Abstract
Objective: To identify differentially expressed long non-coding RNA (lncRNA) genes in human myometrium in women with spontaneous labor at term.
Materials and methods: Myometrium was obtained from women undergoing cesarean deliveries who were not in labor (n = 19) and women in spontaneous labor at term (n = 20). RNA was extracted and profiled using an Illumina® microarray platform. We have used computational approaches to bound the extent of long non-coding RNA representation on this platform, and to identify co-differentially expressed and correlated pairs of long non-coding RNA genes and protein-coding genes sharing the same genomic loci.
Results: We identified co-differential expression and correlation at two genomic loci that contain coding-lncRNA gene pairs: SOCS2--AK054607 and LMCD1--NR_024065 in women in spontaneous labor at term. This co-differential expression and correlation was validated by qRT-PCR, an experimental method completely independent of the microarray analysis. Intriguingly, one of the two lncRNA genes differentially expressed in term labor had a key genomic structure element, a splice site, that lacked evolutionary conservation beyond primates.
Conclusions: We provide, for the first time, evidence for coordinated differential expression and correlation of cis-encoded antisense lncRNAs and protein-coding genes with known as well as novel roles in pregnancy in the myometrium of women in spontaneous labor at term.
Introduction
While the process of labor is vital to the survival of viviparous species, the understanding of its physiology and pathology remains incomplete. Dysfunctional labor (arrest of dilatation and/or descent) is often an indication for cesarean delivery [Citation1–7], which is being performed in approximately one of every three pregnant women in the United States [Citation8,Citation9]. Elucidation of the mechanism responsible for the spontaneous onset of labor is considered essential for developing strategies to prevent and treat labor disorders at term, and also for the prevention of spontaneous preterm birth, the leading cause of perinatal morbidity and mortality worldwide [Citation10–15].
High-throughput post-genomic biology has been used to gain insight into the mechanisms of disease, and develop diagnostic and prognostic tests for a wide range of physiologic and pathologic states [Citation16–40]. Genomics, transcriptomics and proteomics have been applied to understand pregnancy complications [Citation41–52] as well as normal and abnormal parturition [Citation53–70]. To harness the promise of post-genomic biology and the knowledge gained, we have undertaken an unbiased whole transcriptome approach to the expression of known and novel gene classes in the myometrium. After the completion of the Human Genome Project, and in the course of the past decade, long non-coding RNA (lncRNA) genes have been revealed to comprise the most frequent, prevalent, and abundantly expressed novel class of human genes [Citation71–85].
This study has focused on the myometrium because uterine contractility is a key feature of spontaneous labor at term. We have already established a beachhead in this field by studying the protein encoding genes and the regulatory networks therein within the framework of the myometrium at term [Citation64]. Moreover, we have characterized these changes in the most important and common labor disorders, arrest of descent [Citation67] and arrest of dilatation [Citation70].
Materials and methods
Study design
A cross-sectional study was designed to compare the expression of lncRNAs in the myometrium of women not in labor at term versus those in labor at term. We previously reported the original analysis of the protein-coding genes using microarrays [Citation64].
Briefly, myometrium was obtained from women undergoing cesarean sections at term (≥37 weeks) in the following groups: (1) women not in labor (n = 20); and (2) those in spontaneous labor (n = 19). Labor was diagnosed in the presence of spontaneous regular uterine contractions occurring at a minimum frequency of 2 every 10 min with cervical change that required hospital admission. Women in the term not in labor group underwent a cesarean section due to the fetus in non-cephalic presentation, previous uterine surgery, previous classical cesarean section, non-reassuring fetal status, or an elective cesarean section with no more than one previous cesarean section. Women in the spontaneous term labor group underwent cesarean delivery because of a non-reassuring fetal heart rate tracing (as determined by the physician) or fetal malpresentation. Only women who delivered an appropriate for gestational age (AGA) neonate were included. Patients with clinical or histologic chorioamnionitis, those undergoing induction of labor, and those who underwent cesarean section for arrest of dilatation or arrest of descent were excluded. Histologic chorioamnionitis was diagnosed using previously described criteria [Citation86,Citation87]. Clinical chorioamnionitis was diagnosed using criteria proposed by Gibbs et al. [Citation88]. An AGA neonate was defined as a birthweight between the 10th and 90th percentile for the gestational age at birth [Citation89].
Eligible patients were enrolled at Hutzel Women’s Hospital (Detroit, MI). All women provided written informed consent prior to the collection of myometrial samples. The collection and utilization of the samples for research purposes was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS, Bethesda, MD), and the Human Investigation Committee of Wayne State University (Detroit, MI).
Sample collection
Myometrial tissue samples were collected during cesarean section following delivery of the placenta. Biopsies were obtained from the midpoint of the superior aspect of the uterine incision using Metzenbaum scissors. Specimens measured approximately 1.0 cm3. Tissues were snap-frozen in liquid nitrogen and kept at −80 °C until use.
RNA isolation and microarray analysis of RNA expression
The methods for RNA isolation and microarray data generation have been previously reported [Citation64]. Briefly, we used the Illumina® HumanHT-12 v3 expression microarray platform (Illumina®, San Diego, CA) to assess the expression levels in each individual specimen following the manufacturer’s instructions.
Data analysis for lncRNA
The goal of the analysis was to use a commercially-available microarray platform to interrogate the expression of putative lncRNA genes that we had determined to be represented by the commercial platform (Illumina®). All microarray probes were mapped to the collection of lncRNAs that we describe in the next paragraph. Pairs of lncRNAs and neighbor or overlapping protein coding genes were then identified such that a coding gene and a lncRNA gene were co-differentially expressed in the same locus (definition: the distance between the nearest pair of lncRNA gene boundaries and protein-coding gene boundaries had to be 10 000 bases or less) and correlated based on microarray expression.
