications of the pregnancy, and delivered a normal term (≥37 weeks) neonate whose birth weight was between the 10th and 90th percentile for gestational age without complications [Citation162]. Preeclampsia was defined as hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) and proteinuria (≥300 mg in a 24-hours urine collection or one dipstick measurement ≥2+) [Citation163]. Early-onset and late-onset preeclampsia were defined as cases diagnosed before and after 34 weeks of gestation, respectively [Citation51]. Severe preeclampsia was defined as previously described [Citation164].
Doppler velocimetry
Pulse-wave and color Doppler ultrasound examinations of the uterine and umbilical arteries were performed in a subset of patients with preeclampsia as previously described (Acuson, Sequoia, Mountain View, CA) [Citation104,Citation164]. Uterine artery Doppler velocimetry was defined as abnormal if the mean (average of right and left) resistance index (RI) was above the 95th percentile for gestational age (using the reference range proposed by Kurmanavicius et al.) [Citation165]. Umbilical artery Doppler velocimetry was defined as abnormal if either the pulsatility index (PI) was above the 95th percentile for gestational age (using the reference range proposed by Arduini and Rizzo) [Citation166] or in the presence of abnormal waveforms (absent or reversed end-diastolic velocities) [Citation167]. The patients were classified into the following groups: (1) normal Doppler velocimetry in the uterine and umbilical arteries; (2) Doppler abnormalities in the uterine arteries alone; (3) Doppler abnormalities in umbilical arteries alone; or (4) Doppler abnormalities in both vessels.
Sample collection and human sST2 and IL-33 immunoassay
The blood collected by venipuncture was stored in tubes containing EDTA. Following centrifugation, the samples were stored at −70 °C. Specific enzyme-linked immunoassays were used for the determination of maternal plasma concentrations of sST2 (R&D Systems, Minneapolis, MN) and IL-33 (BioLegend, San Diego, CA). The quantitative sandwich enzyme immunoassay technique was used. The inter- and intra-assay coefficients of variation (CVs) were 4.6% and 3.9% for sST2 and 14.9% and 12.2% for IL-33, respectively. The sensitivities of the assays for sST2 and IL-33 were 17.5 and 12 pg/mL, respectively.
Angiogenic and anti-angiogenic factor immunoassay
Maternal plasma concentrations of PlGF, sVEGFR-1, and sEng were determined by sensitive and specific immunoassays (R&D Systems, Minneapolis, MN). All three immunoassays utilized the quantitative sandwich enzyme immunoassay technique. The inter- and intra-assay CVs obtained were 6.02% and 4.8% for PlGF; 1.4% and 3.9% for sVEGFR-1, and 2% and 4% for sEng, respectively. The sensitivity of the assays were: PlGF: 9.52 pg/mL; sVEGFR-1: 16.97 pg/mL and sEng: 0.08 ng/mL, respectively. The results of sVEGFR-1 and sEng have been reported in previous publications to examine the relationships between these proteins and Doppler velocimetry in uterine and umbilical arteries [Citation104,Citation164].
Statistical analysis
A Kolmogorov–Smirnov or Shapiro–Wilk test and visual plot inspection were used to assess normality of arithmetic data distributions. For analyte concentrations below the limit of the detection of the assays, the concentration was replaced by 99% of the lowest detectable concentration. A Kruskal–Wallis with post hoc Mann–Whitney U test was used to compare variables that were not normally distributed among and between groups. Comparison of proportions was performed using Chi-square or Fisher’s exact test, as appropriate. Correlation between two continuous variables was determined using Spearman’s rank correlation.
Logistic regression models were used to determine the magnitude of association between biochemical markers and preeclampsia. They were also used to construct receiver-operating characteristic (ROC) curves to assess the performance of plasma concentrations of sST2, PlGF, sVEGFR-1 and sEng in identifying preeclampsia at the time of diagnosis. Covariables included in adjusted models were selected based on clinical knowledge. Model reduction was performed additionally based on the plausibility of regression coefficients, association with independent/dependent variables and the magnitude of change in the main effect parameter estimates. Models constructed to represent clinical factors, selected biomarkers, Doppler velocimetry in the uterine and umbilical arteries, both independently and in combination, were evaluated overall and additionally stratified by gestational age at the diagnosis of preeclampsia (≤34 weeks; >34 to <37 weeks; ≥37 weeks). Paired sample non-parametric statistical techniques were used to compare area under the ROC curves (AUC) of models constructed using logistic regression for the identification of preeclampsia at the time of diagnosis.
A general linear model was fit to determine whether the predicted geometric mean analyte concentration varied by more than 5% when adjusted for one or more of the following: gestational age at venipuncture, maternal age, race, nulliparity and smoking. A p value <0.05 was considered statistically significant. Analyses were performed with SPSS, version 19 (IBM Corp, Armonk, NY) and SAS version 9.3 (Cary, NC).
Results
Demographic and clinical characteristics of the study population
Demographic and obstetric characteristics of women with uncomplicated pregnancies (n = 131) and patients with preeclampsia (n = 106) are summarized in . The proportion of nulliparous women was greater among patients diagnosed with preeclampsia than in those with uncomplicated pregnancies [61% (65/106) versus 28% (36/131); p < 0.0001]. As expected, the median gestational age at delivery and the median birth weight were significantly lower in women with preeclampsia than in those without preeclampsia. No differences were observed by age, race, pre-pregnancy weight, smoking habit or gestational age at venipuncture. Among patients with preeclampsia, 39% (41/106) had early-onset disease and 80% (85/106) were diagnosed with severe preeclampsia ().
