Abstract
Objective: The objectives of this study were to: (1) determine the amniotic fluid (AF) microbiology of patients with preterm prelabor rupture of membranes (PROM); and (2) examine the relationship between intra-amniotic inflammation with and without microorganisms (sterile inflammation) and adverse pregnancy outcomes in patients with preterm PROM.
Methods: AF samples obtained from 59 women with preterm PROM were analyzed using cultivation techniques (for aerobic and anaerobic bacteria as well as genital mycoplasmas) and with broad-range polymerase chain reaction coupled with electrospray ionization mass spectrometry (PCR/ESI-MS). AF concentration of interleukin-6 (IL-6) was determined using ELISA. Results of both tests were correlated with AF IL-6 concentrations and the occurrence of adverse obstetrical/perinatal outcomes.
Results: (1) PCR/ESI-MS, AF culture, and the combination of these two tests each identified microorganisms in 36% (21/59), 24% (14/59) and 41% (24/59) of women with preterm PROM, respectively; (2) the most frequent microorganisms found in the amniotic cavity were Sneathia species and Ureaplasma urealyticum; (3) the frequency of microbial-associated and sterile intra-amniotic inflammation was overall similar [ 29% (17/59)]: however, the prevalence of each differed according to the gestational age when PROM occurred; (4) the earlier the gestational age at preterm PROM, the higher the frequency of both microbial-associated and sterile intra-amniotic inflammation; (5) the intensity of the intra-amniotic inflammatory response against microorganisms is stronger when preterm PROM occurs early in pregnancy; and (6) the frequency of acute placental inflammation (histologic chorioamnionitis and/or funisitis) was significantly higher in patients with microbial-associated intra-amniotic inflammation than in those without intra-amniotic inflammation [93.3% (14/15) versus 38% (6/16); p = 0.001].
Conclusions: (1) The frequency of microorganisms in preterm PROM is 40% using both cultivation techniques and PCR/ESI-MS; (2) PCR/ESI-MS identified microorganisms in the AF of 50% more women with preterm PROM than AF culture; and (3) sterile intra-amniotic inflammation was present in 29% of these patients, and it was as or more common than microbial-associated intra-amniotic inflammation among those presenting after, but not before, 24 weeks of gestation.
Introduction
Prelabor rupture of membranes (PROM) is defined as the spontaneous rupture of the chorioamniotic membranes occurring before the onset of labor [Citation1–14]. When the rupture takes place before 37 weeks of gestation, the condition is known as preterm PROM, which affects approximately 2% of all pregnancies [Citation1,Citation8,Citation9,Citation11,Citation15,Citation16]. The main consequence of preterm PROM is the onset of premature labor and delivery [Citation17–19]. Indeed, preterm PROM occurs in 40% of all spontaneous preterm deliveries, representing a significant contribution to perinatal morbidity and mortality worldwide [Citation2,Citation4,Citation12,Citation20–23].
The clinical management of preterm PROM relies on balancing the benefits of prolonging gestation to reduce adverse events related to prematurity against the risk of intra-amniotic infection and its potential consequences for both mother and infant. The frequency of intra-amniotic infection in patients with preterm PROM in the absence of labor is 20–40% [Citation7,Citation24–34]. In contrast, when amniocentesis is performed at the time of the onset of labor, the prevalence of intra-amniotic infection as high as 75% has been reported [Citation7]. The identification of microorganisms in the amniotic fluid (AF) presents a major diagnostic challenge: the results of culture require several days to be obtained, and this is too long to inform clinical care.
Here, we describe a recently-developed method (PCR-ESI-MS) which combines broad-range real-time PCR with electrospray ionization mass spectrometry (ESI-MS) for the detection and characterization of amplified DNA from bacteria and viruses in AF. The PCR/ESI-MS assay detects and identifies 3400 bacteria and over 40 Candida species within 8 h [Citation35–57]. Early detection of microorganisms in the AF of patients with preterm PROM would allow for timely intervention in order to reduce the risk of maternal infection and perinatal complications. The objectives of this study were to: (1) determine the AF microbiology of patients with preterm PROM; and (2) examine the relationship between intra-amniotic inflammation with and without microorganisms and adverse pregnancy outcomes in patients with preterm PROM using both cultivation and PCR/ESI-MS.
Methods
Study population
This was a retrospective cohort study of women with singleton pregnancies with a diagnosis of preterm PROM. Patients were identified by searching the clinical database and Bank of Biological Samples of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD) (Detroit, MI). The inclusion criteria were: (1) singleton gestation; (2) amniocentesis (trans abdominal amniocentesis) between 20 and 35 weeks performed for microbiological studies; (3) availability of AF for the performance of molecular microbiologic studies; and (4) neonatal outcome. Patients were excluded from the study if they had: (1) a chromosomal or structural fetal anomaly; or (2) placenta previa.
Patients with the diagnosis of preterm PROM were counseled by their treating physicians about the potential value of identifying microorganisms in amniotic fluid. Women who agreed to undergo an amniocentesis were asked to donate additional AF other than that required for clinical studies and allow collection of clinical information for research purposes. Further management of these patients was at the discretion of the attending physician. All patients provided written informed consent and the use of biological specimens and clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
Clinical definitions
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination, or by ultrasound examination alone if the sonographic determination of gestational age was not consistent with menstrual dating. Preterm PROM was diagnosed with a sterile speculum examination with documentation of pooling of amniotic fluid in the vagina in association with a positive nitrazine test and/or and positive ferning tests when necessary. Clinical chorioamnionitis was diagnosed when maternal temperature was elevated to 37.8 °C and two or more of the following criteria were present: uterine tenderness, malodorous vaginal discharge, maternal leukocytosis (>15 000 cells/mm3), maternal tachycardia (>100 beats/min), and fetal tachycardia (>160 beats/min) [Citation58,Citation59].
The presence of microorganisms in the amniotic cavity was defined according to the results of AF culture and PCR/ESI-MS (Ibis® Technology – Athogen, Carlsbad, CA) [Citation60–63]. Intra-amniotic inflammation was diagnosed when AF interleukin (IL)-6 concentration was ≥ 2.6 ng/mL [Citation64,Citation65]. Based on the results of AF culture, PCR/ESI-MS and AF concentration of IL-6, patients were classified as: (1) no intra-amniotic inflammation/infection (either using AF culture or PCR/ESI-MS); (2) microbial invasion of the amniotic cavity (MIAC; identification of microorganisms by either AF cultures or PCR/ESI-MS without intra-amniotic inflammation); (3) microbial-associated intra-amniotic inflammation (combination of MIAC and intra-amniotic inflammation); or (4) sterile intra-amniotic inflammation (an elevated AF IL-6 concentration without evidence of microorganisms using cultivation and molecular methods). Acute histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes [Citation66,Citation67], and acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly, using the previously described criteria [Citation66–68]. For all newborns, data records regarding morbidity and mortality were reviewed. Neonatal outcome was assessed by measuring composite neonatal morbidity and mortality, defined as the presence of one or more of the following: bronchopulmonary dysplasia, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage ≥ grade III, and respiratory failure requiring mechanical ventilation. Perinatal mortality (stillbirth and neonatal death) were documented separately.
Sample collection
Patients with the diagnosis of preterm PROM who underwent transabdominal ultrasound-guided amniocentesis for evaluation of intra-amniotic infection were eligible for the study. AF was transported in capped sterile syringes to the clinical laboratory and cultured for aerobic and anaerobic bacteria, as well as genital mycoplasmas. Evaluation of white blood cell (WBC) count, AF glucose concentration and Gram stain of AF were also performed shortly after collection. AF not required for clinical assessment was centrifuged for 10 min at 4 °C shortly after the amniocentesis, and the supernatant was aliquoted and stored at −70 °C until analysis. Following delivery, the placenta, umbilical cord and chorioamniotic membranes were collected and the presence or absence of acute histologic chorioamnionitis and/or funisitis was determined.