Description of the lncRNA dataset
To construct a non-redundant lncRNA gene set (a single reference transcript per gene), we considered at least one base pair overlap in the entire genomic span (including exons and introns along the hg19 human genome reference assembly coordinates) among all transcripts located on the same strand in the same locus. We assembled 18 498 experimentally supported [with full-length cDNA or manually-curated high-quality expressed sequence tag (EST) evidence], non-redundant (with respect to genomic position and orientation) lncRNA genes from multiple public sources: Gencode v3 [Citation90] and v7 lncRNAs [Citation78]; the human lncRNA catalog from our previous work [Citation73]; NCBI Refseq non-coding transcripts; all human ESTs submitted to Genbank by RIKEN, Japan; manually annotated lncRNAs from human sense–antisense pairs [Citation91,Citation92]; and Broad Institute lncRNAs [Citation75]. We used genomic positional overlap of UCSC all_mrna, all_est, and ref_all files from http://genome.ucsc.edu [Citation93] as well as Gencode transcripts from www.gencodegenes.org [Citation90,Citation94] to define transcriptional unit boundaries according to the FANTOM3 definition of a transcriptional unit [Citation95] along the hg19 assembly, in the UCSC Genome Brower (http://genome.ucsc.edu) [Citation96], collapsing the cDNA/EST/Gencode/Broad transcript-to-genome alignments into genomic positionally non-redundant transcriptional units with one randomly selected reference transcript per transcriptional unit.
Mapping microarray probes to lncRNAs
The data measured by microarray probes that were not assigned by the manufacturer to genes having a valid Genbank identifier had been discarded in the original analysis. Here, we considered all probes on the Human HT-12 V3 array. The 50 base pair sequence of the probes was used to match these against the human genome (hg19) using BLAT software (University of California, Santa Cruz, CA) [Citation97]. The probes that had a perfect match (100%) were retained. The microarray probe alignments were then matched against the genomic coordinates of all human cDNAs from the “Genbank mRNA” track of the UCSC Human Genome Database [Citation96], and hence, having a match between the Illumina® probes and the cDNAs from the UCSC database. The list of cDNAs from the UCSC database was intersected by complete name string matching with the list of cDNAs corresponding to lncRNAs described above.
Assessing differential expression of coding and non-coding RNAs
We used an Illumina® BeadArray Reader to image the microarrays, and the BeadStudio Software V.3.4.0 (Illumina®, San Diego, CA) to extract raw expression data from the array images. The raw probe intensity data were log (base 2) transformed, then quantile normalized [Citation98] to make expression levels comparable among arrays. Probes with intensity above the background (p value <0.1) in more than 5 of the 39 samples were retained for further analysis. A moderated t-test was used at probe level to test for differential expression between groups, and the resulting p values were corrected using the false discovery rate (FDR) method [Citation99] to obtain q values. Probes with a q value <0.1 and fold change >1.25 were defined as differentially expressed.
Determining the significance of pairwise correlations between coding and non-coding genes
The correlation between lncRNA expression level on a log2 scale (denoted with Y) and the pair coding gene (denoted with X) was tested by fitting a linear model in which the response was Y, while the independent variables were: X, the group variable, and their interaction term (Y = a + b · X + c · Group + d · X · Group + ε). Then, a second model was fit including only the group variable (Y = a + c · Group + ε). By comparing the quality of the data fit between these two models using an F-test, we assessed whether the expression of the lncRNA (Y) was significantly correlated with the expression level of the coding gene (X) within a same-locus gene pair while allowing that the slopes (Y versus X) be different between the two groups (not in labor versus in labor). The correlation p values obtained in this way were further adjusted for multiple tests, and significance was inferred using q value <0.1. We defined “adjacent to or overlapping” as a lncRNA having a genomic distance of 10 000 base pairs or less from the nearest boundary of a protein-coding gene; antisense overlaps were considered as zero distance [Citation100]. After identifying differentially-expressed lncRNAs significantly correlated with protein-coding genes adjacent to or overlapping the lncRNA genes on opposite strands, validation was undertaken using quantitative real-time reverse transcription PCR (qRT-PCR) (see below).
Validation of microarray results using qRT-PCR experiments
A subset of samples from each group (spontaneous term labor: n = 16; term not in labor: n = 18) was obtained for qRT-PCR assay of a selected group of genes found to be differentially expressed by microarray analysis. Taqman® (Life Technologies, Inc., Foster City, CA) qRT-PCR analysis was performed as described elsewhere [Citation100]. Taqman primers/probesets were: Hs00205871_m1 (LMCD1), Hs00705981_s1 (NR_024065), Hs04274293_g1 (SOCS2), Hs00919620_m1(AK054607). The Ct values were averaged over the three replicates per primer and sample, and ΔCt =Cttarget − Ctreference were computed for each sample. −ΔCt values, surrogate for mRNA abundance in a given sample were compared between groups using a t-test. The correlation between the lncRNA and the coding gene within each gene pair was tested using the same model as described for the microarray data.
Results
The clinical and demographic characteristics of the population included in this study are displayed in .
Table 1. Demographic and clinical characteristics of the study groups.
We matched 2995 Illumina® probes to 2634 lncRNAs from our lncRNA dataset of 18 498 lncRNAs, which are described above. Of the 2995 probes, 1868 (62%) were expressed above background in the myometrium samples and assigned to 1692 unique lncRNAs. Of the 1692 lncRNAs, 13 were differentially expressed between the myometrium of women not in labor and those in labor (fold change > 1.25, q value < 0.1) (see Table S1). Of these 13 lncRNAs, seven were computationally identified as residing in putative sense–antisense and neighbor-gene pairs, where they were co-differentially expressed (fold change > 1.25, q value < 0.1) and correlated (q value < 0.1) with the corresponding coding genes (see Table S2). Those seven loci collapsed into only four that were genomically unique, due to probe redundancy. Our UCSC Genome Browser-based manual annotation of these four loci identified two bona fide lncRNA pairs, which we then attempted to validate by qRT-PCR. The microarray gene expression data for the two manually curated bona fide lncRNA-coding gene pairs (AK054607–SOCS2) and (NR_024065–LMCD1) are displayed in . For the AK054607–SOCS2 pair, we found that both the lncRNA and the protein-coding gene were significantly down-regulated in term labor versus non-labor myometrium (see ). For the NR_024065–LMCD1 pair, we demonstrated that both the lncRNA and the protein-coding gene were significantly up-regulated in the myometrium of women in term labor compared to that of women not in labor at term (see ).