Table 1. Clinical characteristics of the study population.
Table 2. Clinical characteristics of patients with preeclampsia (n = 106).
Plasma concentrations of sST2 in preeclampsia, early- and late-form of preeclampsia and clinical severity
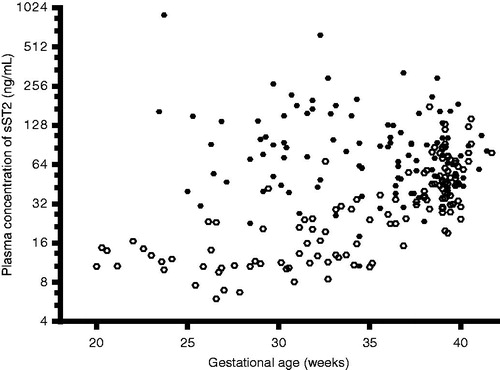
Patients with preeclampsia had a higher median plasma concentration of sST2 than women with uncomplicated pregnancies (median 76.1 ng/mL, interquartile range (IQR) 48.4–130.3 versus median 30.8 ng/mL, IQR 14–52.3; p < 0.0001). The magnitude of the difference in sST2 plasma concentrations between women with preeclampsia and women with uncomplicated pregnancies was greater among patients diagnosed at ≤34 weeks, >34 but <37 weeks compared to those diagnosed at term (≥37 weeks; ). There was no significant difference in the median plasma sST2 concentrations among patients with preeclampsia diagnosed at different gestational age categories (p = 0.87; ). While plasma sST2 concentrations in uncomplicated pregnancies increased as a function of gestational age (Spearman’s Rho = 0.77, p < 0.0001), there was no significant relationship between plasma sST2 concentration and gestational age at the diagnosis of preeclampsia (Spearman’s Rho = −0.09; p = 0.38; ).
Figure 1. Plasma concentrations of sST2 sub-classified according to gestational age at the diagnosis of preeclampsia. The median plasma concentration of sST2 in women with preeclampsia was significantly higher than in those with uncomplicated pregnancies (≤34 weeks, median 93 ng/mL, interquartile range (IQR) 48.4–159.3 ng/mL versus median 12.7 ng/mL, IQR10.6–20; p < 0.0001; between >34 and <37 weeks, median 62.4 ng/mL, IQR 39.8–101.3 ng/mL versus median 22.5 ng/mL, IQR 12.2–32.5 ng/mL; p < 0.0001; and ≥37 weeks, median 73.1 ng/mL, IQR 51–101.7 ng/mL versus median 49 ng/mL, IQR 35.1–73.4 ng/mL; p ≤ 0.027). The magnitude of the difference was more pronounced in earlier gestation than at term. However, no differences were observed in plasma concentrations of sST2 in women with preeclampsia stratified by gestational age at diagnosis (p = 0.87). The y axis is presented in log 2 scale.
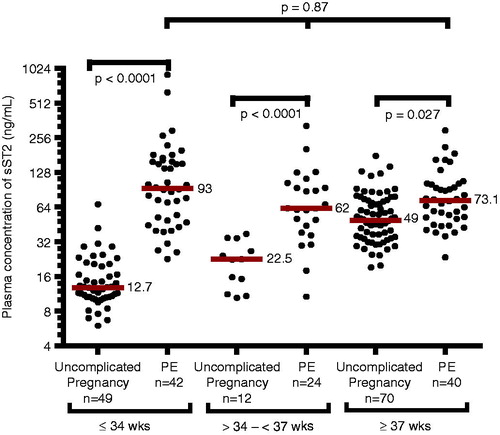
Figure 2. Relationship between gestational age at venipuncture (weeks) and plasma concentrations of sST2 (ng/mL) in women with uncomplicated pregnancies (ˆ; Spearman’s Rho = 0.77, p < 0.0001) and those with preeclampsia (•; Spearman’s Rho = −0.09, p = 0.38). The y axis is presented in log 2 scale.
Patients with severe preeclampsia had a significantly higher median plasma concentration of sST2 than those with mild preeclampsia (median 88.9 ng/mL, IQR 52–145 versus median 52.4 ng/mL, IQR 42–85, p = 0.01). Among women diagnosed with preeclampsia, plasma concentrations of sST2 were significantly correlated with the amount of urine protein in 24 h (Spearman’s Rho = 0.4, p = 0.02), gestational age at delivery (Spearman’s Rho = 0.17, p < 0.0001) and neonatal birth weight (Spearman’s Rho = 0.13, p = 0.001). In contrast, sST2 was not associated with mean arterial pressure (Spearman’s Rho = −0.2, p = 0.4) or platelet count (Spearman’s Rho = −0.2, p = 0.4).
Use of general linear models to evaluate the need for multivariable adjustment (gestational age at venipuncture, maternal race, maternal age, nulliparity, and smoking) revealed little change (<5%) in the predicted geometric mean of sST2 concentrations compared to unadjusted estimates, meaning the results shown above are robust and reliable in that they persist after multivariable adjustment.
