Abstract
Objective: Intra-amniotic infection/inflammation (IAI) is causally linked with spontaneous preterm labor and delivery. The ST2L receptor and its soluble form (sST2) are capable of binding to interleukin (IL)-33, a member of the IL-1 superfamily. Members of this cytokine family have been implicated in the onset of spontaneous preterm labor in the context of infection. Soluble ST2 has anti-inflammatory properties, and plasma concentrations are elevated in systemic inflammation, such as sepsis, acute pyelonephritis in pregnancy and the fetal inflammatory response syndrome. The aims of this study were to examine: (1) whether amniotic fluid concentrations of sST2 change with IAI, preterm, and term parturition; and (2) if mRNA expression of ST2 in the chorioamniotic membranes changes with acute histologic chorioamnionitis in women who deliver preterm.
Method: A cross-sectional study was conducted to determine amniotic fluid concentrations of sST2 in: (1) women with preterm labor (PTL) who delivered at term (n = 49); (2) women with PTL who delivered preterm without IAI (n = 21); (3) women with PTL who delivered preterm with IAI (n = 31); (4) term pregnancies not in labor (n = 13); and (5) term pregnancies in labor (n = 43). The amniotic fluid concentration of sST2 was determined by ELISA. The mRNA expression of ST2 in the chorioamniotic membranes of women who delivered preterm with (n = 24), and without acute histologic chorioamnionitis (n = 19) was determined by qRT-PCR.
Results: (1) Patients with PTL who delivered preterm with IAI had a lower median amniotic fluid concentration of sST2 compared to those with PTL who delivered preterm without IAI [median 410 ng/mL, inter-quartile range (IQR) 152–699 ng/mL versus median 825 ng/mL, IQR 493–1216 ng/mL; p = 0.0003] and those with PTL who delivered at term [median 410 ng/mL, IQR 152–699 ng/mL versus median 673 ng/mL, IQR 468–1045 ng/mL; p = 0.0003]; (2) no significant differences in the median amniotic fluid concentration of sST2 were observed between patients with PTL who delivered at term and those who delivered preterm without IAI (p = 0.4), and between women at term in labor and those at term not in labor (p = 0.9); (3) the mean mRNA expression of ST2 was 4-fold lower in women who delivered preterm with acute histologic chorioamnionitis than in those without this lesion (p = 0.008).
Conclusions: The median sST2 amniotic fluid concentration and mRNA expression of ST2 by chorioamniotic membranes is lower in PTL associated with IAI and acute histologic chorioamnionitis than in PTL without these conditions. Changes in the median amniotic fluid sST2 concentration are not observed in preterm and term parturition without IAI. Thus, amniotic fluid sST2 in the presence of IAI behaves differently when compared to sST2 in the plasma of individuals affected by fetal inflammatory response syndrome, acute pyelonephritis in pregnancy, and adult sepsis. Decreased concentrations of sST2 in IAI are likely to promote a pro-inflammatory response, which is important for parturition in the context of infection.
Introduction
Intra-amniotic infection is associated with spontaneous preterm parturition [Citation1–38]. The gold standard for determining the presence of microbial invasion in the amniotic fluid is a positive culture for microorganisms [Citation20,Citation39]. The frequency of positive amniotic fluid cultures in patients with preterm labor (PTL) and intact membranes is 12.8% [Citation39], and 32.4% in patients with preterm premature rupture of the membranes (pPROM) [Citation39]. The earlier the gestational age at preterm delivery, the higher the prevalence of intra-amniotic infection [Citation40,Citation41]. With the use of molecular microbiologic techniques, the frequency of microbial invasion of the amniotic cavity has been determined to be higher than that previously reported by culture [Citation42–47].
The mechanisms responsible for preterm parturition in the context of infection involve the production of pro-inflammatory cytokines and chemokines [Citation14,Citation20,Citation22,Citation25,Citation48–72]. The first cytokine to be implicated in the mechanism of parturition was interleukin (IL)-1β [Citation17,Citation48,Citation51,Citation73,Citation74]. This cytokine is produced by the human decidua in response to microbial products, can stimulate prostaglandin production, can induce the onset of labor in pregnant mice, and administration of the IL-1 receptor antagonist can abrogate the effect of IL-1 in the induction of parturition [Citation48,Citation75,Citation76]. Tumor necrosis factor-α has similar properties as IL-1β, and a role in preterm labor associated with infection has also been proposed [Citation22,Citation50,Citation51,Citation59,Citation73,Citation77–81]. Among chemokines, IL-8 [Citation82–95], monocyte chemoattractant protein-1 [Citation96–100], macrophage inflammatory protein-1α [Citation53,Citation101,Citation102], and growth regulated oncogene-α [Citation103–105] have also been shown to play a role in preterm parturition.