Detection of microorganisms with cultivation and molecular methods
AF was analyzed using cultivation techniques (for aerobic and anaerobic bacteria as well as genital mycoplasmas) and with PCR/ESI-MS (Ibis® Technology – Athogen, Carlsbad, CA). Briefly, DNA was extracted from 300 µL of AF using a method that combines bead-beating cell lysis with a magnetic-bead based extraction method [Citation69,Citation70]. The extracted DNA was amplified on the bacterial artificial chromosome (BAC) spectrum assay according to the manufacturer's instructions. PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp. which are represented in the platform's signature database [Citation43,Citation50,Citation71]. A total of 200 µL of extract was used per sample.
For viral detection, the nucleic acids were extracted from 300 µL of AF using a method that combines chemical lysis with a magnetic-bead-based extraction method. The extracted RNA/DNA was amplified on the broad viral assay according to the manufacturer's instructions. In the eight wells, there were fourteen primer pairs used to detect the following viruses: Herpes simplex virus 1 (HHV-1), Herpes simplex virus 2 (HHV-2), Varicella-zoster virus (HHV-3), Epstein-Barr virus (HHV-4), Cytomegalovirus (HHV-5), Kaposi's sarcoma-associated herpes virus (HHV-8), Human adenoviruses, Human enteroviruses, BK polyomavirus, JC polyomavirus and Parvovirus B19 [Citation71].
After PCR amplification, 30-μL aliquots of each PCR product were desalted and analyzed via ESI-MS as previously described [Citation39,Citation43]. The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. Along with organism identification, the ESI-MS analysis includes a Q-score and level of detection (LOD). The Q-score, a rating between 0 (low) and 1 (high), represents a relative measure of the strength of the data supporting identification; only Q-scores ≥0.90 were reported for the BAC Spectrum assay [Citation51]. The LOD describes the amount of amplified DNA present in the sample: this is calculated with reference to an internal calibrant, as previously described [Citation38], and is reported herein as genome equivalents per PCR reaction well (GE/well). The sensitivity (LOD) of the Ibis assay for the detection of bacteria in blood is on average 100 CFU/mL (95% CI, 6–600 CFU/mL) [Citation50]. A comparison of detection limits between blood and amniotic fluid showed that the assays have comparable detection limits (100 CFU/mL). The sensitivity (LOD) for the broad viral in plasma ranges from 400 copies/mL to 6600 copies/mL [Citation72]. Detection limits in AF were similar to plasma, ranging from ∼800 to 1600 copies/mL (depending upon the specific microorganism).
Determination of IL-6 in amniotic fluid
AF concentrations of IL-6 were determined to assess the magnitude of the intra-amniotic inflammatory response. We used a sensitive and specific enzyme immunoassay from R&D Systems (Minneapolis, MN). Briefly, the immunoassay utilized was the quantitative sandwich enzyme immunoassay technique, and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7 and 4.6%, respectively. The detection limit of the IL-6 assay was 0.09 pg/mL. AF IL-6 concentrations were determined for research purposes, and such results were not used in patient management. We have previously reported the use of IL-6 for the assessment of intra-amniotic inflammation [Citation30,Citation54,Citation60,Citation62,Citation64,Citation73–88].
Statistical analysis
The Kolmogorov–Smirnov test and visual plot inspection were used to assess the normality of continuous data distributions. Patients were stratified by the gestational age at which the rupture of the membranes (ROM) occurred and according to the presence of intra-amniotic inflammation with or without detectable microorganisms. Spearman's non-parametric correlation coefficients were calculated. Between-group comparisons were performed using the Kruskal–Wallis and Mann–Whitney U-tests to examine the differences in arithmetic variable distributions. The χ2 or Fischer's exact test was used to test for differences in proportions, as appropriate. A two-tailed p value of <0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS, Chicago, IL).
Results
Characteristics of the study population
Fifty-nine patients with the diagnosis of preterm PROM were identified. Demographic and clinical characteristics of the study population are displayed in . The median interquartile range (IQR) of gestational age at amniocentesis was 28 (25–31) weeks. The distribution of patients according to the gestational age at diagnosis was 23.7% (14/59) before 25 weeks, 66.1% (39/59) between 25 and <33 weeks, and 10.2% (6/59) between 33 and 35 weeks of gestation ().
Table 1. Maternal characteristics and demographic data of the study population.
Upon admission, 37.3% (22/59) of the patients delivered within 48 h of membrane rupture. The remaining 37 (62.7%) patients had a latent phase of greater than 48 h, with 19 patients (32.2%) having a latent phase of more than 14 days. Labor began spontaneously in 28.8% (17/59) of the women and was induced in 50.8% (30/59) of the patients. The route of delivery was vaginal in 56% (33/59), and 44% (26/59) of the patients were delivered by cesarean section.
Microbial prevalence and diversity using PCR/ESI-MS and cultivation techniques
Among the study population, 24% (14/59) had a positive microbial culture in AF, and in 36% (21/59) of the cases, PCR/ESI-MS detected genomic material from bacteria or viruses. Microorganisms in AF were identified in 40.6% (24/59) of the patients when combining the results from the two techniques. shows that both PCR/ESI-MS and AF cultures were positive in 18.6% (11/59) of patients, whereas three culture-positive samples (5.1%) were negative by PCR/ESI-MS, and 10 (17%) PCR/ESI-MS positive samples were negative by AF culture.
Figure 1. Bacteria and viruses detected in amniotic fluid of patients with preterm PROM standard cultivation techniques versus PCR/ESI-MS. Amniotic fluid culture includes routine cultivation techniques for bacteria (aerobes, anaerobes and genital mycoplasmas). PCR/ESI-MS refers to broad range PCR and ESI-MS.
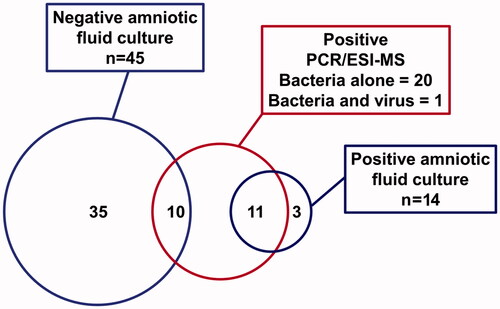
shows the microorganisms identified, the microbial burden (GE/well) reported by PCR/ESI-MS, concentrations of inflammatory markers in AF and pregnancy outcomes for each patient with a positive AF culture and/or PCR/ESI-MS. The most frequent microorganism identified in AF by PCR/ESI-MS was Sneathia spp. [28.5% (6/21)], followed by Ureaplasma parvum [14.3% (3/21)] and Ureaplasma urealyticum [14.3% (3/21)]; the latter was the most common microorganism identified by AF culture (). Among the 24 patients whose AF tested positive by AF culture or PCR/ESI-MS, 15 bacterial species, one fungus and one virus were identified. Of the 15 bacterial taxa identified, four were detected by both AF culture and PCR/ESI-MS (Ureaplasma spp., Streptococcus pneumoniae, Prevotella bivia, Haemophilus influenzae), three were detected only by AF culture (Bacteroides ureolyticus, Lactobacillus spp., Saccharomyces cerevisiae), and eight were detected by only PCR/ESI-MS (Sneathia species, Ureaplasma parvum, Mycoplasma hominis, Streptococcus mitis, Gardnerella vaginalis, Bacteroides fragilis, Rothia mucilaginosa, Neisseria gonorrhoeae). Three cases had positive detection for Candida albicans, which was detected by both techniques. One patient had positive detection of Human enterovirus [1.7% (1/59)] which was also positive for Mycoplasma hominis ().