Plasma concentrations of IL-33 in women with uncomplicated pregnancies and those with preeclampsia
Plasma concentration of IL-33 was below the detection limit in 8% (10/131) of women with uncomplicated pregnancies and in 5% (5/106) of women with preeclampsia. There was no significant difference in the median plasma concentration of IL-33 between patients with preeclampsia (92.5 pg/mL, IQR 34.9–258.2) and women with uncomplicated pregnancies (90.9 pg/mL, IQR 42.6–179.4) overall, or in any of the gestational age intervals (p = 1.0 for all comparisons).
Plasma concentrations of sST2 and IL-33 do not correlate with the result of Doppler velocimetry of the uterine and umbilical arteries among women with preeclampsia
Information about Doppler velocimetry was available in 61% (65/106) of patients with preeclampsia. The median uterine artery RI was 0.68 (IQR 0.58–0.75) and the median umbilical artery PI was 1.2 (IQR 1.0–1.4). Abnormal uterine artery Doppler velocimetry was observed in 82% (53/65) of patients, while abnormal umbilical artery Doppler velocimetry was present in 18% (12/65) of patients diagnosed with preeclampsia.
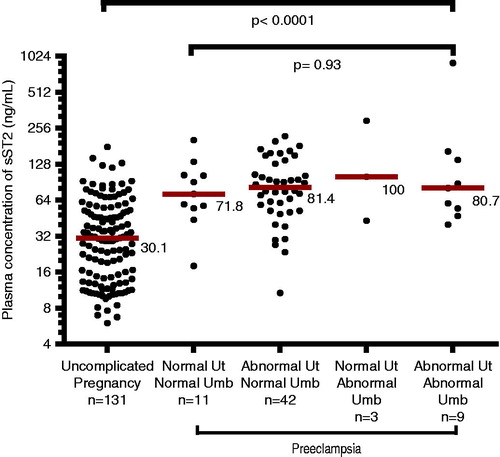
Plasma concentrations of sST2 were not correlated with uterine artery RI (Spearman’s Rho = 0.05, p = 0.7), or umbilical artery PI (Spearman’s Rho = −0.001, p = 1). Similarly, plasma concentrations of IL-33 were not correlated with uterine artery RI (Spearman’s Rho = 0.21, p = 0.12), or umbilical artery PI (Spearman’s Rho = −0.03, p = 0.85). There were no significant differences in the median plasma concentration of sST2 among patients with preeclampsia with and without abnormalities in the uterine and/or umbilical artery Doppler velocimetry (). Similar results were observed after taking into account the effect modification term including gestational age at venipuncture and abnormalities in Doppler velocimetry.
Figure 3. Plasma concentrations of sST2 in uncomplicated pregnancies and patients with preeclampsia sub-classified according to the results of uterine (Ut) and umbilical (Umb) artery Doppler velocimetry. When compared to women with uncomplicated pregnancies, each sub-group of preeclampsia had a significantly higher median plasma concentration of sST2 (Kruskal–Wallis test p < 0.0001). There was no significant difference in the median plasma concentration of sST2 among each subgroup of preeclampsia (Kruskal–Wallis test p = 0.93). The y axis is presented in log 2 scale.
The performance of sST2 and angiogenic/anti-angiogenic factors in identifying patients with preeclampsia at the time of diagnosis
Nineteen percent (20/106) of patients with preeclampsia had plasma concentrations of PlGF below the detection limit. The median plasma concentrations of PlGF, sEng, and sVEGFR1 and the ratio between PlGF/sVEGFR1 and PlGF/sEng in women with uncomplicated pregnancies and those with preeclampsia are shown in . There was a positive correlation between plasma concentrations of sST2 and anti-angiogenic factors (for sEng: Spearman’s Rho = 0.63; for sVEGFR-1: Spearman’s Rho = 0.72; each p < 0.0001) and a negative correlation between sST2 and PlGF (Spearman’s Rho = −0.56), PlGF/sVEGFR1 ratio (Spearman’s Rho = −0.69), and PlGF/sEng ratio (Spearman’s Rho = −0.63; each p < 0.0001; ). No correlation was observed either between IL-33 and sST2, or between IL-33 and angiogenic/anti-angiogenic factors ().
Table 3. Plasma concentrations of angiogenic and anti-angiogenic factors in women with uncomplicated pregnancies and patients with preeclampsia.
Table 4. Correlation between sST2, IL-33 and angiogenic/anti-angiogenic factors.
Table 5. Area under the ROC curves (AUC) achieved by sST2 and angiogenic/anti-angiogenic factors (adjusted for clinical factors) in identifying women with preeclampsia at the time of diagnosis.