Acute inflammation of the chorioamniotic membranes is the maternal response to the presence of intra-amniotic infection [Citation70,Citation106–108]. In 72% of placentas with acute histologic chorioamnionitis, bacteria are isolated from the subchorionic plate [Citation109–112]. Funisitis and chorionic vasculitis are the histologic hallmarks of the fetal inflammatory response syndrome (FIRS) [Citation113–115]. Fetuses with FIRS are at increased risk for the development of short and long-term complications [Citation116–118] such as sepsis [Citation54,Citation113,Citation119–121], intraventricular hemorrhage [Citation122–124], periventricular leukomalacia [Citation125–128], cerebral palsy [Citation129–139], chronic lung disease [Citation140–148], and retinopathy of prematurity [Citation149–151]. In this context, the onset of preterm labor mediated by inflammation most likely represents the host defense mechanism against infection, and could be of survival value for both mother and fetus [Citation113,Citation152].
Toll-like receptors (TLRs), a family of pattern-recognition receptors, represent one of the mechanisms by which the innate immune system recognizes the presence of microorganisms [Citation153–157] and triggers inflammation [Citation158,Citation159]. TLR-2 and TLR-4 are expressed in the dendritic cells, monocytes/macrophages [Citation160], and in a wide range of epithelial cells [Citation161–163]. The amniotic epithelium also expresses TLR-2 and TLR-4, and this increases in the presence of acute histologic chorioamnionitis [Citation164]. This observation indicates that the innate immune system is part of the host defense in the presence of intra-amniotic infection [Citation164]. Indeed, the spontaneous deletion of TLR-4 protects against lipopolysaccharide (LPS) induced preterm labor [Citation165].
ST2 is a gene located on chromosome 2 in humans [Citation166,Citation167], and its two main products are a trans-membranous receptor expressed on Th2, but not, Th1 immune cells (ST2L) [Citation168–170], and a decoy receptor, soluble ST2 (sST2) [Citation168,Citation171]. IL-33 is the ligand for the ST2L receptor, and it stimulates the Th2 type immune response [Citation172]. This interaction can be interrupted by sST2, which binds IL-33 and suppress its stimulating properties [Citation171]. Soluble ST2 is involved in regulation of the Th1/Th2-associated immune response and modulation of the inflammatory response in the presence of infection [Citation170,Citation173–175], while it has anti-inflammatory properties through negative regulation of TLR-2 and TLR-4 [Citation176,Citation177]. Soluble ST2 has been implicated in several pathological conditions such as allergic asthma [Citation178–183], lung fibrosis [Citation184,Citation185], chronic obstructive pulmonary disease [Citation186], acute myocardial infarction [Citation187,Citation188] and preeclampsia [Citation189,Citation190]. Moreover, plasma concentrations of sST2 are elevated in patients with systemic inflammation (e.g. sepsis) [Citation191,Citation192], acute pyelonephritis in pregnancy (unpublished observation), and in the presence of FIRS [Citation193].
The objectives of this study were to examine: (1) whether amniotic fluid concentrations of sST2 change during IAI and preterm or term parturition; and (2) whether ST2 mRNA expression in the chorioamniotic membranes changes in the presence of acute histologic chorioamnionitis in women who deliver preterm.
Material and method
Study design and population
This is a retrospective cross-sectional study conducted by searching our clinical database and Bank of Biologic Samples, including 157 patients who had amniotic fluid samples obtained by trans-abdominal amniocentesis. Women were included into the following groups: (1) patients with PTL who delivered at term (n = 49); (2) those with PTL who delivered preterm with IAI (n = 31) and without (n = 21); and (3) term pregnancies in labor (n = 43) and not in labor (n = 15). Women with multiple gestations, or those who had fetuses affected with chromosomal and/or sonographic abnormalities were excluded.
In a separate cohort of 43 patients with spontaneous preterm delivery, ST2 mRNA expression was determined in the chorioamniotic membranes collected from those with (n = 24), and without acute histologic chorioamnionitis (n = 19).
All women provided written informed consent before the collection of biological samples. The collection and utilization of the samples was approved by the Human Investigation Committee of the participating institutions and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS). Many of these samples have been used in previous studies of inflammation and growth factors in pregnancy complications.
Clinical definitions
The diagnosis of preterm labor was made in the presence of regular uterine contractions (at least 3 in 30 min) and a documented cervical change in patients with a gestational age of 20 to 36 6/7 weeks who required hospitalization [Citation194]. Women at term in labor consisted of those admitted for suspected preterm labor because of uncertain dates. However, those who delivered a neonate ≥2500 g without neonatal complications of prematurity were considered likely to represent patients in spontaneous labor at term [Citation195]. IAI was defined as a positive culture for microorganisms in amniotic fluid and/or an elevated amniotic fluid IL-6 concentration (≥2.6 ng/mL) [Citation196]. Acute histologic chorioamnionitis was diagnosed in the presence of neutrophil infiltration into the chorionic plate or extra-placental membranes according to criteria previously described [Citation114].
Amniotic fluid sample collection
Amniotic fluid samples were obtained by trans-abdominal amniocentesis under ultrasound guidance. In patients with preterm labor, amniotic fluid samples were obtained to evaluate the microbial status of the amniotic cavity. Women at term (≥37 weeks) not in labor underwent amniocentesis to assess fetal lung maturity prior to cesarean delivery. Women at term in labor underwent amniocentesis to assess fetal lung maturity and the presence or absence of microbial invasion of the amniotic cavity (as described in the previous paragraph).
Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital Mycoplasmas. White blood cell (WBC) count [Citation16], glucose concentration [Citation197], and Gram stain [Citation198] were performed shortly after collection [Citation16,Citation197]. The results of these tests were used for clinical management, while the amniotic fluid IL-6 concentration results were used for research purposes only. Amniotic fluid not used for clinical management was centrifuged at 1300 g for 10 min at 4 °C, and the supernatant was stored at −70 °C.
Determination of sST2 in amniotic fluid
Concentrations of sST2 and IL-6 in amniotic fluid were determined by sensitive and specific enzyme immunoassays obtained from R&D Systems (Minneapolis, MN). The initial assay validation was performed in our laboratory prior to the conduction of this study. The quantitative sandwich enzyme immunoassay technique was utilized and concentrations were determined by interpolation from standard curves. The inter- and intra-assay coefficients of variation for sST2 in amniotic fluid were 4.5% and 3.3%, respectively, and for IL-6, these were 8.7% and 4.6%, respectively. The sensitivity of the assay for sST2 and IL-6 was 20.1 pg/mL and 0.09 pg/mL, respectively.
ST2 mRNA expression in chorioamniotic membranes in acute histologic chorioamnionitis
Total RNAs were isolated from snap-frozen chorioamniotic membrane tissue samples by using a TRIzol reagent (Invitrogen, Carlsbad, CA) and Qiagen RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations. Five hundred nanograms of RNA samples were reverse transcribed using the SuperScript III First-Strand Synthesis System and oligo (dT)20 primers (Invitrogen). All qRT-PCR analyses were carried out using TaqMan Assays (ST2/IL1RL1: Hs01073300_m1; GAPDH: Hs99999905_m1; Applied Biosystems, Foster City, CA) on a BioMark™ Real-Time PCR System (Fluidigm, South San Francisco, CA) according to the manufacturers’ instructions.
Statistical analysis
Kolmogorov–Smirnov and Shapiro–Wilk tests were used to determine if the data were normally distributed. Kruskal–Wallis and “post-hoc” Mann–Whitney U test were used to compare continuous non-parametric variables among and between groups. Comparisons between proportions were performed using Chi-square or Fisher’s exact tests when appropriate. Correlation between two continuous variables was determined using Spearman’s rank correlation test. A p value <0.05 was considered statistically significant. Analysis was performed using SPSS, version 19 (IBM Corp, Armonk, NY).
Gene expression analysis
The fold change between groups was estimated using a linear model in which the dependent variable was the −ΔCt value of the gene, while the independent variable was the study group. All data analysis was performed using the R statistical language and environment (www.r_project.org).
Results
Demographic and clinical characteristics of the study population
Demographic and clinical characteristics of the preterm and term labor groups are displayed in and , respectively. Among women in the preterm labor group, there were no significant differences in the median maternal age, body mass index (BMI) and gestational age at amniocentesis. As expected, due to the study design, the median interval to delivery, gestational age at delivery and birthweight were significantly different among the PTL subgroups (). The median maternal age, gestational age at amniocentesis and gestational age at delivery in women at term in labor were not significantly different from those at term not in labor ().
Table 1. Demographic and clinical characteristics of the preterm labor sub-groups.
Table 2. Demographic and clinical characteristics of the women at term sub-groups.
Intra-amniotic infection/inflammation is associated with decreased median concentrations of sST2 in amniotic fluid
Patients with PTL who delivered preterm with IAI had a lower median amniotic fluid concentration of sST2 than: (1) women with PTL who delivered preterm without IAI [median 410 ng/mL, inter-quartile range (IQR) 152–699 ng/mL versus median 825 ng/mL, IQR 493–1216 ng/mL; p = 0.0003], and (2) women with PTL who delivered at term (median 410 ng/mL, IQR 152–699 ng/mL versus median 673 ng/mL, IQR 468–1045; p = 0.0003]; ). The median sST2 concentration of women with PTL who delivered at term did not differ significantly from women with PTL who delivered preterm without IAI (median 673 ng/mL; IQR 468–1045 ng/mL versus median 825 ng/mL; IQR 493–1216; p = 0.4; ). Amniotic fluid sST2 concentrations did not correlate with amniotic fluid concentrations of IL-6, WBC count, or glucose (Spearman’s Rho −0.17 [p = 0.09], −0.06 [p = 0.6], −0.02 [p = 0.8], respectively).
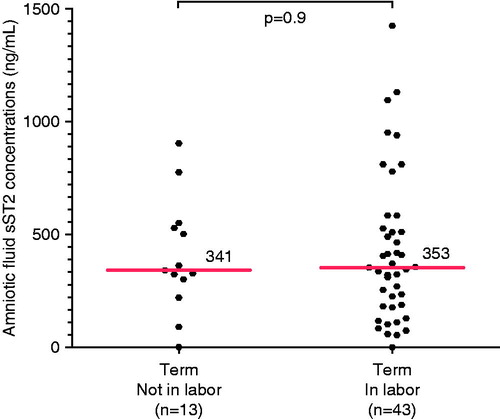
Figure 1. Amniotic fluid concentrations of sST2 in preterm labor sub-groups. The median amniotic fluid concentration of sST2 was significantly lower in women with PTL with intra-amniotic infection/inflammation (IAI) than in those who delivered preterm without IAI (median 410 ng/mL; IQR 152-699 versus median 825 ng/mL; IQR 493-1216 [p = 0.0003]) and those with PTL who delivered at term (median 410 ng/mL; IQR 152-699 versus median 673 ng/mL; IQR 468-1045 [p = 0.0003]). In the absence of IAI, there was no significant difference in the median amniotic fluid concentration of sST2 between women with PTL who delivered at term and those who delivered preterm without IAI (p = 0.4).