Figure 1. Correlation and co-differential expression of mRNA–lncRNA sense–antisense pairs based on microarray data. (A) Log (base 2) microarray expression of SOCS2 gene (probed by Illumina® probe ILMN_2131861) are plotted against the expression of lncRNA AK054607 (probed by Illumina® probe ILMN_1699188). (B) Log (base 2) microarray expression of LMCD1 gene (probed by Illumina® probe ILMN_1754969) are plotted against the expression of lncRNA NR_024065 (probed by Illumina® probe ILMN_2052135). In both panels, samples from the term not in labor group are shown as filled circles while samples from the term in labor group are represented by empty triangles. A linear fit through the expression data is also shown (term not in labor: solid line; term in labor: interrupted line).
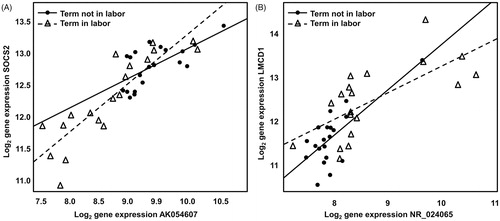
Table 2. Co-differential expression and correlation of lncRNA and coding gene pair based on microarray and qRT-PCR data.
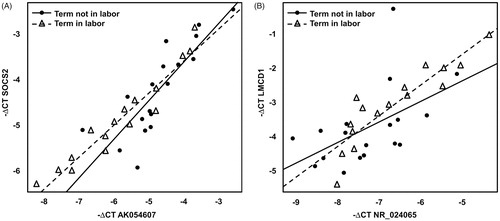
These pairs were tested by qRT-PCR in a subset of 34 of the 39 (see ) samples used in the microarray analysis and their co-differential expression and correlation were confirmed (see and ).
Figure 2. Correlation and co-differential expression of mRNA–lncRNA sense–antisense pairs based on qRT-PCR data. (A) The −ΔCt values, surrogate for log (base 2) expression of SOCS2 gene (probed by Taqman primer Hs0091962_m1) are plotted against the −ΔCt values of lncRNA AK054607 (probed by Taqman primer Hs04274293_g1). (B) −ΔCt values of LMCD1 gene (probed by Taqman primer Hs00205871) are plotted against the −ΔCt values of lncRNA NR_024065 (probed by Taqman primer Hs00705981_s1). In both panels, samples from the term not in labor group are shown as filled circles while samples from the term in labor group are represented by empty triangles. A linear fit through the expression data is also shown (term not in labor: solid line; term in labor: interrupted line).
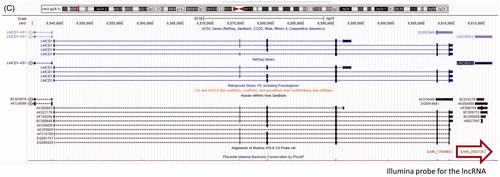
AK054607–SOCS2 is an mRNA–lncRNA sense–antisense pair such that the two genes are divergently transcribed and the transcription start site of each gene is embedded in the first interim of the other gene, leading to an intronic divergent sense–antisense pair. This locus is characterized by substantial transcriptional complexity: both the sense protein-coding gene SOCS2 and the lncRNA gene AK054607 have multiple alternatively spliced isoforms evident in public cDNA data (see ). The Illumina® probe corresponding to the lncRNA is in the constitutively expressed last exon of the lncRNA gene; therefore, it is genomically distant from the region of sense–antisense overlap and clearly allows specific profiling of this lncRNA gene. LMCD1–NR_024065 is a same-strand, neighbor gene mRNA-lncRNA sense–antisense pair. The single-exon lncRNA is genomically located downstream of the 3′ end of the mRNA. Multiple independent sources of experimental evidence (cDNA and EST datasets in the UCSC Genome Browser as well as Broad Institute lncRNAs) indicate that the lncRNA is distinct from its upstream protein-coding neighbor, since there are no overlapping or bridging transcripts between the two.
Figure 3. Genomic structure and evolutionary conservation of mRNA–lncRNA sense–antisense pairs. (A) Sense–antisense transcriptional units and cognate Illumina® probe positions at the SOCS2--AK054607 locus. Arrows indicate: the differentially-expressed lncRNA probe (bottom), and genomic boundaries of the antisense and sense transcriptional units (middle and top, respectively). (B) Multi-species sequence alignment of a splice acceptor site in an intron of AK054607. The splice site is on the antisense strand, corresponding to sense strand nucleotides AG. Obtained from the UCSC Genome Browser. (C) The LMCD1 transcriptional unit and its downstream sense-strand neighbor, lncRNA NR_024065 (synonym: LINC00312). Arrow indicates the differentially-expressed Illumina® probe corresponding to this lncRNA.