The AUC for models constructed to identify women with preeclampsia at the time of diagnosis using sST2 alone significantly exceeded that achieved by clinical factors (age, smoking, nulliparity and race) [0.85 (95% CI 0.80–0.90) versus 0.70 (95% CI 0.63–0.78), respectively (p = 0.0007)]. An addition of clinical factors to sST2 did not significantly improve the performance of sST2 in identifying women with preeclampsia at the time of diagnosis as reflected by change in AUC (0.86 vs. 0.85; p = 0.5). The AUCs for PlGF, sEng, sVEGFR1, PlGF/sVEGFR1 and PlGF/sEng ratio (adjusted for clinical factors) are shown in ; each performed better than sST2 in identifying women with preeclampsia at the time of diagnosis (). The combination of sST2 with the PlGF/sVEGFR1 ratio or the PlGF/sEng ratio did not result in significant improvement (AUC for PlGF/sVEGFR1 alone: 0.977 versus AUC for PlGF/sVEGFR1 plus sST2: 0.978; p = 0.7; and AUC for PlGF/sEng alone: 0.971 versus for sST2 plus PlGF/sEng 0.974; p = 0.6).
Figure 4. Receiver operating characteristic (ROC) curves of plasma sST2 concentrations, each angiogenic/anti-angiogenic factor and their ratios (adjusted for clinical factors) for the identification of women with preeclampsia at the time of diagnosis. Area under the ROC curve for each analyte was also presented (sST2 = 0.86, PlGF = 0.96, sEng = 0.94, sVEGFR1 = 0.97, PlGF/sVEGFR1 = 0.98, and PlGF/sEng = 0.97).
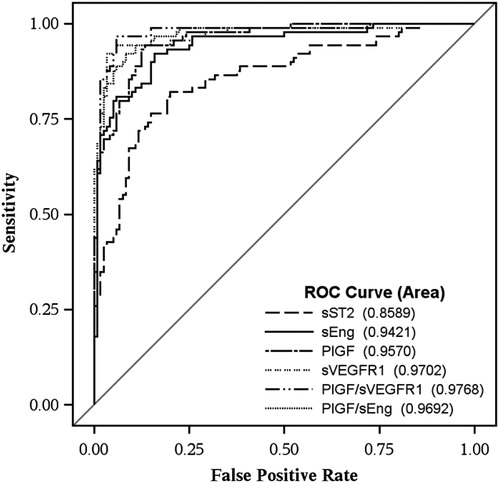
Results stratified according to gestational age at the diagnosis of preeclampsia showed that in women diagnosed before 37 gestational weeks, sST2 performed similarly to angiogenic/anti-angiogenic factors in the identification of patients with preeclampsia at the time of diagnosis (). In contrast, in women evaluated at or beyond 37 gestational weeks, the AUC achieved by sST2 in identifying women with preeclampsia at the time of diagnosis (AUC 0.73) was significantly lower than that achieved by angiogenic/anti-angiogenic factors (AUCs ranging from 0.85 for sEng to 0.97 for PlGF/sVEGFR-1 ratio).
Table 6. Area under the ROC curves (AUC) for sST2 and angiogenic/anti-angiogenic factors (without adjustment for clinical factors) sub-classified according to gestational age at the diagnosis of preeclampsia.
Discussion
Principal findings of this study
(1) Preeclampsia is associated with a higher plasma sST2 concentration than in uncomplicated pregnancies; the magnitude of this difference is greater in early- compared to late-onset disease, and in severe than in mild preeclampsia; (2) among patients with preeclampsia, there was no association between plasma sST2 concentrations and abnormalities of Doppler velocimetry of the uterine and umbilical arteries; (3) the performance of sST2 in identifying patients with preeclampsia diagnosed at <37 weeks of gestation was similar to that of angiogenic (PlGF), anti-angiogenic (sVEGFR-1, sEng) factors and their ratios (PlGF/sVEGFR-1, PlGF/sEng). However, the performance of angiogenic/anti-angiogenic factors and their ratios was better than that of sST2 plasma concentrations in patients who were diagnosed at term; and (4) no significant differences in plasma concentrations of IL-33 were observed between women with and without preeclampsia.
Plasma concentrations of soluble ST2 is elevated in preeclampsia
The finding that the median plasma concentration of sST2 in preeclampsia was higher than in uncomplicated pregnancies are consistent with a previous study by Granne et al. [Citation132]. Moreover, the current study extends these observations by demonstrating that the magnitude of the difference between preeclampsia and uncomplicated pregnancies is markedly higher in early- than in late-onset disease, (Figure 1), and in severe than in mild preeclampsia.
Two hypotheses can be invoked to explain these findings. First, a higher plasma sST2 concentration may be a consequence of an intravascular inflammatory response which is exacerbated in preeclampsia when compared to an uncomplicated pregnancy [Citation23,Citation25,Citation168–170]. Indeed, it has been demonstrated that pro-inflammatory cytokines are increased in the plasma or serum of women with early-onset preeclampsia [Citation35,Citation41,Citation42] and severe preeclampsia [Citation36,Citation40,Citation41] when compared to women with uncomplicated pregnancies. Moreover, IL-1β or TNF-α (pro-inflammatory cytokines which are increased in preeclampsia) are able to stimulate vascular endothelial cells to secrete sST2 into supernatant [Citation146,Citation147]. This hypothesis is also consistent with the view that preeclampsia is characterized by a Th1/Th2 imbalance towards a Th1 type immune response [Citation160,Citation161,Citation171], since sST2 can inhibit IL-33 function, thus favoring a shift toward a Th1 response.