Table 2. Amniotic fluid IL-6 concentrations, white blood cell count, placenta pathology results, pregnancy outcome, microorganisms and microbial burden detected in the amniotic fluid of patients with PPROM using cultivation techniques versus PCR/ESI-MS.
The frequency of microbial-associated and sterile intra-amniotic inflammation in patients with preterm PROM
Intra-amniotic inflammation (defined as AF IL-6 ≥ 2.6 ng/mL) was identified in 57.6% (34/59) of the cases. When combining the results of AF culture, PCR/ESI-MS and AF IL-6 concentrations, 30.5% (18/59) of patients did not have either intra-amniotic inflammation or infection, 12% (7/59) had MIAC, and 29% (17/59) had microbial-associated intra-amniotic inflammation. Twenty-nine percent (17/59) of the patients had intra-amniotic inflammation without detection of bacteria or viruses using both PCR/ESI-MS and AF cultures, and were thus categorized as having sterile intra-amniotic inflammation.
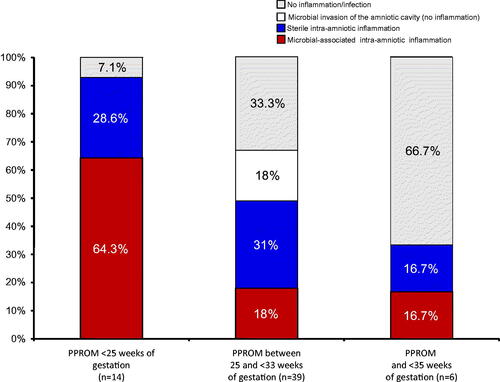
The prevalence of microbial-associated and sterile intra-amniotic inflammation differed according to the gestational age in which the rupture of the membranes (ROM) occurred (). The earlier the gestational age at which ROM occurred, the higher the prevalence of microbial-associated inflammation. Among patients with ROM at <25 weeks of gestation, the frequency of microbial-associated intra-amniotic inflammation was significantly higher than that of sterile intra-amniotic inflammation [64.3% (9/14) versus 28.6% (4/14); p = 0.005]. In contrast, microbial-associated intra-amniotic inflammation was present in only 18% (7/39) of patients when ROM occurred between 25 and <33 weeks of gestation (). Two-thirds (4/6) of patients who had ROM between 33 and 35 weeks of gestation did not have evidence of intra-amniotic inflammation ().
Figure 2. Prevalence of microbial – associated and sterile intra-amniotic inflammation in patients with preterm PROM according to the gestational age at diagnosis. The earlier the gestational age at which rupture of the membranes occurs, the higher the frequency of both microbial-associated and sterile intra-amniotic inflammation.
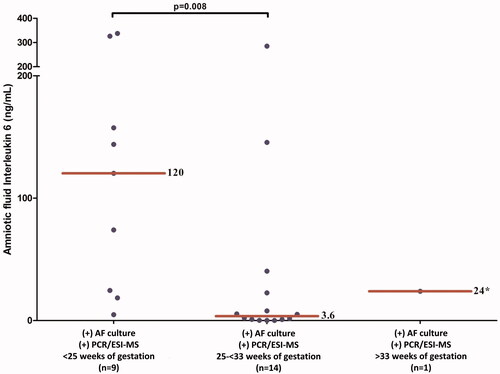
Among patients with a positive PCR/ESI-MS, there was no correlation between the microbial load from bacteria or viruses [genome copies per PCR well reaction (GE/well)] and the intensity of the intra-amniotic inflammatory response (defined by AF concentration of IL-6 and AF WBC count; p = 0.6 and p = 0.7, respectively). However, the median AF IL-6 concentration was significantly higher in patients who presented with preterm PROM at <25 weeks and either a positive AF culture or PCR/ESI-MS than in those who presented between 25 and <33 weeks of gestation [120 (21.5–241.8) versus 3.6 (0.7–24) ng/mL; p = 0.008] ().
Figure 3. Amniotic fluid concentrations of interleukin 6 in patients with a positive AF culture or PCR/ESI-MS according to the gestational age at which the rupture of the membranes occurred. Patients who presented with preterm PROM <25 weeks of gestation with a positive AF culture or PCR/ESI-MS had a significantly higher AF IL-6 concentrations than those who presented with a positive AF culture or PCR/ESI-MS between 25 and <33 weeks of gestation.
Pregnancy outcome according to the results of cultivation and molecular techniques
To determine the clinical relevance of detecting microbial-associated or sterile intra-amniotic inflammation using AF IL-6 concentrations, PCR/ESI-MS and AF culture, pregnancy outcomes were compared among the groups according to the test results. The median (IQR) AF IL-6 concentrations and WBC count in patients with sterile intra-amniotic inflammation were significantly higher than those of patients without intra-amniotic inflammation [AF IL-6: 12 (4.7–137) versus 0.7 (0.5–1.1) ng/mL; p < 0.001; and WBC count: 175 (21–395) versus 1 (0–4) cells/mm3; p < 0.001]. However, there were no significant differences in those parameters (AF IL-6 and WBC) between patients with sterile intra-amniotic inflammation and those with microbial-associated intra-amniotic inflammation (IL-6; p = 0.1 and WBC; p = 0.4) (). The median amniocentesis-to-delivery interval of women with microbial-associated intra-amniotic inflammation was significantly shorter than that of women without intra-amniotic inflammation [median, 3 IQR: 1–4 days versus median, 12 IQR: 1–22 days; p = 0.02].
Table 3. Inflammatory markers in amniotic fluid, pregnancy outcome and placental pathology results in patients with preterm PROM according to the results of amniotic fluid culture and PCR/ESI-MS.
Neonatal outcomes were known in 96.6% (57/59) of the patients. Neonatal morbid events (assessed by composite neonatal morbidity) were significantly more common in patients with microbial-associated intra-amniotic inflammation than in those without intra-amniotic inflammation [82.4% (14/17) versus 43.8% (7/16); p = 0.02] (). Importantly, there was no significant difference in the prevalence of neonatal morbid events between neonates born to mothers with sterile intra-amniotic inflammation and those born to mothers with microbial-associated intra-amniotic inflammation [64.7% (11/17) versus 82.4% (14/17); p = 0.2]. Additionally, 17.5% (10/57) of the patients had a neonatal death – five were periviable gestations, and all were born to mothers with microbial-associated intra-amniotic inflammation.
The relationship between detectable microorganisms in the amniotic fluid and acute histological chorioamnionitis
The extraplacental membranes and umbilical cord were examined in 91.5% (54/59) of the cases; 57.4% (31/54) had acute histologic chorioamnionitis and 42.6% (23/54) had funisitis. The prevalence of acute placental inflammation (histologic chorioamnionitis and/or funisitis) was significantly higher in patients with microbial-associated intra-amniotic inflammation than in patients with either sterile intra-amniotic inflammation or no intra-amniotic inflammation [93% (14/15) versus 50% (8/16); p = 0.01 and 93% (14/15) versus 38% (6/16); p = 0.001]. However, there were no significant differences in the frequency of acute placental inflammation between patients with sterile intra-amniotic inflammation and those without intra-amniotic inflammation (p = 0.5; ).