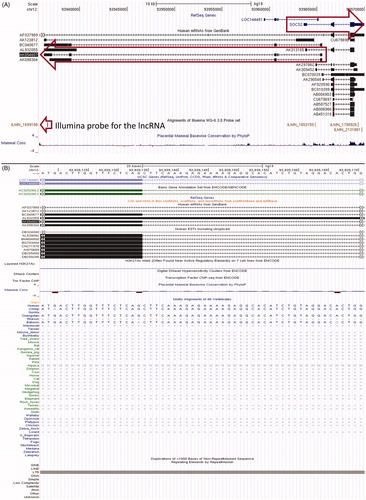
Conservation assessment of validated antisense lncRNA transcription in spontaneous labor at term
Because lncRNAs do not have protein-coding sequences, evolutionary conversation of their gene structure can be analyzed using key features of the gene structure itself: promoters, splice sites and polyadenylation signals, as reviewed by Lipovich et al. [Citation74] and Johnsson et al. [Citation101]. Therefore, we compared the genomic sequence of this lncRNA’s splice sites between humans and the 45 non-human vertebrates in the UCSC genome browser public multispecies sequence alignment [Citation96]. We found that a canonical splice acceptor (-AG) of an AK054607 intron resides within a non-conserved, primate-specific retroviral insertion in the human genome (). The insertion was a long terminal repeat inside an ERV1 element, which the multispecies alignment suggests originated in Old World monkeys after the prosimian split. On the contrary, NR_024065 is an unspliced gene that has its canonical hexamer polyadenylation signal conserved all the way through marsupials and monotremes, attesting to an origin of the lncRNA before the emergence of placental mammals.
Discussion
Principal findings of the study
Here, for the first time, we have intersected a meta-collection of experimentally-supported lncRNA genes with a clinical microarray dataset from myometrium of women not in labor and those in labor. We have demonstrated co-differential expression at two genomic loci that contain coding–noncoding gene pairs. At one of these loci, the sense gene (SOCS2) has been implicated in reproductive disorders (recurrent spontaneous pregnancy loss [Citation102] and fetal growth restriction [Citation103]); whereas in the other locus, the protein-coding gene (LMCD1), although well-characterized as a heart muscle regulator [Citation104,Citation105], has no known association with human reproduction. Both genes were previously not understood to be relevant to or regulated by the lncRNA genes that reside at the same loci, and that previously lacked annotation. The key finding of this study is that two lncRNA genes, which are genomically antisense to those two known protein-coding genes, are co-differentially expressed with the protein-coding genes in human spontaneous labor at term.
What are long non-coding RNAs?
The final draft of the human genome was released in 2003 [Citation106]. In subsequent years, experimental analyses of the mammalian transcriptome using high-throughput cDNA construction and next-generation sequencing by the ENCODE [Citation107] and FANTOM Consortia [Citation95] have led to a key unexpected finding of post-genomic biology: that approximately 70% of the human genome is transcribed [Citation108] but only 2% corresponds to messenger RNA of protein-coding genes [Citation82]. The term “junk DNA” had been coined to refer to the major part of the genome, the part that resides outside of the protein-coding genes [Citation109,Citation110]. This derogatory term has now been repudiated by substantial experimental evidence from multiple high-throughput projects (transcriptome sequencing), indicating that the majority of this fraction of the genome is transcribed into diverse and functional classes of non-coding RNA [Citation76,Citation111–115]. Although these classes include previously well-characterized classical housekeeping RNAs such as transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), microRNAs (miRNAs) and piRNAs, which are constitutively expressed and play critical roles in protein biosynthesis [Citation83], the majority of this transcriptional output consists of a new class of RNA: lncRNA. In fact, the study of lncRNAs is not new; rather, it is the ubiquity of lncRNA expression that has only recently been grasped [Citation78]. The estimated number of lncRNA genes in the human genome is more than 20 000 and has now surpassed the number of protein-coding genes [Citation94]. Although only a fraction of lncRNAs has been mechanistically and functionally characterized in human cells and tissues, it is already evident that lncRNAs have important, often essential roles in normal cellular function (including but not limited to: proliferation, differentiation, pluripotency [Citation116], apoptosis and energy metabolism) [Citation71,Citation72,Citation74,Citation77,Citation83,Citation85,Citation112,Citation117–123], human development, and disease (i.e. epilepsy [Citation100], cancer [Citation82,Citation101,Citation124], Alzheimer’s disease [Citation125], diabetes and obesity [Citation126–128]).
Why study lncRNAs in parturition?
Despite the central importance of lncRNA in the cellular economy [Citation78], there is a paucity of information about lncRNA function in normal labor at term. We undertook this study to examine the extent to which known and novel lncRNA may or may not be differentially expressed in this essential mammalian biological process. This is important, not only to gain an understanding of physiologic parturition, but also to leverage upon our new post-genomic understanding of lncRNAs as an unprecedented window into preterm labor and delivery.
Strong evidence that two lncRNA genes with putative cis-regulatory functions are differentially expressed in the myometrium of women in spontaneous labor at term
In this study, we have analyzed approximately 18 498 human lncRNA genes using a computational approach that intersects their genomic coordinates with Illumina® microarray data. Of these, 2634 were represented by probes on the commercial catalog Illumina® platform that we used. Upon computational enrichment for putative cis-regulatory lncRNAs, meaning those at or near protein-coding genes with potential to regulate those genes, we converged upon two bona fide mRNA–lncRNA gene pairs, one sense–antisense, and one neighbor–gene.
