Abstract
Objective: Acute histologic chorioamnionitis (HCA) is associated with an increased risk of perinatal mortality and morbidity. The purpose of this study was to determine the relationship between the intensity of intra-amniotic inflammation (IAI) and the severity of acute HCA in preterm gestation.
Methods: The relationship between the intensity of IAI and the presence and severity of acute HCA was examined in 412 patients with singleton gestations who delivered within 120 h of transabdominal amniocentesis. The concentration of amniotic fluid (AF) matrix metalloproteinase (MMP)-8 was assayed to determine the presence and intensity of IAI. Acute HCA was defined as the presence of inflammatory change in any tissue samples according to the criteria previously reported. The total grade of acute HCA was used to determine the severity of HCA.
Results: (1) Patients with IAI had a significantly higher rate of acute HCA than those without IAI [76.9% (133/173)] versus 20.9% (50/239), p < 0.001]. The AF MMP-8 concentration was significantly higher in patients with acute HCA than in those without acute HCA (median [range]; 188.3 ng/ml [0.3–6142.6] versus 1.8 ng/ml [0.3–2845.5], p < 0.001); (2) Of 183 patients with acute HCA, the AF MMP-8 concentration was positively correlated with the severity of acute HCA (p < 0.001).
Conclusions: AF MMP-8 concentration was not only a predictor of the presence of acute HCA, but its concentration also correlated with the severity of acute HCA. The higher the intensity of IAI, the worse the degree of acute HCA in preterm gestation.
Introduction
Acute histologic chorioamnionitis (HCA) is an inflammatory lesion of the placenta frequently observed after preterm or term births [Citation1–18]. The presence of acute HCA is associated with an increased risk of perinatal mortality and morbidity, including neonatal sepsis, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia and cerebral palsy [Citation19–53]. However, not all newborns whose placentas had acute HCA have adverse outcomes. In a previous study, when placental inflammation was classified according to the tissue involved (e.g. amnion, choriodecidua and/or chorionic plate), inflammation of the amnion was the most advanced stage of the maternal inflammatory response and a good predictor of early-onset neonatal sepsis [Citation54].
Intra-amniotic inflammation (IAI) and/or infection is present in about one-third of patients with preterm labor and intact membranes [Citation6, Citation20, Citation55–92] and in about half of patients with preterm premature rupture of membranes [Citation9, Citation93–101], and it is a risk factor for impending preterm delivery and adverse perinatal outcomes [Citation6, Citation27, Citation71, Citation78, Citation98, Citation99, Citation102–152]. Matrix metalloproteinase-8 (MMP-8) is a matrix-degrading enzyme stored within specific granules of neutrophils and released during activation [Citation153], and an increased concentration of MMP-8 in the amniotic fluid (AF) has been extensively used to define IAI [Citation9,Citation92,Citation98,Citation100,Citation123,Citation128,Citation137,Citation148,Citation150,Citation154–162]. Thus, this study was performed to analyze the relationship between the concentration of AF MMP-8 and the grading of HCA to examine the intensity of IAI and the severity of acute HCA.
Materials and methods
Study population
The relationship between the intensity of IAI and the presence and severity of acute HCA was examined in 412 cases delivered at Seoul National University Hospital, Seoul, Korea between January 1993 and February 2009. Patients included met the following criteria: (1) singleton gestation; (2) preterm birth at gestational age between 24 and 35 weeks; (3) transabdominal amniocentesis for microbiologic studies in AF or assessment of fetal lung maturity; (4) delivery within 120 h of amniocentesis (to preserve a meaningful temporal relationship between the results of AF studies and the histologic findings of placenta); and (5) placental histological examination after preterm delivery.
Amniocentesis was performed with written informed consent, and the Institutional Review Board of Seoul National University Hospital, Seoul, Korea approved the collection and utilization of the biological materials and clinical data for the research purposes. The Seoul National University has a Federal Wide Assurance (FWA) with the Office for Human Research Protections (OHRP) of the Department of Health and Human Services (DHHS) of the United States.
Retrieval of amniotic fluid and amniotic fluid MMP-8 concentration measurements
AF was obtained by transabdominal amniocentesis with ultrasound guide and aseptic technique. AF was cultured for aerobic and anaerobic bacteria, as well as genital mycoplasmas (ureaplasmas [Ureaplasma urealyticum & Ureaplasma parvum] and Mycoplasma hominis) or used for assessment of fetal lung maturity. The remaining AF was centrifuged, and supernatant was stored at −70 °C until assayed.
MMP-8 concentrations were measured with a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Inc., Little Chalfont, Bucks, UK) with sensitivity of 0.3 ng/ml. Both inter-and intra- assay coefficients of variation were <10%. IAI was defined as an elevated AF MMP-8 concentration (>23 ng/ml) according to the previous study [Citation154,Citation155].