An alternative view is that an elevation of sST2 in preeclampsia could be a response of the myocardium and vascular endothelium to pressure and/or volume (hemodynamic) overload. Indeed, patients with preeclampsia have increased plasma concentrations of natriuretic peptides that reflect changes in ventricular size and volume load [Citation172–175]. In chronic heart disease, serum sST2 concentration increases as natriuretic peptides and indexes of hemodynamic load increase [Citation146,Citation153]. In this case, the source of sST2 could be cardiac myocytes, as shown by the up-regulated ST2 gene expression in neonatal rat myocytes exposed to mechanical strain [Citation176]. However, Bartunek et al. provided evidence that adult human myocardium is not a source of an increased sST2 concentration in patients with cardiac hypertrophy induced by pressure overload or those with congestive cardiomyopathy [Citation146]. These investigators have postulated that elevated plasma sST2 concentrations in patients with diastolic overload were more likely to originate from endothelial cells rather than cardiac myocytes [Citation146]. Collectively, these observations support the hypothesis that the increased plasma sST2 concentration in preeclampsia may derive from myocardial and endothelial cells stimulated by hemodynamic changes in pregnancy, which becomes exacerbated in women with preeclampsia.
Relationship between plasma sST2 concentrations and Doppler velocimetry, gestational age at diagnosis, and severity of preeclampsia
The involvement of the placenta in early-onset preeclampsia is more extensive than in the late-onset disease [Citation15,Citation52,Citation177]. The finding that the magnitude of the difference in plasma sST2 concentrations between preeclampsia and uncomplicated pregnancies was greater in early- than in late-onset disease may indicate a greater contribution of sST2 from an injured placenta in preeclampsia. Indeed, the ST2 gene [Citation178] and protein [Citation132] expressions were observed in the placenta, and sST2 can be released into the maternal circulation in a placental perfusion model [Citation132]. Although sST2 secretion from the placenta is increased by hypoxia/reperfusion or treatment with pro-inflammatory cytokines (TNF-α or IL-1β), there was no significant difference in ST2 and IL-33 protein expression in placentas obtained from patients with preeclampsia when compared to placentas from uncomplicated pregnancies [Citation132]. Moreover, the findings that there was no significant relationship between plasma concentrations of sST2 and abnormalities in Doppler velocimetry in the uterine and umbilical arteries do not support the view that the increased impedance to blood flow in the placenta is responsible for the increased plasma sST2 concentration in preeclampsia. Consistent with this hypothesis, plasma concentrations of sST2 in preeclampsia is only mildly correlated with neonatal birth weight (Spearman’s Rho = 0.13, p = 0.001). Therefore, the finding that the plasma concentrations of sST2 were higher in severe than in mild preeclampsia may reflect a greater degree of endothelial dysfunction and intravascular inflammation, which characterizes severe preeclampsia [Citation168].
Relationship between sST2 and angiogenic/anti-angiogenic factors
A novel finding of the current study is that plasma concentrations of sST2 in preeclampsia had a strong correlation with plasma concentrations of anti-angiogenic factors (sVEGFR-1 and sEng), and a moderate inverse relationship with the angiogenic factor (PlGF). Although individual angiogenic/anti-angiogenic factors reported herein can identify patients with preeclampsia at the time of diagnosis, the ratio between angiogenic and anti-angiogenic factor concentrations seems to provide a slightly larger AUC than the individual marker alone. The performance of sST2 is better in preterm than in term preeclampsia, with the AUC comparable to that of angiogenic/anti-angiogenic factors for preeclampsia diagnosed in preterm gestations. sST2 did not perform as well when compared to angiogenic/anti-angiogenic factors in identifying preeclampsia diagnosed at term. The integration of sST2 to models using PlGF/sVEGFR1 or PlGF/sEng to identify preeclampsia at the time of diagnosis did not improve the performance compared to the latter biomarker ratios alone. This indicates that the combination of these markers does not result in a clinical advantage over using each marker alone, most likely due to their moderate to strong correlation.
Strengths and limitations:
This study is the first to examine the association between plasma concentrations of sST2 and abnormalities of Doppler velocimetry in uterine and/or umbilical arteries, gestational age at diagnosis, and severity of preeclampsia. The cross-sectional design, however, prevents inferences about temporal changes. Also, like all observational studies, we are unable to separate causation from association in this study.
Conclusion
Preeclampsia is associated with higher plasma sST2 concentrations than in uncomplicated pregnancies. The magnitude of this difference is markedly increased in early- than in late-onset disease, and in severe than mild preeclampsia. Among women with preeclampsia, the plasma sST2 concentration does not correlate with uterine or umbilical artery Doppler velocimetry. This indicates that elevated maternal plasma sST2 concentrations in preeclampsia are not related to the increased impedance to flow in the utero-placental circulation. The performance of sST2 in identifying preeclampsia at the time of diagnosis is similar to that of angiogenic/anti-angiogenic factors among women diagnosed prior to 37 weeks of gestation, but worse in identifying women diagnosed with preeclampsia thereafter. It remains to be elucidated if an elevation of maternal plasma sST2 concentrations in pregnancy is specific to preeclampsia.
Declaration of interest
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Notes
*Presented at the 33rd Annual Meeting of the Society for Maternal Fetal-Medicine on February 11–16, 2013, in San Francisco, CA, USA.