One of these pairs is comprised of an antisense lncRNA, AK054607, which overlaps the protein-coding gene SOCS2. There is compelling evidence that physiologic parturition is associated with cellular and molecular signatures of inflammation in the chorioamniotic membranes [Citation65,Citation129–134], myometrium [Citation53,Citation54,Citation56,Citation57,Citation59,Citation62,Citation64,Citation69,Citation131,Citation134–140] and cervix [Citation58,Citation61,Citation63,Citation131,Citation134,Citation141–145]. Indeed, the participation of chemokines and pro-inflammatory cytokines [Citation146–164], eicosanoids [Citation137,Citation165–175] and lipooxygenase arachidonate products [Citation176–178] in physiologic labor is well established. Blumenstein et al. reported the behavior of the suppression of cytokine signaling of molecules in spontaneous labor at term and found differential expression for SOCS1 and SOCS3 [Citation179]. SOCS2 protein levels increased following labor [Citation179]. Here, we found that both SOCS2 and its antisense lncRNA encoded in the same locus were down-regulated in the myometrium of women experiencing spontaneous labor at term versus those not in labor. Our previous analyses of human sense–antisense pairs have found that in most pairs, the two transcript expression levels changed in the same direction in a given biological process (synergistic pairs), while in a minority of pairs, the sense and antisense levels changed in the opposite direction (reciprocal pairs) [Citation180]. Here, both of the pairs that we found were, in fact, synergistic, concordant with our earlier findings of Katayama et al. [Citation180]. This represents the first evidence that a lncRNA in the same locus as SOCS2 is a putative novel regulator of SOCS2, because there is a vast amount of literature showing that antisense lncRNAs are not merely co-expressed with their sense partners, but directly regulate them, as recently reviewed [Citation74,Citation83,Citation92,Citation100,Citation181].
The other lncRNA–mRNA pair identified and validated in our study is LMCD1–NR_024065. LMCD1 is well-known for its regulatory function in the heart [Citation105] and in smooth muscle [Citation104], although it has never been previously connected to myometrium or the process of labor. For the first time herein, we show a direct and significant connection of LMCD1 expression with spontaneous parturition at term. Moreover, we report that NR_024065, a neighboring lncRNA in the LMCD1 locus, is significantly and synergistically up-regulated in concert with LMCD1 in term labor, strongly suggesting a regulatory function for this lncRNA. Unlike the SOCS2 locus where the protein-coding gene and lncRNA share a substantial sense–antisense overlap, LMCD1 and NR_024065 are neighbor genes, which share close genomic positional proximity, although their boundaries do not overlap. Emerging mechanisms of lncRNA function include epigenetic cis-regulation and co-regulation of protein-coding genes by genomic-neighbor lncRNAs [Citation182,Citation183]. In this study, we demonstrated that a lncRNA–mRNA sense-strand neighbor-gene pair is functionally relevant in spontaneous parturition at term.
A window into the evolutionary complexity of the lncRNA transcriptome in human parturition
Unlike protein-coding genes, which are highly conserved in evolution, a key and bewildering property of the lncRNA transcriptome (lncRNAome) is its lack of evolutionary conservation: one-third of human lncRNA genes are not conserved beyond primates [Citation78], and both sequences and structures of other lncRNA are poorly conserved across metazoa [Citation101]. Accordingly, we examined the extent of evolutionary conservation in the two lncRNA that we validated in this study, and found that the genomic structure of the AK054607 lncRNA gene relies upon a primate-specific splice site, consistent with the reported human and primate exclusivity of thousands of lncRNAs. This has important implications for rodent models of labor, highlighting that key cis-regulatory lncRNAs that impact protein-coding functional genes may be altered or absent in those models.
The future of lncRNAs in reproductive biology
Little is known about the role of lncRNAs in pregnancy. This year, an analysis of several pedigrees in a Dutch study converged on an autosomal recessive gene (HELLP), a lncRNA which is functional in trophoblasts and causes HELLP syndrome [Citation184]. This lncRNA is expressed in extravillous trophoblast obtained from first trimester placentas, and its knockdown activated a large set of genes involved in the cell cycle. By blocking mutation sites, transcription of the lncRNA itself was up-regulated and led to a reduction in trophoblast cell invasion [Citation185]. While the disease causing mutation is detected in the fetus, the disease phenotype is diagnosed in the mother.
In contrast to the limited power of Mendelian pedigree approaches, our work has established a precedent for transcriptomic analysis of groupwise and case-control study designs to discover additional lncRNAs functional in labor. Future approaches in this field should assess lncRNA transcriptomes in complications of pregnancy, as well as utilize the broad power and scope of published genome-wide association studies of reproductive diseases to emerge with causative SNP variants in additional lncRNAs.
Conclusions
We have harnessed and leveraged upon the power of postgenomic transcriptome biology to set forth a precedent of clinically relevant discoveries of lncRNAs functionally related to parturition. We have canvassed computational and experimental data integration, as well as multiple independent validation approaches, to establish a new methodology that is capable of transforming our understanding of human reproduction through lncRNAomics.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C. L.L. is a member of the ENCODE Consortium; however, no funds or material resources from ENCODE were used in this study.