Placental examination
Placental tissue samples were obtained from a chorioamniotic membrane roll (amnion and choriodecidua), chorionic plate and umbilical cord. These samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of tissue blocks were stained with hematoxylin and eosin. The degree of acute inflammation was classified as grade 1 or 2 in each tissue (amnion, choriodecidua, umbilical cord and chorionic plate) according to previously published criteria [Citation23]. Grade 1 inflammation of the choriodecidua or amnion was diagnosed as the presence of at least 1 focus of >5 neutrophils, and grade 2 inflammation of the choriodecidua or amnion was diagnosed as the presence of diffuse neutrophilic inflammation; in the chorionic plate, grade 1 inflammation was diagnosed in the presence of more than 1 focus of at least 10 neutrophilic foci or diffuse inflammation in sub-chorionic fibrin, and grade 2 inflammation was diagnosed as diffuse and dense inflammation, neutrophilic infiltration into connective tissue of placental plate or placental vasculitis. Acute HCA was defined as the presence of inflammatory change in any part of the tissue samples (amnion, choriodecidua and chorionic plate) and the highest total grade of acute HCA could be 6 if each score was 2 in all three sections. Funisitis was diagnosed as the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly. Funisitis was classified separately from acute HCA, as it is a fetal (rather than maternal) inflammatory response [Citation163–165].
Statistical analysis
Comparison of the continuous variables that could not be assumed as normal distribution was performed using the Mann–Whitney U test. Proportions were compared with the use of the Chi-square test or Fisher’s exact test. Among three or more groups, the Kruskal–Wallis test and Jonckheere–Terpstra test were used for comparison of continuous variables and linear-by-linear association was used for comparison of the proportions. Logistic regression analysis was used to examine the relationship between the presence of HCA and outcome of interest after adjusting for potential confounding factors. A probability value of <0.05 was considered statistically significant.
Results
Four hundred and twelve patients met the inclusion criteria. The prevalence of acute HCA was 44.4% (183/412). The overall rate of IAI was 42.0% (173/412); proven intra-amniotic infection was found in 18.5% (74/400). The most common microorganism cultured from AF was genital mycoplasmas (ureaplasmas [U. urealyticum & U. parvum] and M. hominis) (42/74). Other microorganisms, such as Candida spp., Streptococcus spp., Staphylococcus spp., Lactobacillus spp., Corynebacterium spp., Actinetobacter bauman, Klebsiella pneumonia, Burkholderia cepacia, Gardnerella vaginalis, Enterococcus faecalis and Escherichia coli were also isolated.
Patients with IAI had a significantly higher rate of acute HCA than those without IAI (76.9% [133/173] versus 20.9% [50/239], p < 0.001). presents the characteristics of the study population according to the presence of acute HCA. The AF MMP-8 concentration was significantly higher in patients with acute HCA than in those without HCA (median, 188.3 ng/ml [range, 0.3–6142.6] versus 1.8 ng/ml [range, 0.3–2845.5], p < 0.001). IAI and infection were also more common in patients with acute HCA than in those without HCA (72.7% [133/183] versus 17.5% [40/229] and 31.1% [56/180] versus 8.2% [18/220], p < 0.001). This difference remained significant after adjusting the gestational age at amniocentesis by logistic regression analysis.
Table 1. Clinical characteristics of patients according to the presence or absence of acute histologic chorioamnionitis.
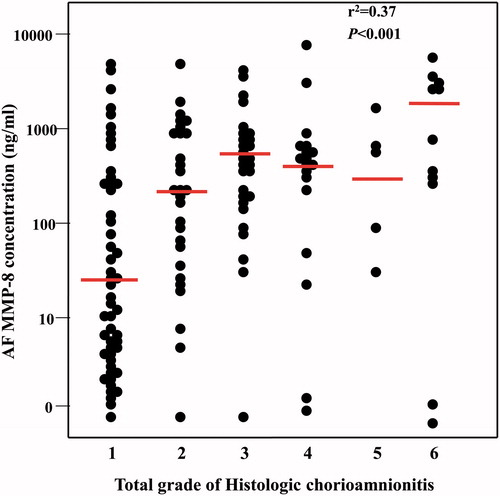
and show the relationship between AF MMP-8 concentration and the AF white blood cell (WBC) count and the prevalence of funisitis, amnionitis, proven intra-amniotic infection and IAI according to the total grade of acute HCA. The AF concentration of MMP-8 and the AF WBC count, the prevalence of funisitis, amnionitis, positive amniotic culture and IAI increased significantly as a function of the severity of acute HCA (total grade of HCA).
Table 2. The prevalence of funisitis, amnionitis and positive amniotic fluid culture according to the total grade of acute histologic chorioamnionitis.
Table 3. The prevalence of intra-amniotic inflammation and amniotic fluid matrix metalloproteinase-8 concentration, amniotic fluid white blood cell count according to the total grade of acute histologic chorioamnionitis.