![Figure 1. Amniotic fluid concentrations of sST2 in preterm labor sub-groups. The median amniotic fluid concentration of sST2 was significantly lower in women with PTL with intra-amniotic infection/inflammation (IAI) than in those who delivered preterm without IAI (median 410 ng/mL; IQR 152-699 versus median 825 ng/mL; IQR 493-1216 [p = 0.0003]) and those with PTL who delivered at term (median 410 ng/mL; IQR 152-699 versus median 673 ng/mL; IQR 468-1045 [p = 0.0003]). In the absence of IAI, there was no significant difference in the median amniotic fluid concentration of sST2 between women with PTL who delivered at term and those who delivered preterm without IAI (p = 0.4).](/cms/asset/2ea757a1-9e86-472e-9614-7c59d4b50e81/ijmf_a_806894_f0001_b.jpg)
Labor at term is not associated with an elevation of amniotic fluid sST2 median concentration
When comparing women at term not in labor to those at term in labor, there was no significant difference in the median amniotic fluid concentration of sST2 (median 341 ng/mL; IQR 260–539 ng/mL versus median 353 ng/mL; IQR 181–527 ng/mL; p = 0.9; ).
ST2 mRNA expression is down-regulated in chorioamniotic membranes in the presence of acute histologic chorioamnionitis
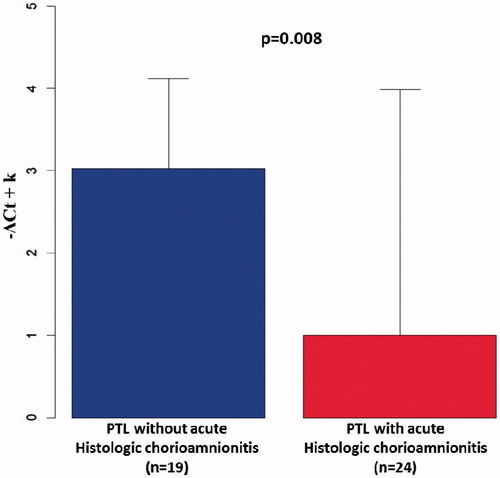
A case–control study was conducted to determine ST2 mRNA expression by the fetal membranes in patients with, and without acute histologic chorioamnionitis. The median gestational age at delivery was not significantly different between women with PTL and acute histologic chorioamnionitis and women with PTL without this condition (median 31.4 weeks; IQR 30.4–32.6 weeks versus median 32 weeks; IQR 30–33.1 weeks; p = 0.6). The mean ST2 mRNA expression in the chorioamniotic membranes of patients with acute histologic chorioamnionitis was 4-fold lower compared to those without the lesion (p = 0.008; ).
Figure 3. ST2 mRNA expression by the chorioamniotic membranes. The mean ST2 mRNA expression by the chorioamniotic membranes of patients who delivered preterm with acute histologic chorioamnionitis was 4-fold lower than those without this lesion (p = 0.008). −ΔCt: fold change between groups estimated by a generalized linear model.
Discussion
Principal findings of the study
(1) Women with PTL who delivered preterm with IAI have a lower median amniotic fluid sST2 concentration compared to women with PTL who delivered preterm, and compared to those who delivered at term without IAI; (2) median amniotic fluid sST2 concentration did not differ between women at term not in labor and women at term in labor; and (3) ST2 mRNA expression in the chorioamniotic membranes is down-regulated in women with acute histologic chorioamnionitis who delivered preterm compared to women who delivered preterm without this lesion.
Soluble ST2 decreases in preterm labor with intra-amniotic infection/inflammation
ST2L receptor and sST2 are generated by mRNA transcript alternative splicing from a single gene [Citation168,Citation199]. Therefore, the expression of sST2 and ST2L can be independent of each other, and the two proteins have different roles. ST2L stimulates, while sST2 has a suppressive role on the Th2 immune response [Citation170,Citation173,Citation174]. Moreover, sST2 down-regulates the inflammatory response through the modulation of TLR expression [Citation176,Citation177]. TLRs are of crucial importance for the recognition of microorganisms and activation of the innate immune response (inflammation) [Citation15,Citation153–158]. Indeed, mice with a spontaneous mutation for TLR-4 are less likely to deliver preterm after intrauterine inoculation of heat-killed bacteria or LPS than wild-type mice [Citation165].
If the initial host response fails to recognize microbial products and does not initiate an inflammatory response, overwhelming sepsis can develop with widespread infection and multiple organ damage [Citation200]. The administration of sST2 fusion protein in ST2 knock-out mice results in the suppression of pro-inflammatory cytokine production by macrophages in response to LPS [Citation201], and it attenuates septic shock and collagen-induced arthritis [Citation201,Citation202].