Supplementary Material
Download PDF (256.5 KB)References
- Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010;203:326.e1–10
- Boyle A, Reddy UM, Landy HJ, et al. Primary cesarean delivery in the United States. Obstet Gynecol 2013;122:33–40
- Brennan DJ, Robson MS, Murphy M, O'Herlihy C. Comparative analysis of international cesarean delivery rates using 10-group classification identifies significant variation in spontaneous labor. Am J Obstet Gynecol 2009;201:308.e301–8
- Gifford DS, Morton SC, Fiske M, et al. Lack of progress in labor as a reason for cesarean. Obstet Gynecol 2000;95:589–95
- Getahun D, Strickland D, Lawrence JM, et al. Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. Am J Obstet Gynecol 2009;201:422.e421–7
- Barber EL, Lundsberg LS, Belanger K, et al. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol 2011;118:29–38
- Branch DW, Silver RM. Managing the primary cesarean delivery rate. Clin Obstet Gynecol 2012;55:946–60
- Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. National Vital Stat Reports 2013;62:1–33
- Simon AE, Uddin SG. National trends in primary cesarean delivery, labor attempts, and labor success, 1990--2010. Am J Obstet Gynecol 2013;209:554.e1--8
- World Health Organization. Born too soon: the global action report in preterm birth. WHO Library Cataloguing-in-Publication: World Health Organization; 2012
- Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–8
- Goldenberg RL, McClure EM. The epidemiology of preterm birth. In: Berguella V, ed. Preterm birth: prevention & management. Oxford: Willey-Blackwell; 2010:22–38
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72
- Hamilton BE, Hoyert DL, Martin JA, et al. Annual summary of vital statistics: 2010–2011. Pediatrics 2013;131:548–58
- PeriStats [online database]. White Plains (NY): March of Dimes; 2006. Available from: http://www.marchofdimes.com/peristats/ [last accessed Dec 2013]
- Wahlestedt C. Natural antisense and noncoding RNA transcripts as potential drug targets. Drug Discov Today 2006;11:503–8
- Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov 2013;12:433–46
- Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–7
- Hedenfalk I, Duggan D, Chen Y, et al. Gene-expression profiles in hereditary breast cancer. N Engl J Med 2001;344:539–48
- Tubbs RR, Hicks DG, Cook J, et al. Fluorescence in situ hybridization (FISH) as primary methodology for the assessment of HER2 Status in adenocarcinoma of the breast: a single institution experience. Diagn Mol Pathol 2007;16:207–10
- Inaki K, Hillmer AM, Ukil L, et al. Transcriptional consequences of genomic structural aberrations in breast cancer. Genome Res 2011;21:676–87
- Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 2010;11:685–96
- Hondow HL, Fox SB, Mitchell G, et al. A high-throughput protocol for mutation scanning of the BRCA1 and BRCA2 genes. BMC Cancer 2011;11:265
- Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature 2011;472:90–4
- Previati M, Manfrini M, Galasso M, et al. Next generation analysis of breast cancer genomes for precision medicine. Cancer Lett 2013;339:1–7
- Mudvari P, Ohshiro K, Nair V, et al. Genomic insights into triple-negative and HER2-positive breast cancers using isogenic model systems. PLoS One 2013;8:e74993
- Donahue HJ, Genetos DC. Genomic approaches in breast cancer research. Brief Funct Genomics 2013;12:391–6
- Kaur H, Mao S, Shah S, et al. Next-generation sequencing: a powerful tool for the discovery of molecular markers in breast ductal carcinoma in situ. Expert Rev Mol Diagn 2013;13:151–65
- Kosir MA, Jia H, Ju D, Lipovich L. Challenging paradigms: long non-coding RNAs in breast ductal carcinoma in situ (DCIS). Front Genet 2013;4:50
- Auffray C, Chen Z, Hood L. Systems medicine: the future of medical genomics and healthcare. Genome Med 2009;1:2
- Stewart JJ, White JT, Yan X, et al. Proteins associated with Cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Mol Cell Proteomics 2006;5:433–43
- Lin B, White JT, Lu W, et al. Evidence for the presence of disease-perturbed networks in prostate cancer cells by genomic and proteomic analyses: a systems approach to disease. Cancer Res 2005;65:3081–91
- Pascal LE, Vencio RZ, Page LS, et al. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer 2009;9:452
- Li R, Guo Y, Han BM, et al. Proteomics cataloging analysis of human expressed prostatic secretions reveals rich source of biomarker candidates. Proteomics Clin Appl 2008;2:543–55
- True L, Coleman I, Hawley S, et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA 2006;103:10991–6
- Sung J, Kim PJ, Ma S, et al. Multi-study integration of brain cancer transcriptomes reveals organ-level molecular signatures. PLoS Comput Biol 2013;9:e1003148
- Li XJ, Hayward C, Fong PY, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med 2013;5:207ra142
- Horikawa Y, Oda N, Cox NJ, et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet 2000;26:163–75
- Simon R, Roychowdhury S. Implementing personalized cancer genomics in clinical trials. Nat Rev Drug Discov 2013;12:358–69
- McKillop AM, Flatt PR. Emerging applications of metabolomic and genomic profiling in diabetic clinical medicine. Diabetes Care 2011;34:2624–30
- Tromp G, Kuivaniemi H, Romero R, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol 2004;191:1331–8
- Romero R, Espinoza J, Gotsch F, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006;113:118–35
- Khoury MJ, Romero R. The integration of genomics into obstetrics and gynecology: a HuGE challenge. Am J Obstet Gynecol 2006;195:1503–5
- Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet Gynecol 2006;195:360–3
- Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med 2010;23:1344–59
- Toft JH, Lian IA, Tarca AL, et al. Whole-genome microarray and targeted analysis of angiogenesis-regulating gene expression (ENG, FLT1, VEGF, PlGF) in placentas from pre-eclamptic and small-for-gestational-age pregnancies. J Matern Fetal Neonatal Med 2008;21:267–73
- Rajakumar A, Chu T, Handley DE, et al. Maternal gene expression profiling during pregnancy and preeclampsia in human peripheral blood mononuclear cells. Placenta 2011;32:70–8
- Vaiman D, Calicchio R, Miralles F. Landscape of transcriptional deregulations in the preeclamptic placenta. PLoS One 2013;8:e65498
- Varkonyi T, Nagy B, Fule T, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta 2011;32:S21–9
- Chaiworapongsa T, Romero R, Whitten A, et al. Differences and similarities in the transcriptional profile of peripheral whole blood in early and late-onset preeclampsia: insights into the molecular basis of the phenotype of preeclampsiaa. J Perinat Med 2013;41:485–504
- Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92
- Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol 2013;70:265–84
- Aguan K, Carvajal JA, Thompson LP, Weiner CP. Application of a functional genomics approach to identify differentially expressed genes in human myometrium during pregnancy and labour. Mol Hum Reprod 2000;6:1141–5