References
- Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med 2009;22:636–9
- Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med 2009;22:633–5
- Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol 1988;12:302–23
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–4
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302–9
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22:203–12
- Jelin AC, Cheng YW, Shaffer BL, et al. Early-onset preeclampsia and neonatal outcomes. J Matern Fetal Neonatal Med 2010;23:389–92
- Schneider S, Freerksen N, Maul H, et al. Risk groups and maternal-neonatal complications of preeclampsia–current results from the national German Perinatal Quality Registry. J Perinat Med 2011;39:257–65
- Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol 1977;4:573–93
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 2002;187:1416–23
- Pijnenborg R, Anthony J, Davey DA, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991;98:648–55
- Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens 1976;5:115–27
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201
- George EM, Granger JP. Linking placental ischemia and hypertension in preeclampsia: role of endothelin 1. Hypertension 2012;60:507–11
- Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med 2011;39:641–52
- Gilbert JS, Ryan MJ, LaMarca BB, et al. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008;294:H541–50
- Cindrova-Davies T. Gabor than Award lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. Placenta 2009;30:S55–65
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999;222:222–35
- Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy 2002;21:205–23
- Myatt L, Kossenjans W, Sahay R, et al. Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med 2000;9:79–82
- Zhou X, Zhang GY, Wang J, et al. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am J Obstet Gynecol 2012;206:447 e447–16
- Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009;30:S43–8
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–6
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506
- Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–7
- Friedman SA, Schiff E, Emeis JJ, et al. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol 1995;172:202–3
- Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev Reprod 1996;1:107–16
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30:S32–7
- Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989;161:1200–4
- Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens 1991;4:700–8
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:5–15
- Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:17–31
- Chaiworapongsa T, Romero R, Yoshimatsu J, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002;12:19–27
- Petrozella L, Mahendroo M, Timmons B, et al. Endothelial microparticles and the antiangiogenic state in preeclampsia and the postpartum period. Am J Obstet Gynecol 2012;207:140.e120–46
- Vitoratos N, Economou E, Iavazzo C, et al. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm 2010;2010:908649
- Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol 2007;58:21–30
- Szarka A, Rigo J Jr, Lazar L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 2010;11:59
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 1997;37:240–9
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 1998;40:102–11
- Xiao JP, Yin YX, Gao YF, et al. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine 2012;60:856–60
- Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol 2006;70:83–91
- Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004;44:708–14
- Borekci B, Aksoy H, Al RA, et al. Maternal serum interleukin-10, interleukin-2 and interleukin-6 in pre-eclampsia and eclampsia. Am J Reprod Immunol 2007;58:56–64
- Kucuk M, Sezer SD, Yenisey C, et al. Comparison of interleukin-6 levels in maternal and umbilical cord blood in early- and late-onset preeclampsia. Gynecol Endocrinol 2012;28:640–3
- Piering WF, Garancis JG, Becker CG, et al. Preeclampsia related to a functioning extrauterine placenta: report of a case and 25-year follow-up. Am J Kidney Dis 1993;21:310–3
- Kanter D, Lindheimer MD, Wang E, et al. Angiogenic dysfunction in molar pregnancy. Am J Obstet Gynecol 2010;202:184.e181–5
- Hou JL, Wan XR, Xiang Y, et al. Changes of clinical features in hydatidiform mole: analysis of 113 cases. J Reprod Med 2008;53:629–33
- Speroff L. Toxemia of pregnancy. Mechanism and therapeutic management. Am J Cardiol 1973;32:582–91
- Toxaemias of pregnancy. General considerations. Q Med Rev 1968;18:1–36
- Morrison JC, Whybrew DW, Wiser WL, et al. Laboratory characteristics in toxemia. Obstet Gynecol 1972;39:866–72
- von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy 2003;22:143–8
- Egbor M, Ansari T, Morris N, et al. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG 2006;113:580–9
- Moldenhauer JS, Stanek J, Warshak C, et al. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol 2003;189:1173–7
- Romero R, Vizoso J, Emamian M, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol 1988;5:146–51
- Romero R, Mazor M, Lockwood CJ, et al. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am J Perinatol 1989;6:32–8
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785–99
- Tuffnell DJ, Jankowicz D, Lindow SW, et al. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG 2005;112:875–80
- Haddad B, Barton JR, Livingston JC, et al. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome versus severe preeclampsia: onset at < or =28.0 weeks’ gestation. Am J Obstet Gynecol 2000;183:1475–9
- Langenveld J, Jansen S, van der Post J, et al. Recurrence risk of a delivery before 34 weeks of pregnancy due to an early onset hypertensive disorder: a systematic review. Am J Perinatol 2010;27:565–71
- Sep S, Verbeek J, Koek G, et al. Clinical differences between early-onset HELLP syndrome and early-onset preeclampsia during pregnancy and at least 6 months postpartum. Am J Obstet Gynecol 2010;202:271.e271–5
- Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol 2006;30:16–9
- Ales KL, Charlson ME. Epidemiology of preeclampsia and eclampsia. Am J Obstet Gynecol 1991;165:238
- Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 2003;111:600–2
- Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med 2005;18:9–16