The finding that patients with IAI have a lower median amniotic fluid concentration of sST2 than those without IAI could indicate that the cytokine network and its receptors are organized, so that a pro-inflammatory response promotes preterm delivery. The observation that the amniotic fluid concentration of sST2 are decreased in the context of infection differs from that made in other compartments, such as peripheral blood in sepsis [Citation191,Citation192], FIRS [Citation193], and acute pyelonephritis in pregnancy (unpublished observation). These differences could represent distinct homeostatic mechanisms in the amniotic cavity and circulation. The increased concentration of sST2 in peripheral blood may represent the activation of the anti-inflammatory limb of the immune response to prevent the deleterious effect of an exaggerated pro-inflammatory response of a cytokine storm. Indeed, some of the molecules that have modulatory properties on the immune response have different functions, depending on the tissue localization [Citation203]. Nevertheless, pregnant women with intra-amniotic infection represent a unique condition in which there is a potential conflict between mother and fetus. Prolonging the pregnancy by inducing an anti-inflammatory response could decrease the risk of prematurity, and thus benefit the fetus; however this exposes both the mother and fetus to risks of uncontrolled infection and sepsis. Three different host defense strategies to infection have recently been proposed: avoidance, resistance and tolerance [Citation204]. Low concentrations of sST2 in amniotic fluid in the presence of IAI, along with other immune changes, suggest that the host (both mother and fetus) favors preterm labor by inducing an inflammatory response. Accordingly, the mechanism of preterm labor in the presence of intraamniotic infection could represent the execution of avoidance by the initiation of labor.
ST2 mRNA expression is down-regulated in the chorioamniotic membranes of women who delivered preterm and have acute histologic chorioamnionitis
The observation that ST2 mRNA expression is down-regulated in the chorioamniotic membranes of women who delivered preterm and have acute histologic chorioamnionitis further strengthens our hypothesis. Indeed, acute histologic chorioamnionitis often results from intra-amniotic infection [Citation53,Citation205–209], and it is the most common placental lesion in early spontaneous preterm birth [Citation210–212]. Moreover, in vitro experiments have shown that ST2 mRNA expression is decreased in amnion mesenchymal cells, but is increased in human umbilical vein endothelial cells after treatment with IL-1β [Citation213]. This evidence is consistent with the observed changes of sST2 concentrations in amniotic fluid (lower concentration in IAI) and in umbilical cord plasma (elevated concentration in FIRS) [Citation193].
Amniotic fluid sST2 concentrations do not change with preterm and term parturition in the absence of intra-amniotic infection/inflammation
To determine whether changes in amniotic fluid sST2 concentration are related to labor per se, we examined the concentration of this soluble receptor in the amniotic fluid of women at term (with and without labor), and in those with PTL in the absence of IAI. There were no differences in the median amniotic fluid concentration of sST2 between women at term in labor and those at term not in labor. Similarly, amniotic fluid sST2 concentrations in patients without IAI who delivered preterm did not differ from those with PTL who delivered at term. These findings represent further evidence that changes in amniotic fluid concentrations of sST2 in patients with IAI are most likely due to the presence of infection/inflammation, rather than parturition.
Strengths and limitations of the study
This is the first study to examine the changes of amniotic fluid sST2 concentration in preterm labor in the presence of IAI, as well as parturition. The observations herein were supported by evaluation of ST2 mRNA expression in the chorioamniotic membranes of preterm deliveries with, and without acute histologic chorioamnionitis. We have previously studied IL-33 concentrations in amniotic fluid of patients in preterm labor, and in all patients, IL-33 was below the sensitivity of the assay (n = 10; unpublished observation).
Conclusions
The median amniotic fluid concentration of sST2 and ST2 mRNA expression by the chorioamniotic membranes is lower in patients with IAI and acute histologic chorioamnionitis than in women without infection/inflammation in the amniotic cavity or acute inflammation in the chorioamniotic membranes. Term or preterm labor without IAI is not associated with changes in the median amniotic fluid concentration of sST2. Down-regulation of ST2 in the chorioamniotic membranes and amniotic fluid in the presence of infection-induced preterm labor may have a role in enhancing pro-inflammatory response in the amniotic fluid of these patients.