- Chan EC, Fraser S, Yin S, et al. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab 2002;87:2435–41
- Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab 2002;87:2431–4
- Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 2005;26:661–71
- Havelock JC, Keller P, Muleba N, et al. Human myometrial gene expression before and during parturition. Biol Reprod 2005;72:707–19
- Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–86
- Bukowski R, Hankins GD, Saade GR, et al. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med 2006;3:e169
- Romero R, Tarca AL, Tromp G. Insights into the physiology of childbirth using transcriptomics. PLoS Med 2006;3:e276
- Hassan SS, Romero R, Tarca AL, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2007;197:250.e251–7
- O'Brien M, Morrison JJ, Smith TJ. Upregulation of PSCDBP, TLR2, TWIST1, FLJ35382, EDNRB, and RGS12 gene expression in human myometrium at labor. Reprod Sci 2008;15:382–93
- Hassan SS, Romero R, Tarca AL, et al. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med 2009;22:1183–93
- Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010;38:617–43
- Nhan-Chang CL, Romero R, Tarca AL, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol 2010;202:462.e1–41
- Hassan SS, Romero R, Tarca AL, et al. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol 2010;203:472.e1–14
- Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol 2011;204:177.e115–33
- Mosher AA, Rainey KJ, Giembycz MA, et al. Prostaglandin E2 represses interleukin 1 beta-induced inflammatory mediator output from pregnant human myometrial cells through the EP2 and EP4 receptors. Biol Reprod 2012;87:7 (1–10)
- Lim S, MacIntyre DA, Lee YS, et al. Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS One 2012;7:e34707
- Chaemsaithong P, Madan I, Romero R, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med 2013;41:665--81
- Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet 2006;15:R17–29
- Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet 2009;5:e1000459
- Jia H, Osak M, Bogu GK, et al. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA 2010;16:1478–87
- Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta 2010;1799:597–615
- Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 2011;25:1915–27
- Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol 2011;22:366–76
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43:904–14
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012;22:1775–89
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012;81:145–66
- Hacisuleyman E, Cabili MN, Rinn JL. A Keystone for ncRNA. Genome Biol 2012;13:315
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339–46
- Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 2012;9:703–19
- Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics 2013;193:651–69
- Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol 2013;20:300–7
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 2013;152:1298–307
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol 1980;136:709–13
- Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–8
- Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 2012;22:1760–74
- Grinchuk OV, Jenjaroenpun P, Orlov YL, et al. Integrative analysis of the human cis-antisense gene pairs, miRNAs and their transcription regulation patterns. Nucleic Acids Res 2010;38:534–47
- Wood E, Chin-Inmanu K, Jia H, Lipovich L. Sense-antisense gene pairs: sequence, transcription, and structure are not conserved between human and mouse. Frontiers Genetics 2013;4:183 . doi: 10.3389/fgene.2013.00183
- UCSC Genome Bioinformatics. Available from: http://genome.ucsc.edu/. [last accessed Oct 2013]
- The GENCODE Project: Encyclopædia of genes and gene variants. Available from: www.gencodegenes.org [last accessed Oct 2013]
- Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science 2005;309:1559–63
- Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res 2002;12:996–1006
- Kent WJ. BLAT – the BLAST-like alignment tool. Genome Res 2002;12:656–64
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003;19:185–93
- Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–84
- Lipovich L, Dachet F, Cai J, et al. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics 2012;192:1133–48
- Johnsson P, Lipovich L, Grandér D, Morris KV. Evolutionary conservation of long noncoding RNAs; sequence, structure, function. Biochim Biophys Acta. 2013. In press: http://dx.doi.org/10.1016/j.bbagen.2013.10.035
- Taylor DD, Bohler HC, Gercel-Taylor C. Pregnancy-linked suppression of TcR signaling pathways by a circulating factor absent in recurrent spontaneous pregnancy loss (RPL). Mol Immunol 2006;43:1872–80
- Street ME, Viani I, Ziveri MA, et al. Impairment of insulin receptor signal transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal growth. Eur J Endocrinol 2011;164:45–52
- Rath N, Wang Z, Lu MM, Morrisey EE. LMCD1/Dyxin is a novel transcriptional cofactor that restricts GATA6 function by inhibiting DNA binding. Mol Cell Biol 2005;25:8864–73
- Bian ZY, Huang H, Jiang H, et al. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension 2010;55:257–63
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 2004;431:931–45
- Bernstein BE, Birney E, Dunham I, et al. An integrated encyclopedia of DNA elements in the human genome. Nature 2012;489:57–74
- Kapranov P, Willingham AT, Gingeras TR. Genome-wide transcription and the implications for genomic organization. Nat Rev Genet 2007;8:413–23
- Comings DE. The structure and function of chromatin. Adv Hum Genet 1972;3:237–431
- Ohno S. So much “junk” DNA in our genome. In: Smith H, ed. Evolution of genetic systems. New York: Gordon & Breach; 1972:366--70
- Willingham AT, Gingeras TR. TUF love for “junk” DNA. Cell 2006;125:1215–20
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009;10:155–9
- Knowling S, Morris KV. Non-coding RNA and antisense RNA. Nature's trash or treasure? Biochimie 2011;93:1922–7
- Mattick JS. Long noncoding RNAs in cell and developmental biology. Semin Cell Dev Biol 2011;22:327
- Magistri M, Faghihi MA, St Laurent 3rd G, Wahlestedt C. Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet 2012;28:389–96
- Sheik Mohamed J, Gaughwin PM, Lim B, et al. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA 2010;16:324–37
- Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome 2008;19:454–92
- Mercer TR, Dinger ME, Sunkin SM, et al. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci USA 2008;105:716–21
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009;136:629–41
- Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009;458:223–7
- Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 2009;23:1494–504
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol 2011;21:354–61
- Ng SY, Bogu GK, Soh BS, Stanton LW. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell 2013;51:349–59