Discussion
Principal findings of the study
(1) PCR/ESI-MS, AF culture, and the combination of these two tests each identified microorganisms in 36% (21/59), 24% (14/59) and 41% (24/59) of women presenting with preterm PROM, respectively; (2) the most frequent microorganisms found in the amniotic cavity were Sneathia species, Ureaplasma parvum and Ureaplasma urealyticum; (3) the frequency of microbial-associated and sterile intra-amniotic inflammation was overall similar [29% (17/59)]: however, the prevalence of each differed according to the gestational age when PROM occurred; (4) the earlier the gestational age at rupture of the membranes, the higher the frequency of both microbial-associated and sterile intra-amniotic inflammation; (5) the intensity of the intra-amniotic inflammatory response (as measured by the AF concentration of IL-6) in the presence of microorganisms was stronger in patients in whom preterm PROM occurred at <25 weeks as opposed to ≥25 weeks; and (6) the frequency of acute placental inflammatory lesions (histologic chorioamnionitis and/or funisitis) was significantly higher in patients with microbial-associated intra-amniotic inflammation than in those without intra-amniotic inflammation [93.3% (14/15) versus 38% (6/16); p = 0.001]. A major finding of this study is that intra-amniotic inflammation without demonstrable bacteria (sterile inflammation) was frequently identified (31%) in patients with preterm PROM between 25 and <33 weeks of gestation.
The importance of microbial invasion of the amniotic cavity in preterm PROM
Microorganisms may gain access to the amniotic cavity in patients with intact membranes, and induce an inflammatory response leading to the production of cytokines [Citation73,Citation75,Citation76,Citation81,Citation89–107], chemokines [Citation108–114], other inflammatory mediators [Citation115–124], and thrombin [Citation125–130]. Microorganisms and their products can also induce the production of matrix – degrading enzymes [Citation65,Citation131–137], which have been implicated in the mechanisms responsible for membrane rupture. Matrix metalloproteinases, elastases, catepsin, etc., can degrade the extracellular matrix, weakening the membranes [Citation136,Citation138–144]. Cytokines which induce apoptosis, such as members of the tumor necrosis factor (TNFα) super family, may also participate in the mechanisms responsible for membrane rupture, as they can induce programmed cell death (TNFα, TNFα soluble receptors [Citation145], FAS and FAS ligand [Citation145–147]). Why some patients with microbial invasion have preterm labor with intact membranes and others have preterm PROM is not clear. It is possible that genetic factors controlling the composition and quality of extracellular matrix and/or the host inflammatory response (maternal and fetal) play a vital role. We previously reported that AF and fetal plasma concentrations of MMP-9 are higher in fetuses with preterm PROM than in those with preterm labor with intact membranes [Citation148]. We have also reported that polymorphisms in MMP-1 [Citation149,Citation150], MMP-8 [Citation151], MMP-9 [Citation152], and serpin peptidase inhibitor, clade H, member 1 (SERPINH1) [Citation153,Citation154] are associated with preterm PROM.
Rupture of the chorioamniotic membranes can also favor secondary microbial invasion of the amniotic cavity [Citation155–158]. Indeed, microorganisms are detected more frequently in the AF as the duration of the latency period lengthens [Citation159]. Specifically, we have previously reported that the frequency of microbial invasion in patients with preterm PROM who were not in labor at admission was 25%; yet, when an amniocentesis was repeated when patients began contracting after a quiescent period, the frequency of a positive culture was close to 75% [Citation7]. Thus, microbial invasion of the amniotic cavity in patients with preterm PROM may also lead to the onset of preterm labor. The long – held belief that the initiation of preterm labor in patients with preterm PROM is a sign of infection is grounded in clinical and microbiologic studies. Intra-amniotic infection may also lead to fetal invasion; approximately 30% of pregnancies with preterm PROM have evidence of fetal bacteremia determined by cordocentesis [Citation160] or umbilical cord blood culture [Citation68,Citation93]. In turn, these microorganisms may elicit a systemic fetal inflammatory response syndrome (FIRS) and place neonates at risk for short- and long-term adverse outcomes [Citation68,Citation93,Citation161–205]. Collectively, the relationship between intra-amniotic infection, preterm labor, fetal infection and puerperal complications justifies the systematic study of microorganisms in preterm PROM.
Molecular microbiologic techniques to detect microorganisms in the amniotic cavity
The introduction of molecular microbiologic techniques was expected to improve the detection of microorganisms in the amniotic cavity in patients with complications of pregnancy. We previously reported that 50% of patients with preterm PROM have microbial invasion of the amniotic cavity using a combination of cultivation and molecular techniques [Citation61]. However, such study was conducted using research techniques which are not available for clinical microbiology of the studies in a hospital setting. This is the first study to use PCR/ESI-MS to characterize microbial invasion of the amniotic cavity in patients with preterm PROM. We found that PCR/ESI-MS identified genomic material from bacteria, fungi and viruses in 36% (21/59) of the participants, whereas AF culture was positive for only 24% (14/59) of these women. Thus, these results indicate that the use of PCR/ESI-MS in the AF from patients with preterm PROM results in an increase in the detection of microorganisms in AF by 50%.
PCR/ESI-MS has the potential to reduce the time required to obtain results to 8 h, compared to 48–72 h for standard AF cultivation. Another advantage of the use of PCR/ESI-MS is the ease of detecting multiple organisms simultaneously, even compared to molecular techniques including specific or broad range PCR. Organisms identified in this study include common pathogens in intra-amniotic infection (Sneathia amnii spp., Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis), and uncommon microorganisms, such as Rothia mucilaginosa Prevotella bivia. PCR/ESI-MS has also been used to identify infection in other body sites and fluids, such as blood in cases of bacterial endocarditis [Citation51] or culture negative infections of the central nervous system, such as meningitis [Citation48].
Sneathia amnii was the most commonly identified organism in our study, found in 28.5% of patients. Sneathia, a Gram-negative non-motile rod, was previously named “Leptotrichia sanguinegens” and is found in the lower genital tract of normal women and those with bacterial vaginosis. We have previously found this microorganism in the amniotic fluid of women with preterm labor [Citation54,Citation60], preterm PROM [Citation61], preeclampsia [Citation62], a short cervix [Citation56] and clinical chorioamnionitis at term [Citation57]. Moreover, this microorganism has been isolated in postpartum bacteremia [Citation206,Citation207]. Harwich et al. [Citation208] reported the genomic sequence of Sneathia, its morphology, growth requirements and antibiotic sensitivity. Sneathia is sensitive to metronidazole and vancomycin (in contrast to other Gram-negative bacteria, which are resistant to vancomycin) [Citation208]. Our observations highlight the importance of Sneathia in intra-amniotic infection.
Ureaplasma species were the second most common microorganism (14.3%) in the amniotic fluid. In previous studies, Ureaplasma spp. was the microorganism most frequently isolated from the amniotic fluid with standard microbiologic techniques in patients with preterm PROM [Citation83,Citation209–214], as well as other complications of pregnancy associated with intra-amniotic inflammation [Citation215–219]. Isolation of Ureaplasma spp. in the mid-trimester is associated with an increased risk of subsequent development of preterm PROM [Citation159,Citation220,Citation221]. Yoon et al. [Citation211] reported that patients with preterm PROM and a positive PCR assay for Ureaplasma urealyticum but a negative AF culture had a worse pregnancy outcome and higher frequency of histological chorioamnionitis and funisitis than patients with a sterile culture and negative PCR.
Candida species are common saprophytes in the genital tract present in up to 20–25% of pregnant women [Citation222], and have been associated with intra-amniotic infection in patients with and without intrauterine devices [Citation223–225]. In the current study, C. albicans was detected in 5.1% (3/59) of patients with preterm PROM, and two of these three patients had acute histologic chorioamnionitis: this is are consistent with our prior reports [Citation61,Citation226]. Fungal infections are important because they are well-recognized pathogens implicated in fetal death and serious neonatal complications [Citation227–237], and they require specific anti-microbial agents not generally used in the context of preterm PROM.