Declaration of interest
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
References
- Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79
- Kullander S. Fever and parturition. An experimental study in rabbits. Acta Obstet Gynecol Scand Suppl 1977;66:77–85
- Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52
- Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302
- Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol 1983;62:137–44
- Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol 1984;149:726–30
- Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43
- Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol 1986;67:500–6
- Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6
- Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24
- Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol 1989;161:813–16
- Dombroski RA, Woodard DS, Harper MJ, Gibbs RS. A rabbit model for bacteria-induced preterm pregnancy loss. Am J Obstet Gynecol 1990;163:1938–43
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–71
- Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–30
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28
- Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1992;167:1231–42
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29
- Fidel PL Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75
- Gravett MG, Witkin SS, Haluska GJ, et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–7
- Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11
- Gomez R, Ghezzi F, Romero R, et al. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995;22:281–342
- Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995;172:1598–603
- Horowitz S, Mazor M, Romero R, et al. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995;40:375–9
- Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol 1996;174:1725–31; discussion 1731–1723
- Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–7
- Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13
- Gibbs RS, McDuffie RS Jr, Kunze M, et al. Experimental intrauterine infection with Prevotella bivia in New Zealand White rabbits. Am J Obstet Gynecol 2004;190:1082–6
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39
- Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70
- Kalan AM, Simhan HN. Mid-trimester cervical inflammatory milieu and sonographic cervical length. Am J Obstet Gynecol 2010;203:126 e121–5
- Trivedi S, Joachim M, McElrath T, et al. Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstet Gynecol 2012;206:236 e231–8
- Kiefer DG, Keeler SM, Rust O, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol 2012;206:68 e61–6
- Hsu TY, Lin H, Lan KC, et al. High interleukin-16 concentrations in the early second trimester amniotic fluid: an independent predictive marker for preterm birth. J Matern Fetal Neonatal Med 2013;26:285–9
- Park CW, Hyun Yoon B, Shin Park J, Kwan Jun J. An elevated maternal serum c-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and AF inflammatory response are likely in patients with preterm labor: clinical implications. J Matern Fetal Neonatal Med 2013;26:847–53
- Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–7
- Gomez R, Romero R, Nien JK, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2005;192:678–89
- Pao CC, Kao SM, Wang HC, Lee CC. Intraamniotic detection of Chlamydia trachomatis deoxyribonucleic acid sequences by polymerase chain reaction. Am J Obstet Gynecol 1991;164:1295–9
- Jalava J, Mantymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol 1996;103:664–9
- Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis 1997;24:1228–32
- Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA 2004;292:462–9
- Miralles R, Hodge R, McParland PC, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res 2005;57:570–7
- DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57
- Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 220–203
- Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–87
- McDuffie RS, Jr Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol 1992;167:1583–8
- Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83
- Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994;32:108–13
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12
- Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol 1996;174:1855–61; discussion 1861–1852
- Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust N Z J Obstet Gynaecol 1998;38:403–6
- Arntzen KJ, Kjollesdal AM, Halgunset J, et al. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998;26:17–26
- Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999;181:1142–8
- Baud O, Emilie D, Pelletier E, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–7
- Wenstrom KD, Andrews WW, Hauth JC, et al. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–50
- Keelan JA, Marvin KW, Sato TA, et al. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999;181:1530–6
- Zaga-Clavellina V, Garcia-Lopez G, Flores-Herrera H, et al. In vitro secretion profiles of interleukin (IL)-1beta, IL-6, IL-8, IL-10, and TNF alpha after selective infection with Escherichia coli in human fetal membranes. Reprod Biol Endocrinol 2007;5:46
- Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–61
- Krolak-Olejnik B, Olejnik I. Late-preterm cesarean delivery and chemokines concentration in the umbilical cord blood of neonates. J Matern Fetal Neonatal Med 2012;25:1810–13
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:34–47
- Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–19
- Hassanein SM, El-Farrash RA, Hafez HM, et al. Cord blood interleukin-6 and neonatal morbidities among preterm infants with PCR-positive Ureaplasma urealyticum. J Matern Fetal Neonatal Med 2012;25:2106–10
- Vaisbuch E, Romero R, Gomez R, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2011;24:391–6
- Tsiartas P, Kacerovsky M, Musilova I, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;Apr 16 [e-pub ahead of print]
- Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:795–801
- Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med 2012;25:29–31
- Sadowsky DW, Adams KM, Gravett MG, et al. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006;195:1578–89
- Marconi C, de Andrade Ramos BR, Peracoli JC, et al. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol 2011;65:549–56
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–5
- Romero R, Sepulveda W, Mazor M, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol 1992;167:863–72
- Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41
- Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol 1993;168:1318–22
- Baumann P, Romero R, Berry S, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol 1993;30:184–93
- Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol 2002;187:1159–62