- Crispi F, Dominguez C, Llurba E, et al. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol 2006;195:201–7
- Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008;21:41–52
- Chaiworapongsa T, Romero R, Tarca AL, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol 2010;202:550.e551–10
- Benton SJ, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol 2011;205:469.e461–8
- Ghosh SK, Raheja S, Tuli A, et al. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet 2012;285:417–22
- Hagmann H, Thadhani R, Benzing T, et al. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem 2012;58:837–45
- Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;122:43–52
- Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005;46:1077–85
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med 2012;25:2651--7
- Bahado-Singh RO, Akolekar R, Mandal R, et al. Metabolomics and first-trimester prediction of early-onset preeclampsia. J Matern Fetal Neonatal Med 2012;25:1840–7
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med 2011;24:1187–207
- Mazaki-Tovi S, Vaisbuch E, Romero R, et al. Maternal and neonatal circulating visfatin concentrations in patients with pre-eclampsia and a small-for-gestational age neonate. J Matern Fetal Neonatal Med 2010;23:1119–28
- Sibai B, Romero R, Klebanoff MA, et al. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. Am J Obstet Gynecol 2009;200:630.e631–8
- Romero R, Kusanovic JP, Than NG, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol 2008;199:122.e1–11
- Serin IS, Ozcelik B, Basbug M, et al. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2002;100:143–5
- Kronborg CS, Gjedsted J, Vittinghus E, et al. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand 2011;90:791–6
- Bodova KB, Biringer K, Dokus K, et al. Fibronectin, plasminogen activator inhibitor type 1 (PAI-1) and uterine artery Doppler velocimetry as markers of preeclampsia. Dis Markers 2011;30:191–6
- Dusse LM, Carvalho MG, Getliffe K, et al. Increased circulating thrombomodulin levels in pre-eclampsia. Clin Chim Acta 2008;387:168–71
- Boffa MC, Valsecchi L, Fausto A, et al. Predictive value of plasma thrombomodulin in preeclampsia and gestational hypertension. Thromb Haemost 1998;79:1092–5
- Shaarawy M, Didy HE. Thrombomodulin, plasminogen activator inhibitor type 1 (PAI-1) and fibronectin as biomarkers of endothelial damage in preeclampsia and eclampsia. Int J Gynaecol Obstet 1996;55:135–9
- Hsu CD, Iriye B, Johnson TR, et al. Elevated circulating thrombomodulin in severe preeclampsia. Am J Obstet Gynecol 1993;169:148–9
- Minakami H, Takahashi T, Izumi A, Tamada T. Increased levels of plasma thrombomodulin in preeclampsia. Gynecol Obstet Invest 1993;36:208–10
- Hsu CD, Copel JA, Hong SF, Chan DW. Thrombomodulin levels in preeclampsia, gestational hypertension, and chronic hypertension. Obstet Gynecol 1995;86:897–9
- Bontis J, Vavilis D, Agorastos T, et al. Maternal plasma level of thrombomodulin is increased in mild preeclampsia. Eur J Obstet Gynecol Reprod Biol 1995;60:139–41
- Thilaganathan B, Wormald B, Zanardini C, et al. Early-pregnancy multiple serum markers and second-trimester uterine artery Doppler in predicting preeclampsia. Obstet Gynecol 2010;115:1233–8
- Meads CA, Cnossen JS, Meher S, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2008;12:iii–iv, 1–270
- Mei-Dan E, Wiznitzer A, Sergienko R, et al. Prediction of preeclampsia: liver function tests during the first 20 gestational weeks. J Matern Fetal Neonatal Med 2013;26:250--3
- Torry DS, Wang HS, Wang TH, et al. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–44
- Tidwell SC, Ho HN, Chiu WH, et al. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001;184:1267–72
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–58
- Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–51
- Taylor RN, Grimwood J, Taylor RS, et al. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–82
- Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–63
- Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–7
- Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–9
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, et al. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–22
- Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension 2012;59:555–7
- Molvarec A, Szarka A, Walentin S, et al. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res 2010;33:892–8
- Staff AC, Braekke K, Johnsen GM, et al. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol 2007;197:176.e171–6
- Chaiworapongsa T, Espinoza J, Gotsch F, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40
- Crispi F, Llurba E, Dominguez C, et al. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303–9
- Asvold BO, Vatten LJ, Romundstad PR, et al. Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am J Epidemiol 2011;173:630–9
- Gourvas V, Dalpa E, Konstantinidou A, et al. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol Med Report 2012;6:23–7
- Espinoza J, Chaiworapongsa T, Romero R, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507
- Chaiworapongsa T, Kusanovic JP, Savasan ZA, et al. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med 2010;23:960–72
- Chaiworapongsa T, Romero R, Kusanovic JP, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern Fetal Neonatal Med 2010;23:794–805
- Bixel K, Silasi M, Zelop CM, et al. Placental origins of angiogenic dysfunction in mirror syndrome. Hypertens Pregnancy 2012;31:211–17
- Espinoza J, Romero R, Nien JK, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome' (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–13
- Koga K, Osuga Y, Tajima T, et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril 2010;94:305–8
- Kusanovic JP, Romero R, Espinoza J, et al. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol 2008;198:382.e381–8
- Llurba E, Marsal G, Sanchez O, et al. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet Gynecol 2012;40:367–9
- Rana S, Venkatesha S, DePaepe M, et al. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol 2007;109:549–52
- Chaiworapongsa T, Romero R, Tarca A, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med 2009;22:1122–39
- Romero R, Chaiworapongsa T, Erez O, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med 2010;23:1384–99
- Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18
- Moore Simas TA, Crawford SL, Solitro MJ, et al. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol 2007;197:244.e241–8
- Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–87
- Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23
- Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol 2007;197:211.e211–14
- Widmer M, Villar J, Benigni A, et al. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol 2007;109:168–80
- Park CW, Park JS, Shim SS, et al. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–9
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012;60:451–8
- Rana S, Cerdeira AS, Wenger J, et al. Plasma Concentrations of Soluble Endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One 2012;7:e48259
- Wikstrom AK, Larsson A, Eriksson UJ, et al. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol 2007;109:1368–74
- Robinson CJ, Johnson DD, Chang EY, et al. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255–9
- Pedrosa AC, Matias A. Screening for pre-eclampsia: a systematic review of tests combining uterine artery Doppler with other markers. J Perinat Med 2011;39:619–35
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–38
- Granne I, Southcombe JH, Snider JV, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS One 2011;6:e24463
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett 1989;258:301–4
- Tominaga S, Inazawa J, Tsuji S. Assignment of the human ST2 gene to chromosome 2 at q11.2. Hum Genet 1996;97:561–3
- Werenskiold AK, Hoffmann S, Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol 1989;9:5207–14
- Klemenz R, Hoffmann S, Werenskiold AK. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc Natl Acad Sci U S A 1989;86:5708–12
- Meisel C, Bonhagen K, Lohning M, et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol 2001;166:3143–50
- Lohning M, Stroehmann A, Coyle AJ, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A 1998;95:6930–5
- Trajkovic V, Sweet MJ, Xu D. T1/ST2–an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev 2004;15:87–95
- Yanagisawa K, Naito Y, Kuroiwa K, et al. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem 1997;121:95–103
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–90
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 2007;179:2051–4
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, et al. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 2009;113:1526–34
- Bourgeois E, Van LP, Samson M, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol 2009;39:1046–55
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 2007;282:26369–80
- Bartunek J, Delrue L, Van Durme F, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol 2008;52:2166–74
- Topping V, Romero R, Than NG, et al. Interleukin-33 in the Human Placenta. J Matern Fetal Neonatal Med 2013;26:327--38
- Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med 2009;179:772–81
- Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med 2004;30:1468–73
- Hoogerwerf JJ, Tanck MW, van Zoelen MA, et al. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med 2010;36:630–7
- Mildner M, Storka A, Lichtenauer M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res 2010;87:769–77
- Sabatine MS, Morrow DA, Higgins LJ, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation 2008;117:1936–44
- Weinberg EO, Shimpo M, Hurwitz S, et al. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003;107:721–6
- Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117:1538–49
- Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med 2008;205:339–46
- Lecart S, Lecointe N, Subramaniam A, et al. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol 2002;32:2979–87
- Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA 2010;107:8017–22
- Kurokawa M, Matsukura S, Kawaguchi M, et al. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Int Arch Allergy Immunol 2011;155:12–20
- Oshikawa K, Kuroiwa K, Tokunaga T, et al. Acute eosinophilic pneumonia with increased soluble ST2 in serum and bronchoalveolar lavage fluid. Respir Med 2001;95:532–3
- Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol 1996;106:127–33
- Saito S, Sakai M, Sasaki Y, et al. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 1999;117:550–5
- Alexander GR, Himes JH, Kaufman RB, et al. United States national reference for fetal growth. Obstet Gynecol 1966;87:163–8
- Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997;177:1003–10
- Chaiworapongsa T, Romero R, Kusanovic JP, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol 2010;35:155–62
- Kurmanavicius J, Florio I, Wisser J, et al. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24-42 weeks of gestation. Ultrasound Obstet Gynecol 1997;10:112–20
- Arduini D, Rizzo G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med 1990;18:165–72
- Trudinger BJ, Cook CM, Giles WB, et al. Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. Br J Obstet Gynaecol 1991;98:378–84
- Luppi P, Tse H, Lain KY, et al. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol 2006;56:135–44
- Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 2006;11:309–16
- Mihu D, Sabau L, Costin N, et al. Implications of maternal systemic oxidative stress in normal pregnancy and in pregnancy complicated by preeclampsia. J Matern Fetal Neonatal Med 2012;25:944–51
- Borzychowski AM, Croy BA, Chan WL, et al. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol 2005;35:3054–63
- Borghi C, Esposti DD, Immordino V, et al. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am J Obstet Gynecol 2000;183:140–7
- Sala C, Campise M, Ambroso G, et al. Atrial natriuretic peptide and hemodynamic changes during normal human pregnancy. Hypertension 1995;25:631–6
- Castro LC, Hobel CJ, Gornbein J. Plasma levels of atrial natriuretic peptide in normal and hypertensive pregnancies: a meta-analysis. Am J Obstet Gynecol 1994;171:1642–51
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004;44:2349–54
- Weinberg EO, Shimpo M, De Keulenaer GW, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002;106:2961–6
- Mayhew TM, Ohadike C, Baker PN, et al. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta 2003;24:219–26
- Manetti M, Ibba-Manneschi L, Liakouli V, et al. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann Rheum Dis 2010;69:598–605