The role of viral infection in preterm PROM has not been extensively investigated. Previous studies using PCR-based methods have concluded that viruses are uncommon in the amniotic fluid of normal women in the midtrimester [Citation238–242], as well as in women with preterm PROM [Citation243,Citation244]. In the current study, PCR/ESI-MS detected one viral infection with an enterovirus. This patient also had a positive PCR in the amniotic fluid for M. hominis. However, given that viral infection may predispose to bacterial infection [Citation245–248], further investigations of the role of systemic or local viral infections during pregnancy are necessary.
Sterile intra-amniotic inflammation in preterm PROM
Sterile intra-amniotic inflammation, defined by the presence of an acute inflammatory response (elevated IL-6) in the absence of detectable microorganisms, has been reported in a subset of patients with preterm labor with intact membranes [Citation54], a short cervix [Citation56] and clinical chorioamnionitis at term [Citation57]. Sterile intra-amniotic inflammation is a risk factor for preterm delivery in patients with an episode of preterm labor, and among those with a short cervix [Citation56]. In this study, patients with preterm PROM and sterile intra-amniotic inflammation presented at a more advanced gestational age than those with microbial associated intra-amniotic inflammation, but earlier than those without intra-amniotic inflammation. Further studies with a larger sample size would be required to determine the clinical significance of sterile intra-amniotic inflammation in preterm PROM. The present series includes only 17 patients, which is insufficient to draw inferences about neonatal outcome. It is noteworthy that sterile intra-amniotic inflammation was characterized by a normal amniotic fluid white blood cell count and glucose concentration in patients with preterm labor [Citation54], a short cervix [Citation56] and clinical chorioamnionitis at term [Citation57]. However, in patients with preterm PROM, the amniotic fluid white blood cell count was elevated (median 175) and the glucose was low (median 10 mg/dL), suggesting that there may be differences between the sterile intra-amniotic inflammatory process in preterm PROM and other obstetrical syndromes.
The mechanisms responsible for the induction of sterile intra-amniotic inflammation in preterm PROM remain to be determined. We have previously proposed that “danger signals” resulting from cellular stress or necrotic cells may engage pattern recognition receptors (PRR) and stimulate an intra-amniotic inflammatory response [Citation55]. The amniotic fluid concentration of the prototypic alarmin, high mobility group box-1 (HMGB-1), is higher in patients with sterile intra-amniotic inflammation and preterm labor, suggesting that alarmins may play a vital role in this condition [Citation55]. Indeed, IL-1α, an alarmin previously reported in amniotic fluid [Citation91], can induce labor in pregnant animals [Citation249,Citation250]. A role for the inflammasome in parturition and preterm labor has recently been proposed [Citation251–254].
Insight into the origin of preterm PROM
Although preterm PROM is pragmatically considered a single entity, the data reported herein suggest clinical and pathogenic heterogeneity. The gestational age at the diagnosis of preterm PROM was related to the frequency of intra-amniotic inflammation and the specific subtype. Before 25 weeks, intra-amniotic inflammation was present in 90% of patients with preterm PROM, and 64% of cases were due to microbial-associated inflammation. However, between 25 and <33 weeks of gestation, intra-amniotic inflammation was present in 50%, and infection accounted for only 18% of all cases. Importantly, after 33 weeks of gestation, most cases of preterm PROM were not related to intra-amniotic inflammation at the time of presentation (). These observations indicate that preterm PROM is a group of entities which can be classified according to gestational age, the presence of intra-amniotic inflammation and microbial invasion of the amniotic cavity. This has implications for the understanding of the mechanisms of disease in this important obstetrical complication.
Future directions
The assessment of patients with preterm PROM relies largely on maternal clinical signs and biophysical tests of fetal well-being [Citation255–260]. Therapy is mainly aimed at inducing fetal maturation and antibiotic administration [Citation2,Citation16,Citation261]. The data presented herein suggest that there are substantial differences in the mechanisms responsible [Citation262] for preterm PROM. It is possible that the methods to monitor maternal and fetal health may differ according to the pathologic process operative in cases of preterm PROM with and without infection, and also in those without intra-amniotic inflammation. For example, the administration of antibiotics to patients with preterm PROM results in prolongation of the latency period and a reduction in the rate of clinical chorioamnionitis and neonatal sepsis [Citation263–273]. However, studies in which amniocentesis has been performed at the time of admission and after the administration of antibiotics show that antimicrobial agents do not eradicate intra-amniotic infection present at admission, nor prevent subsequent microbial invasion [Citation274]. Whether treatment of patients with antimicrobial agents selected on knowledge of the identity of the microorganism is a more effective strategy remains to be determined. For example, several microorganisms found in the amniotic cavity in patients with preterm PROM are not adequately treated with antimicrobial agents currently administered in the clinical setting. This is also the case for treatment of the infected newborn. Genital mycoplasmas and fungi are not adequately covered by antimicrobial agents generally administered in the neonatal intensive care unit. An important developing area of investigation is unraveling the causes of sterile intra-amniotic inflammation in preterm PROM as well as in cases of PROM in which there is neither infection nor inflammation.
The diagnosis of intra-amniotic infection and inflammation currently relies on analysis of amniotic fluid for Gram stain [Citation24,Citation25,Citation27,Citation28,Citation275–277], and other rapid tests such as white blood cell count and glucose determination [Citation30,Citation209,Citation277,Citation278], as well as microbial cultures for aerobic and anaerobic bacteria including genital mycoplasmas. However, it is now clear that these tests are not adequate for the rapid diagnosis of microbial invasion of the amniotic cavity or intra-amniotic inflammation [Citation277–279]. Cultivation techniques are insensitive, and results are typically not available for clinical decision making. The amniotic fluid white blood cell count and glucose determination are not adequate for the diagnosis of sterile intra-amniotic inflammation. Therefore, determination of cytokines and chemokines appears to be necessary to define the presence of intra-amniotic inflammation, and molecular microbiologic techniques are needed for the rapid detection of bacteria or viruses. PCR/ESI-MS can identify bacteria at the species level in 8 h, bringing state-of-the-art microbiology to clinical obstetrics.
Conclusions
The frequency of microorganisms in preterm PROM is 40% using both cultivation and PCR/ESI-MS. PCR/ESI-MS identified microorganisms in the AF of 50% more women with preterm PROM than AF culture. Sterile intra-amniotic inflammation was present in 29% of these patients, and was as or more common than microbial-associated intra-amniotic inflammation among those presenting after, but not before, 25 weeks of gestation.