- Johnsson P, Ackley A, Vidarsdottir L, et al. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol 2013;20:440–6
- Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 2008;14:723–30
- Xu B, Gerin I, Miao H, et al. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS One 2010;5:e14199
- Moran I, Akerman I, van de Bunt M, et al. Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 2012;16:435–48
- Sun L, Goff LA, Trapnell C, et al. Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci USA 2013;110:3387–92
- Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55
- Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394.e1–24
- Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003;9:41–5
- Mittal P, Romero R, Mazaki-Tovi S, et al. Fetal membranes as an interface between inflammation and metabolism: increased aquaporin 9 expression in the presence of spontaneous labor at term and chorioamnionitis. J Matern Fetal Neonatal Med 2009;22:1167–75
- Than NG, Romero R, Tarca AL, et al. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J Matern Fetal Neonatal Med 2009;22:1000–13
- Bollapragada S, Youssef R, Jordan F, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009;200:104.e101–11
- Hertelendy F, Romero R, Molnar M, et al. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol 1993;30:49–57
- Belt AR, Baldassare JJ, Molnar M, et al. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol 1999;181:359–66
- Molnar M, Rigo Jr J, Romero R, Hertelendy F. Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am J Obstet Gynecol 1999;181:42–9
- Allport VC, Pieber D, Slater DM, et al. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 2001;7:581–6
- Jenkin G, Young IR. Mechanisms responsible for parturition; the use of experimental models. Anim Reprod Sci 2004;82–83:567–81
- Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim 2012;47:344–52
- Liggins G. Cervical ripening as an inflamamtory reaction. In: Ellwood D, Anderson A, eds. The cervix in pregnancy and labor: clinical and biochemical investigations. Edinburgh: Churchill Livingstone; 1981:1–9
- Sennstrom MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 2000;6:375–81
- Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol 2002;57:217–24
- Stjernholm-Vladic Y, Stygar D, Mansson C, et al. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol 2004;2:74
- Dubicke A, Fransson E, Centini G, et al. Pro-inflammatory and anti-inflammatory cytokines in human preterm and term cervical ripening. J Reprod Immunol 2010;84:176–85
- Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990;35:235–8
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab 1993;77:805–15
- Saito S, Kasahara T, Kato Y, et al. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–8
- Austgulen R, Lien E, Liabakk NB, et al. Increased levels of cytokines and cytokine activity modifiers in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 1994;57:149–55
- Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol 1995;86:223–9
- Steinborn A, Kuhnert M, Halberstadt E. Immunmodulating cytokines induce term and preterm parturition. J Perinat Med 1996;24:381–90
- Tanaka Y, Narahara H, Takai N, et al. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol 1998;179:644–9
- Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999;181:989–94
- Keelan JA, Marvin KW, Sato TA, et al. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999;181:1530–6
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000;21:514–50
- Esplin MS, Romero R, Chaiworapongsa T, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med 2003;14:51–6
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Hamill N, Romero R, Gotsch F, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008;36:217–27
- Bogavac MA, Brkic S. Serum proinflammatory cytokine – interleukin-8 as possible infection site marker in preterm deliveries. J Perinat Med 2009;37:707–8
- Nace J, Fortunato SJ, Maul H, Menon R. The expression pattern of two novel cytokines (IL-24 and IL-29) in human fetal membranes. J Perinat Med 2010;38:665–70
- Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology 2012;153:481–91
- Hua R, Pease JE, Cheng W, et al. Human labour is associated with a decline in myometrial chemokine receptor expression: the role of prostaglandins, oxytocin and cytokines. Am J Reprod Immunol 2013;69:21–32
- Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids 1990;41:35–8
- Dowling DD, Romero RJ, Mitchell MD, Lundin-Schiller S. Isolation of multiple substances in amniotic fluid that regulate amnion prostaglandin E2 production: the effects of gestational age and labor. Prostaglandins Leukot Essent Fatty Acids 1991;44:253–5
- Romero R, Gonzalez R, Baumann P, et al. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids 1994;50:97–104
- Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol 1994;171:1613–20
- Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev 1995;7:623–32
- Edwin SS, Romero RJ, Munoz H, et al. 5-Hydroxyeicosatetraenoic acid and human parturition. Prostaglandins 1996;51:403–12
- Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 1996;54:187–91
- Gibb W. The role of prostaglandins in human parturition. Ann Med 1998;30:235–41
- Keelan JA, Sato TA, Gupta DK, et al. Prostanoid stimulation of cytokine production in an amnion-derived cell line: evidence of a feed-forward mechanism with implications for term and preterm labor. J Soc Gynecol Investig 2000;7:37–44
- Mitchell MD, Chang MC, Chaiworapongsa T, et al. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab 2005;90:4244–8
- Lee SE, Romero R, Park IS, et al. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med 2008;21:89–94
- Romero R, Emamian M, Wan M, et al. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol 1987;70:849–51
- Romero R, Wu YK, Mazor M, et al. Increased amniotic fluid leukotriene C4 concentration in term human parturition. Am J Obstet Gynecol 1988;159:655–7
- Romero R, Wu YK, Mazor M, et al. Amniotic fluid concentration of 5-hydroxyeicosatetraenoic acid is increased in human parturition at term. Prostaglandins Leukot Essent Fatty Acids 1989;35:81–3
- Blumenstein M, Bowen-Shauver JM, Keelan JA, Mitchell MD. Identification of suppressors of cytokine signaling (SOCS) proteins in human gestational tissues: differential regulation is associated with the onset of labor. J Clin Endocrinol Metab 2002;87:1094–7
- Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science 2005;309:1564–6
- Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 2013;152:1308–23
- Martianov I, Ramadass A, Serra Barros A, et al. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 2007;445:666–70
- Almada AE, Wu X, Kriz AJ, et al. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 2013;499:360–3
- van Dijk M, Thulluru HK, Mulders J, et al. HELLP babies link a novel lincRNA to the trophoblast cell cycle. J Clin Invest 2012;122:4003–11
- van Dijk M, Oudejans C. (Epi)genetics of pregnancy-associated diseases. Front Genet 2013;4:180