- Lonergan M, Aponso D, Marvin KW, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab 2003;88:3835–44
- Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 1993;169:1299–303
- Saito S, Kasahara T, Kato Y, et al. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–8
- Puchner T, Egarter C, Wimmer C, et al. Amniotic fluid interleukin-8 as a marker for intraamniotic infection. Arch Gynecol Obstet 1993;253:9–14
- Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab 1993;76:404–10
- Fortunato SJ, Menon R, Swan KF. Amniochorion: a source of interleukin-8. Am J Reprod Immunol 1995;34:156–62
- Tanaka Y, Narahara H, Takai N, et al. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol 1998;179:644–9
- Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol 1998;179:1267–70
- Laham N, Brennecke SP, Rice GE. Interleukin-8 release from human gestational tissue explants: effects of gestation, labor, and chorioamnionitis. Biol Reprod 1999;61:823–7
- Dowd J, Laham N, Rice G, et al. Elevated interleukin-8 concentrations in cervical secretions are associated with preterm labour. Gynecol Obstet Invest 2001;51:165–8
- Witt A, Berger A, Gruber CJ, et al. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med 2005;33:22–6
- Jacobsson B, Mattsby-Baltzer I, Hagberg H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG 2005;112:719–24
- Yoneda S, Sakai M, Sasaki Y, et al. Interleukin-8 and glucose in amniotic fluid, fetal fibronectin in vaginal secretions and preterm labor index based on clinical variables are optimal predictive markers for preterm delivery in patients with intact membranes. J Obstet Gynaecol Res 2007;33:38–44
- Kendal-Wright CE, Hubbard D, Gowin-Brown J, Bryant-Greenwood GD. Stretch and inflammation-induced Pre-B cell colony-enhancing factor (PBEF/Visfatin) and Interleukin-8 in amniotic epithelial cells. Placenta 2010;31:665–74
- Yoneda S, Shiozaki A, Yoneda N, et al. Prediction of exact delivery time in patients with preterm labor and intact membranes at admission by amniotic fluid interleukin-8 level and preterm labor index. J Obstet Gynaecol Res 2011;37:861–6
- Jacobsson B, Holst RM, Wennerholm UB, et al. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol 2003;189:1161–7
- Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73
- Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 2005;26:661–71
- Diamond AK, Sweet LM, Oppenheimer KH, et al. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod Sci 2007;14:548–59
- Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 2008;181:1470–9
- Dudley DJ, Spencer S, Edwin S, Mitchell MD. Regulation of human decidual cell macrophage inflammatory protein-1 alpha (MIP-1 alpha) production by inflammatory cytokines. Am J Reprod Immunol 1995;34:231–5
- Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol 1996;87:94–8
- Cohen J, Ghezzi F, Romero R, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol 1996;35:23–9
- Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol 1998;178:428–32
- Krolak-Olejnik B, Beck B, Olejnik I. Umbilical serum concentrations of chemokines (RANTES and MGSA/GRO-alpha) in preterm and term neonates. Pediatr Int 2006;48:586–90
- Romero R, Salafia CM, Athanassiadis AP, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992;166:1382–8
- Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–8
- Steel JH, O'Donoghue K, Kennea NL, et al. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005;26:672–7
- Aquino TI, Zhang J, Kraus FT, et al. Subchorionic fibrin cultures for bacteriologic study of the placenta. Am J Clin Pathol 1984;81:482–6
- Pankuch GA, Appelbaum PC, Lorenz RP, et al. Placental microbiology and histology and the pathogenesis of chorioamnionitis. Obstet Gynecol 1984;64:802–6
- Chellam VG, Rushton DI. Chorioamnionitis and funiculitis in the placentas of 200 births weighing less than 2.5 kg. Br J Obstet Gynaecol 1985;92:808–14
- Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–8
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83
- Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–57
- Romero R, Soto E, Berry SM, et al. Blood pH and gases in fetuses in preterm labor with and without systemic inflammatory response syndrome. J Matern Fetal Neonatal Med 2012;25:1160–70
- Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med 2011;39:653–66
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–9
- Baltimore RS. Neonatal sepsis: epidemiology and management. Paediatr Drugs 2003;5:723–40
- Wolfs TG, Jellema RK, Turrisi G, et al. Inflammation-induced immune suppression of the fetus: a potential link between chorioamnionitis and postnatal early onset sepsis. J Matern Fetal Neonatal Med 2012;25:8–11
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Kuperman AA, Kenet G, Papadakis E, Brenner B. Intraventricular hemorrhage in preterm infants: coagulation perspectives. Semin Thromb Hemost 2011;37:730–6
- Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–40
- Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177:797–802
- Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 1997;177:406–11
- Martinez E, Figueroa R, Garry D, et al. Elevated amniotic fluid interleukin-6 as a predictor of neonatal periventricular leukomalacia and intraventricular hemorrhage. J Matern Fetal Investig 1998;8:101–7
- Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81
- Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–6
- Willoughby RE, Jr Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol 2002;29:603–21
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110:124–7
- Nelson KB, Grether JK, Dambrosia JM, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 2003;53:600–7
- Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med 2006;34:5–12
- Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol 2008;198:466–e1--11
- Nelson KB. Infection in pregnancy and cerebral palsy. Dev Med Child Neurol 2009;51:253–4
- Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res 2010;67:95–101
- Allin MP, Kontis D, Walshe M, et al. White matter and cognition in adults who were born preterm. PLoS One 2011;6:e24525
- Kalpakidou AK, Allin MP, Walshe M, et al. Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: an fMRI study. PLoS One 2012;7:e34858
- Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10
- Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–9
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9
- Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002;140:171–6
- Mittendorf R, Covert R, Montag AG, et al. Special relationships between fetal inflammatory response syndrome and bronchopulmonary dysplasia in neonates. J Perinat Med 2005;33:428–34
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–55
- Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol 2007;21:479–89
- Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med 2008;358:743–5; author reply 745–746