Declaration of interest
The authors report no conflicts of interest.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
References
- Santolaya-Forgas J, Romero R, Espinoza J, et al. (2008) Prelabour rupture of the membranes. In: Reece EA, Hobbins JC, eds. Clinical obstetrics the fetus & mothers. 3rd ed. Malden, MA: Blackwell; 2008:1130–88
- Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005;32:411–28
- Parry S, Strauss 3rd JF. Premature rupture of the fetal membranes. N Engl J Med 1998;338:663–70
- Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol 1982;60:671–9
- Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol 1984;64:615–20
- Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–76
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6
- Maxwell GL. Preterm premature rupture of membranes. Obstet Gynecol Surv 1993;48:576–83
- Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clin Perinatol 2001;28:721–34
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113:17–42
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84
- Iams JD, Romero R, Culhane JF, et al. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008;371:164–75
- Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol 2009;201:230–40
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529–35
- Mercer BM, Goldenberg RL, Meis PJ, et al. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing – The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;183:738–45
- Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003;101:178–93
- Wilson JC, Levy DL, Wilds PL. Premature rupture of membranes prior to term: consequences of nonintervention. Obstet Gynecol 1982;60:601–6
- Cox SM, Williams ML, Leveno KJ. The natural history of preterm ruptured membranes: what to expect of expectant management. Obstet Gynecol 1988;71:558–62
- Nelson LH, Anderson RL, O'Shea TM, et al. Expectant management of preterm premature rupture of the membranes. Am J Obstet Gynecol 1994;171:350–6; discussion 356–8
- Johnson JW, Daikoku NH, Niebyl JR, et al. Premature rupture of the membranes and prolonged latency. Obstet Gynecol 1981;57:547–56
- Daikoku NH, Kaltreider DF, Khouzami VA, et al. Premature rupture of membranes and spontaneous preterm labor: maternal endometritis risks. Obstet Gynecol 1982;59:13–20
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9
- Tsiartas P, Kacerovsky M, Musilova I, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:1332–6
- Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol 1982;59:539–45
- Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol 1984;63:38–43
- Zlatnik FJ, Cruikshank DP, Petzold CR, et al. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med 1984;29:656–60
- Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66:316–21
- Feinstein SJ, Vintzileos AM, Lodeiro JG, et al. Amniocentesis with premature rupture of membranes. Obstet Gynecol 1986;68:147–52
- Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust NZ J Obstet Gynaecol 1991;31:331–6
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Font GE, Gauthier DW, Meyer WJ, et al. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol 1995;85:656–8
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol 1999;11:541–7
- Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 2012;17:12–19
- Ecker DJ, Sampath R, Blyn LB, et al. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc Natl Acad Sci USA 2005;102:8012–17
- Ecker DJ, Sampath R, Willett P, et al. The Microbial Rosetta Stone Database: a compilation of global and emerging infectious microorganisms and bioterrorist threat agents. BMC Microbiol 2005;5:19
- Sampath R, Hofstadler SA, Blyn LB, et al. Rapid identification of emerging pathogens: coronavirus. Emerg Infect Dis 2005;11:373–9
- Hofstadler SA, Sampath R, Blyn LB, et al. TIGER: the universal biosensor. Int J Mass Spectrom 2005;242:23–41
- Ecker JA, Massire C, Hall TA, et al. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol 2006;44:2921–32
- Blyn LB, Hall TA, Libby B, et al. Rapid detection and molecular serotyping of adenovirus by use of PCR followed by electrospray ionization mass spectrometry. J Clin Microbiol 2008;46:644–51
- Ecker DJ, Massire C, Blyn LB, et al. Molecular genotyping of microbes by multilocus PCR and mass spectrometry: a new tool for hospital infection control and public health surveillance. Methods Mol Biol 2009;551:71–87
- Hujer KM, Hujer AM, Endimiani A, et al. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol 2009;47:1436–42
- Ecker DJ, Sampath R, Li H, et al. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn 2010;10:399–415
- Kaleta EJ, Clark AE, Cherkaoui A, et al. Comparative analysis of PCR-electrospray ionization/mass spectrometry (MS) and MALDI-TOF/MS for the identification of bacteria and yeast from positive blood culture bottles. Clin Chem 2011;57:1057–67
- Massire C, Ivy CA, Lovari R, et al. Simultaneous identification of mycobacterial isolates to the species level and determination of tuberculosis drug resistance by PCR followed by electrospray ionization mass spectrometry. J Clin Microbiol 2011;49:908–17
- Gu Z, Hall TA, Frinder M, et al. Evaluation of repetitive sequence PCR and PCR-mass spectrometry for the identification of clinically relevant Candida species. Med Mycol 2012;50:259–65
- Wolk DM, Kaleta EJ, Wysocki VH. PCR-electrospray ionization mass spectrometry: the potential to change infectious disease diagnostics in clinical and public health laboratories. J Mol Diagn 2012;14:295–304
- Bhatia NS, Farrell JJ, Sampath R, et al. Identification of Streptococcus intermedius central nervous system infection by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol 2012;50:4160–2
- Schuetz AN, Huard RC, Eshoo MW, et al. Identification of a novel Acinetobacter baumannii clone in a US hospital outbreak by multilocus polymerase chain reaction/electrospray-ionization mass spectrometry. Diagn Microbiol Infect Dis 2012;72:14–19
- Metzgar D, Frinder M, Lovari R, et al. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol 2013;51:2670–8
- Brinkman CL, Vergidis P, Uhl JR, et al. PCR-Electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J Clin Microbiol 2013;51:2040–6
- Farrell JJ, Sampath R, Ecker DJ, et al. “Salvage microbiology”: detection of bacteria directly from clinical specimens following initiation of antimicrobial treatment. PLoS One 2013;8:e66349
- Jordana-Lluch E, Carolan HE, Gimenez M, et al. Rapid diagnosis of bloodstream infections with PCR followed by mass spectrometry. PLoS One 2013;8:e62108
- Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58
- Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation is more frequent than microbial-associated intra-amniotic inflammation in preterm labor with intact membranes. Am J Reprod Immunol (Submitted). 2014. [Epub ahead of print]
- Romero R, Miranda J, Chaiworapongsa T, et al. Rapid diagnosis of microbial invasion of the amniotic cavity in asymptomatic patients with a sonographic short cervix. J Matern Fetal Neonatal Med (submitted). 2014;1–52
- Romero R, Miranda J, Kusanovic JP, et al. The microbiology of clinical chorioamnionitis at term: a study based on cultivation and molecular microbiologic techniques. J Matern Fetal Neonatal Med (accepted). 2014
- Hauth JC, Gilstrap 3rd LC, Hankins GD, et al. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol 1985;66:59–62
- Gibbs RS, Dinsmoor MJ, Newton ER, et al. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol 1988;72:823–8
- DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056
- DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57
- DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–13
- DiGiulio DB, Gervasi MT, Romero R, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med 2010;38:495–502
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6
- Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292 e291–5
- Redline RW, Heller D, Keating S, et al. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26:S114–17
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Eshoo MW, Crowder CC, Rebman AW, et al. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 2012;7:e36825
- Shin JH, Ranken R, Sefers SE, et al. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J Clin Microbiol 2013;51:136–41
- A broad range of tests to meet your needs. Available from: www.athogen.com/consulting-services/microbial-tests.html [last accessed Sep 2014]
- Legoff J, Feghoul L, Mercier-Delarue S, et al. Broad-range PCR/electrospray ionization mass spectrometry for detection and typing of adenovirus and other opportunistic viruses in stem cell transplant patients. J Clin Microbiol 2013;51:4186–92
- Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine 1991;3:155–63
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 220–30.
- Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Gomez R, Romero R, Galasso M, et al. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–10
- Romero R, Galasso M, Gomez R, et al. A comparative study of the value of amniotic fluid interleukin-6, white blood cell count and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients with spontaneous labor at term. Annual Meeting of the Society of Perinatal Obstetricians; 1994; Las Vegas, NV. A250 p
- Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–30
- Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179:1254–60
- Yoon BH, Romero R, Moon JB, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–42
- Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24
- Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med 2010;38:275–9
- Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med 2012;40:329–43
- Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014;27:757–69
- Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41
- Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–87
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–8
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Athayde N, Romero R, Maymon E, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000;182:135–41
- Leslie KK, Lee SL, Woodcock SM, et al. Acute intrauterine infection results in an imbalance between pro- and anti-inflammatory cytokines in the pregnant rabbit. Am J Reprod Immunol 2000;43:305–11
- Blank V, Hirsch E, Challis JR, et al. Cytokine signaling, inflammation, innate immunity and preterm labour – a workshop report. Placenta 2008;29:S102–4
- Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod 2010;83:767–73
- Cobo T, Kacerovsky M, Palacio M, et al. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS One 2012;7:e43677
- Cobo T, Kacerovsky M, Holst RM, et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012;91:930–5
- Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical and vaginal fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Cobo T, Jacobsson B, Kacerovsky M, et al. Systemic and local inflammatory response in women with preterm prelabor rupture of membranes. PLoS One 2014;9:e85277
- Kacerovsky M, Musilova I, Jacobsson B, et al. Vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor membrane ruptures. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014. [Epub ahead of print]
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325 e321–e310
- Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e121–e125
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 1993;169:1299–303
- Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994;32:108–13
- Cohen J, Ghezzi F, Romero R, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol 1996;35:23–9
- Hsu CD, Meaddough E, Aversa K, et al. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol 1998;178:428–32
- Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Romero R, Quintero R, Emamian M, et al. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454–60
- Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–7
- Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–61
- Mazor M, Wiznitzer A, Maymon E, et al. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990;26:425–8
- Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–96
- Soto E, Romero R, Richani K, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med 2009;22:983–92
- Vaisbuch E, Romero R, Erez O, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–16
- Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–19
- Kacerovsky M, Drahosova M, Krejsek J, et al. Amniotic fluid CD200 levels in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:1416–24
- Andrys C, Kacerovsky M, Drahosova M, et al. Amniotic fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:520–7
- Rosen T, Schatz F, Kuczynski E, et al. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002;11:11–17
- Mackenzie AP, Schatz F, Krikun G, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol 2004;191:1996–2001
- Lockwood CJ, Toti P, Arcuri F, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol 2005;167:1443–9
- Stephenson CD, Lockwood CJ, Ma Y, et al. Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J Matern Fetal Neonatal Med 2005;18:17–22
- Erez O, Romer R, Vaisbuch E, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med 2009;22:971–82
- Erez O, Romero R, Vaisbuch E, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Matern Fetal Neonatal Med 2010;23:23–33
- Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–53
- Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–20
- Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–9
- Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–53
- Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–16
- Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002;12:237–46
- Park KH, Chaiworapongsa T, Kim YM, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med 2003;31:12–22
- Petersen LK, Helmig R, Oxlund H, et al. Relaxin (hRLX-2)-induced weakening of human fetal membranes in vitro. Eur J Obstet Gynecol Reprod Biol 1994;57:123–8
- Lei H, Furth EE, Kalluri R, et al. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest 1996;98:1971–8
- Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, et al. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol 1996;174:1371–6
- Moore RM, Mansour JM, Redline RW, et al. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta 2006;27:1037–51
- Kumar D, Fung W, Moore RM, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod 2006;74:29–34
- Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol 2007;21:467–78
- Strauss 3rd JF. Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci 2013;20:140–53
- Lonergan M, Aponso D, Marvin KW, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab 2003;88:3835–44
- Maymon E, Edwin S, Gomez R, et al. Evidence for dysreglation in the death factor receptor:Fas in premature labor. Am J Obstet Gynecol 1999;180:S26
- Maymon E, Edwin S, Pacora P, et al. A role of the cell death factor system (Fas/Fas ligand) in spontaneous rupture of membranes. Am J Obstet Gynecol 1999;180:S19
- Romero R, Chaiworapongsa T, Espinoza J, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–30
- Fujimoto T, Parry S, Urbanek M, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem 2002;277:6296–302
- Wang H, Ogawa M, Wood JR, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet 2008;17:1087–96
- Wang H, Parry S, Macones G, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet 2004;13:2659–69
- Ferrand PE, Parry S, Sammel M, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod 2002;8:494–501
- Wang H, Parry S, Macones G, et al. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci U S A 2006;103:13463–7
- Wang H, Sammel MD, Tromp G, et al. A 12-bp deletion in the 5′-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat 2008;29:332
- Sachs BP, Stern CM. Activity and characterization of a low molecular fraction present in human amniotic fluid with broad spectrum antibacterial activity. Br J Obstet Gynaecol 1979;86:81–6
- Hadi HA, Hodson CA, Strickland D. Premature rupture of the membranes between 20 and 25 weeks' gestation: role of amniotic fluid volume in perinatal outcome. Am J Obstet Gynecol 1994;170:1139–44
- Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21
- Akinbi HT, Narendran V, Pass AK, et al. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol 2004;191:2090–6
- Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302
- Carroll SG, Ville Y, Greenough A, et al. Preterm prelabour amniorrhexis: intrauterine infection and interval between membrane rupture and delivery. Arch Dis Child Fetal Neonatal Ed 1995;72:F43–6
- Carroll SG, Nicolaides KH. Fetal haematological response to intra-uterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther 1995;10:279–85
- Murphy DJ, Sellers S, MacKenzie IZ, et al. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet 1995;346:1449–54
- Watterberg KL, Demers LM, Scott SM, et al. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–15
- Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–40
- Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 1997;278:207–11
- Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 1997;177:406–11
- Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997;176:77–81
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 1998;5:190–201
- Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–9
- Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 1999;46:566–75
- Gravett MG, Hitti J, Hess DL, et al. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol 2000;182:1404–13
- Jobe AH, Newnham JP, Willet KE, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182:401–8
- Dammann O, Phillips TM, Allred EN, et al. Mediators of fetal inflammation in extremely low gestational age newborns. Cytokine 2001;13:234–9
- Kramer BW, Moss TJ, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 2001;164:982–8
- Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res 2001;2:27–32
- Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001;79:205–9
- Willet KE, Kramer BW, Kallapur SG, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 2002;282:L411–20
- Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–6
- Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003;8:29–38
- Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–57
- Dammann O, Leviton A. Inflammatory brain damage in preterm newborns – dry numbers, wet lab, and causal inferences. Early Hum Dev 2004;79:1–15
- Wharton KN, Pinar H, Stonestreet BS, et al. Severe umbilical cord inflammation – a predictor of periventricular leukomalacia in very low birth weight infants. Early Hum Dev 2004;77:77–87
- Witt A, Berger A, Gruber CJ, et al. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med 2005;33:22–6
- Dammann O, Leviton A, Gappa M, et al. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. BJOG 2005;112:4–9
- Moss TJ, Nitsos I, Ikegami M, et al. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol 2005;192:1179–86
- Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol 2005;25:S31–5
- Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol 2005;18:117–23
- Kim YM, Romero R, Chaiworapongsa T, et al. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology 2006;49:506–14
- Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med 2006;11:363–8
- Di Naro E, Cromi A, Ghezzi F, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol 2006;194:153–9
- Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 2006;11:354–62
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83
- Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol 2008;198:43 e41–5
- Moss TJ, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol 2008;198:122 e121–8
- Kramer BW, Kallapur SG, Moss TJ, et al. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun 2009;15:101–7
- Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92
- Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med 2011;40:19–32
- Leviton A, Hecht JL, Allred EN, et al. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol 2011;90:235–43
- Kemp MW, Saito M, Nitsos I, et al. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci 2011;18:88–98
- Leviton A, O'Shea TM, Bednarek FJ, et al. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr 2012;101:355–9
- Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:795–801
- Leviton A, Fichorova RN, O'Shea TM, et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res 2013;73:362–70
- Kuban KC, O'Shea TM, Allred EN, et al. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J Child Neurol 2014. [Epub ahead of print]
- Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 2014;75:376–80
- Hanff PA, Rosol-Donoghue JA, Spiegel CA, et al. Leptotrichia sanguinegens sp. nov., a new agent of postpartum and neonatal bacteremia. Clin Infect Dis 1995;20:S237–9
- De Martino SJ, Mahoudeau I, Brettes JP, et al. Peripartum bacteremias due to Leptotrichia amnionii and Sneathia sanguinegens, rare causes of fever during and after delivery. J Clin Microbiol 2004;42:5940–3
- Harwich MD, Jr Serrano MG, Fettweis JM, et al. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genomics 2012;13:S4
- Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 1991;165:1105–10
- Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–33
- Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–7
- Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45
- Olomu IN, Hecht JL, Onderdonk AO, et al. Perinatal correlates of Ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics 2009;123:1329–36
- Jacobsson B, Aaltonen R, Rantakokko-Jalava K, et al. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand 2009;88:63–70
- Kim M, Kim G, Romero R, et al. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med 2003;31:146–52
- Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–7
- Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–19
- Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med 2010;38:261–8
- Kacerovsky M, Celec P, Vlkova B, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One 2013;8:e60399
- Horowitz S, Mazor M, Romero R, et al. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995;40:375–9
- Gray DJ, Robinson HB, Malone J, et al. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn 1992;12:111–17
- van Rensburg HJ, Odendaal HJ. The prevalence of potential pathogenic micro-organisms in the endocervix of pregnant women at Tygerberg Hospital. S Afr Med J 1992;81:156–7
- Smith CV, Horenstein J, Platt LD. Intraamniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: a case report. Am J Obstet Gynecol 1988;159:123–4
- Chaim W, Mazor M, Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Arch Gynecol Obstet 1992;251:9–15
- Donders GG, Moerman P, Caudron J, et al. Intra-uterine Candida infection: a report of four infected fetusses from two mothers. Eur J Obstet Gynecol Reprod Biol 1991;38:233–8
- Romero R, Reece EA, Duff GW, et al. Prenatal diagnosis of Candida albicans chorioamnionitis. Am J Perinatol 1985;2:121–2
- Berry DL, Olson GL, Wen TS, et al. Candida chorioamnionitis: a report of two cases. J Matern Fetal Med 1997;6:151–4
- Qureshi F, Jacques SM, Bendon RW, et al. Candida funisitis: a clinicopathologic study of 32 cases. Pediatr Dev Pathol 1998;1:118–24
- Barth T, Broscheit J, Bussen S, et al. Maternal sepsis and intrauterine fetal death resulting from Candida tropicalis chorioamnionitis in a woman with a retained intrauterine contraceptive device. Acta Obstet Gynecol Scand 2002;81:981–2
- Crawford JT, Pereira L, Buckmaster J, et al. Amniocentesis results and novel proteomic analysis in a case of occult candidal chorioamnionitis. J Matern Fetal Neonatal Med 2006;19:667–70
- Kim SK, Romero R, Kusanovic JP, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinat Med 2010;38:45–53
- Canpolat FE, Cekmez F, Tezer H. Chorioamnionitis and neonatal sepsis due to Candida tropicalis. Arch Gynecol Obstet 2011;283:919–20
- Pineda C, Kaushik A, Kest H, et al. Maternal sepsis, chorioamnionitis, and congenital Candida kefyr infection in premature twins. Pediatr Infect Dis J 2012;31:320–2
- DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med 2012;17:2–11
- Jackel D, Lai K. Candida glabrata sepsis associated with chorioamnionitis in an in vitro fertilization pregnancy: case report and review. Clin Infect Dis 2013;56:555–8
- Ito F, Okubo T, Yasuo T, et al. Premature delivery due to intrauterine Candida infection that caused neonatal congenital cutaneous candidiasis: a case report. J Obstet Gynaecol Res 2013;39:341–3
- Iwatani S, Mizobuchi M, Sofue T, et al. Neonatal leukemoid reaction associated with Candida albicans chorioamnionitis. Pediatr Int 2014;56:277–9
- McLean LK, Chehab FF, Goldberg JD. Detection of viral deoxyribonucleic acid in the amniotic fluid of low-risk pregnancies by polymerase chain reaction. Am J Obstet Gynecol 1995;173:1282–6
- Van den Veyver IB, Ni J, Bowles N, et al. Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab 1998;63:85–95
- Wenstrom KD, Andrews WW, Bowles NE, et al. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol 1998;92:420–4
- Baschat AA, Towbin J, Bowles NE, et al. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med 2003;13:381–4
- Gervasi MT, Romero R, Bracalente G, et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med 2012;25:2002–13
- Naresh A, Simhan H. Absence of viruses in amniotic fluid of women with PPROM: a case series. J Reprod Immunol 2012;96:79–83
- Bopegamage S, Kacerovsky M, Tambor V, et al. Preterm prelabor rupture of membranes (PPROM) is not associated with presence of viral genomes in the amniotic fluid. J Clin Virol 2013;58:559–63
- Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 2010;185:1248–57
- Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol 2011;65:110–17
- Racicot K, Cardenas I, Wunsche V, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol 2013;191:934–41
- Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol 2014;71:387–90
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–71
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–5
- Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–16
- Saglam A, Ozgur C, Derwig I, et al. The role of apoptosis in preterm premature rupture of the human fetal membranes. Arch Gynecol Obstet 2013;288:501–5
- Jaiswal MK, Agrawal V, Mallers T, et al. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 2013;191:5702–13
- Lappas M. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol 2014;71:189–201
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes – an early predictor of fetal infection. Am J Obstet Gynecol 1985;152:510–16
- Vintzileos AM, Feinstein SJ, Lodeiro JG, et al. Fetal biophysical profile and the effect of premature rupture of the membranes. Obstet Gynecol 1986;67:818–23
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. Fetal biophysical profile versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol 1986;68:488–94
- Vintzileos AM, Bors-Koefoed R, Pelegano JF, et al. The use of fetal biophysical profile improves pregnancy outcome in premature rupture of the membranes. Am J Obstet Gynecol 1987;157:236–40
- Hovick Jr TJ, Vintzileos AM, Bors-Koefoed R, et al. Use of the fetal biophysical profile in severe oligohydramnios after preterm premature rupture of the membranes. J Reprod Med 1989;34:353–6
- Fleming AD, Salafia CM, Vintzileos AM, et al. The relationships among umbilical artery velocimetry, fetal biophysical profile, and placental inflammation in preterm premature rupture of the membranes. Am J Obstet Gynecol 1991;164:38–41
- Simhan HN, Canavan TP. Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG 2005;112:32–7
- Vidaeff AC, Ramin SM, Gilstrap 3rd LC. Antenatal corticosteroids in women with preterm premature rupture of the membranes. Clin Perinatol 2001;28:797–805
- Johnston MM, Sanchez-Ramos L, Vaughn AJ, et al. Antibiotic therapy in preterm premature rupture of membranes: a randomized, prospective, double-blind trial. Am J Obstet Gynecol 1990;163:743–7
- Christmas JT, Cox SM, Andrews W, et al. Expectant management of preterm ruptured membranes: effects of antimicrobial therapy. Obstet Gynecol 1992;80:759–62
- Owen J, Groome LJ, Hauth JC. Randomized trial of prophylactic antibiotic therapy after preterm amnion rupture. Am J Obstet Gynecol 1993;169:976–81
- Lewis DF, Fontenot MT, Brooks GG, et al. Latency period after preterm premature rupture of membranes: a comparison of ampicillin with and without sulbactam. Obstet Gynecol 1995;86:392–5
- Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet 1995;346:1271–9
- Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA 1997;278:989–95
- Lovett SM, Weiss JD, Diogo MJ, et al. A prospective, double-blind, randomized, controlled clinical trial of ampicillin-sulbactam for preterm premature rupture of membranes in women receiving antenatal corticosteroid therapy. Am J Obstet Gynecol 1997;176:1030–8
- Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001;357:979–88
- Ovalle A, Martinez MA, Kakarieka E, et al. Antibiotic administration in patients with preterm premature rupture of membranes reduces the rate of histological chorioamnionitis: a prospective, randomized, controlled study. J Matern Fetal Neonatal Med 2002;12:35–41
- Kenyon S, Taylor DJ, Tarnow-Mordi WO. ORACLE – antibiotics for preterm prelabour rupture of the membranes: short-term and long-term outcomes. Acta Paediatr Suppl 2002;91:12–15
- Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet 2008;372:1310–18
- Gomez R, Romero R, Nien JK, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med 2007;20:167–73
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol 1986;67:579–83
- Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–19
- Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–5
- Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1992;167:1231–42
- Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 1988;5:64–9