- Lee J, Oh KJ, Yang HJ, et al. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23
- Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10:133–40
- Harrell SN, Brandon DH. Retinopathy of prematurity: the disease process, classifications, screening, treatment, and outcomes. Neonatal Netw 2007;26:371–8
- Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010;67:394–400
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Medzhitov R, Janeway C Jr. The Toll receptor family and microbial recognition. Trends Microbiol 2000;8:452–6
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol 2003;74:479–85
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004;6:1382–7
- Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol 2005;25:503–10
- Castellheim A, Brekke OL, Espevik T, et al. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol 2009;69:479–91
- Beutler B. Innate immune sensing of microbial infection: the mechanism and the therapeutic challenge. Wien Med Wochenschr 2002;152:547–51
- Beutler B. Science review: key inflammatory and stress pathways in critical illness - the central role of the Toll-like receptors. Crit Care 2003;7:39–46
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001;2:675–80
- Cario E, Brown D, McKee M, et al. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 2002;160:165–73
- Baker BS, Ovigne JM, Powles AV, et al. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 2003;148:670–9
- Hertz CJ, Wu Q, Porter EM, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 2003;171:6820–6
- Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55
- Elovitz MA, Wang Z, Chien EK, et al. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 2003;163:2103–11
- Dale M, Nicklin MJ. Interleukin-1 receptor cluster: gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human chromosome 2q. Genomics 1999;57:177–9
- Tominaga S, Inazawa J, Tsuji S. Assignment of the human ST2 gene to chromosome 2 at q11.2. Hum Genet 1996;97:561–3
- Bergers G, Reikerstorfer A, Braselmann S, et al. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J 1994;13:1176–88
- Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med 1998;187:787–94
- Lohning M, Stroehmann A, Coyle AJ, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A 1998;95:6930–5
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 2008;7:827–40
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–90
- Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med 1999;190:895–902
- Townsend MJ, Fallon PG, Matthews DJ, et al. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 2000;191:1069–76
- Mildner M, Storka A, Lichtenauer M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res 2010;87:769–77
- Liu J, Buckley JM, Redmond HP, Wang JH. ST2 negatively regulates TLR2 signaling, but is not required for bacterial lipoprotein-induced tolerance. J Immunol 2010;184:5802–8
- Buckley JM, Liu JH, Li CH, et al. Increased susceptibility of ST2-deficient mice to polymicrobial sepsis is associated with an impaired bactericidal function. J Immunol 2011;187:4293–9
- Oshikawa K, Kuroiwa K, Tago K, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med 2001;164:277–81
- Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy 2002;32:1520–6
- Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun 2002;299:18–24
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 2007;282:26369–80
- Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med 2009;179:772–81
- Kurokawa M, Matsukura S, Kawaguchi M, et al. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Int Arch Allergy Immunol 2011;155:12–20
- Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest 2003;124:1206–14
- Tajima S, Bando M, Ohno S, et al. ST2 gene induced by type 2 helper T cell (Th2) and proinflammatory cytokine stimuli may modulate lung injury and fibrosis. Exp Lung Res 2007;33:81–97
- Hacker S, Lambers C, Pollreisz A, Hoetzenecker K, et al. Increased soluble serum markers caspase-cleaved cytokeratin-18, histones, and ST2 indicate apoptotic turnover and chronic immune response in COPD. J Clin Lab Anal 2009;23:372–9
- Weinberg EO, Shimpo M, Hurwitz S, et al. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003;107:721–6
- Sabatine MS, Morrow DA, Higgins LJ, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation 2008;117:1936–44
- Granne I, Southcombe JH, Snider JV, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS One 2011;6:e24463
- Stampalija T, Chaiworapongsa T, Chaemsaithong P, et al. Maternal Plasma Concentrations of sST2 and Angiogenic/Anti-angiogenic Factors in Preeclampsia. J Matern Fetal Neonatal Med 2013; May 20 [e-pub ahead of print]
- Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med 2004;30:1468–73
- Hoogerwerf JJ, Tanck MW, van Zoelen MA, et al. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med 2010;36:630–7
- Stampalija T Romero R, Korzeniewski S, et al. Soluble ST2 in the fetal inflammatory response syndrome: in-vivo evidence of activation of the anti-inflammatory limb of immune response. J Matern Fetal Neonatal Med 2013: In press
- Guinn DA, Goldenberg RL, Hauth JC, et al. Risk factors for the development of preterm premature rupture of the membranes after arrest of preterm labor. Am J Obstet Gynecol 1995;173:1310–15
- Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med 1993;38:543–8
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6
- Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74
- Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–19
- Iwahana H, Yanagisawa K, Ito-Kosaka A, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem 1999;264:397–406
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50
- Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol 2001;166:6633–9
- Leung BP, Xu D, Culshaw S, et al. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol 2004;173:145–50
- Mitchell MD, Simpson KL, Keelan JA. Paradoxical proinflammatory actions of interleukin-10 in human amnion: potential roles in term and preterm labour. J Clin Endocrinol Metab 2004;89:4149–52
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012;335:936–41
- Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36
- Seong HS, Lee SE, Kang JH, et al. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol 2008;199:375 e371–5
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol 2007;30:706–14
- Lee SE, Romero R, Kim CJ, et al. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med 2006;19:693–7
- Yoon BH, Romero R, Moon J, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med 2003;13:32–8
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113:17–42
- Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010;23:1000–11
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–7
- Topping V, Romero R, Than NG, et al. Interleukin-33 in the Human Placenta. J Matern Fetal Neonatal Med 2013;26:327–38