There were noticeable differences between acute HCA with a total grade 1 and HCA with total grade 2 or more. First, patients with a total grade 1 HCA accounted for over 40% (78/183) of all cases of acute HCA. The prevalence of funisitis was only 19.2% in patients with HCA (grade 1), while it was over 50% in patients with acute HCA (grade 2 or more). There were no cases of amnionitis in patients with a total grade 1 histologic inflammation. Amnionitis was present in patients with total grade 2 or more HCA, and increased as the total grade of HCA became higher. The prevalence of IAI was only 51.3% in patients with total grade 1 HCA, while it was over 80% in patients with total grade 2 or higher acute HCA. The median value of AF MMP-8 concentrations and AF WBC count was also higher in patients with a total grade 2 or more acute HCA than in those with grade 1.
When the placenta was examined by region, inflammation was most common in choriodecidua (43.9%), while the frequency of inflammation of the amnion and chorionic plate was 19.9% and 14.6% for each.
shows the AF MMP-8 concentration according to the total grade of HCA; AF MMP-8 concentration was positively correlated with the total grade of acute HCA for each (r2 = 0.37, p < 0.001 by Spearman’s rho).
Discussion
Principal findings and strengths of this study
The higher the AF MMP-8 concentration, the worse the intensity of the inflammatory response in the placenta. Our study demonstrated that the AF MMP-8 concentration is an indicator of the likelihood and severity of inflammatory changes of the placenta before birth, although the placenta can only be obtained after delivery. Previous studies have suggested that there is an association between intra-amniotic infection and/or inflammation and acute HCA [Citation2,Citation9,Citation21,Citation23,Citation24,Citation54,Citation98,Citation100,Citation166–174], but the current study demonstrated the quantitative correlation between the intensity of IAI (expressed as AF MMP-8 concentration) and the severity of HCA reflected by histopathologic grading.
Severity of histologic chorioamnionitis
In a previous report, when placental inflammation was classified as affecting amnion, choriodecidua and chorionic plate, the involvement of amnion reflected the most advanced stage of the maternal inflammatory response and was a good predictor of early-onset neonatal sepsis [Citation54]. In our study, there were no cases of amnionitis in patients with total grade 1 acute HCA, but amnionitis began to appear in placentas with a total grade 2 or higher HCA and its frequency increased as the degree of acute HCA worsened. These findings were consistent with the findings of a previous study [Citation54] indicating that amnionitis reflects the most advanced form of inflammation of the extra-placental membranes.
Almost half of cases with acute HCA had a total grade 1. Funisitis and IAI were more common in patients with total grade 2 or higher of acute HCA than those with total grade 1 acute HCA, and the median AF MMP-8 concentrations or median AF WBC count was significantly elevated in patients with total grade 2 or higher HCA. Therefore, it is possible to classify HCA into two groups: mild placental inflammation with a total grade 1 and severe placental inflammation with a total grade 2 or higher.
When placentas were examined by region, inflammation was most common in the choriodecidua (43.9% of all cases). Considering that the overall frequency of HCA was 44.4%, this means that inflammation in choriodecidua was present in almost cases with acute HCA. All cases with total grade 1 HCA (except one with inflammation of chorionic plate) had inflammation because of choriodecidual involvement.
Development and progression of histologic chorioamnionitis
The pathway of intrauterine infection has not been fully elucidated; however, two pathways have been proposed [Citation54]. The first suggests that microorganisms from the lower genital tract traverse the cervix and gain access to the decidua. Bacteria can multiply at this site and cross the chorioamniotic membranes to invade the amniotic cavity. The second proposed pathway is that microorganisms traversing the cervix cross intact membranes or the rupture site in the case of preterm PROM to gain access to the amniotic cavity. In this pathway, there is no broad dissemination of the organisms in the decidua. The findings of this current study suggest that inflammation begins in the decidua. This may occur in either pathway. If the bacteria or microorganisms are located in the decidua, maternal inflammatory cells will concentrate there. On the other hand, if the organisms are in the amniotic cavity, a chemotactic gradient would be established that would bring neutrophils from the decidua to invade the chorion, and eventually, the amnion. Therefore, in either pathway, the presence of inflammatory cells in the amnion would represent the most advanced stage of inflammation. Examples of cytokine and chemokines which may be responsible for this gradient include interleukin-6 [Citation23,Citation27,Citation34,Citation60,Citation107,Citation108,Citation118,Citation123,Citation175–193], interleukin-8 [Citation27,Citation34,Citation113,Citation177–180,Citation182,Citation183,Citation186,Citation187,Citation189,Citation190,Citation194–196], interleukin-1 [Citation27,Citation178,Citation179,Citation189,Citation195], interleukin-10 [Citation189], MMP-8 [Citation9,Citation54,Citation98,Citation121,Citation128,Citation155,Citation158,Citation171,Citation197], TNF (tumor necrosis factor)-α [Citation27,Citation187,Citation189,Citation198], macrophage inhibitory cytokine 1 [Citation199], MCP (monocyte chemotactic protein)-1 [Citation185,Citation189,Citation200–204], MCP-2 and -3 [Citation205], MIP (macrophage inflammatory protein)-1α [Citation204,Citation206,Citation207], CXCL 6 [Citation208], CXCL 10 [Citation188], CXCL 13 [Citation136], sTREM-1 [Citation151], soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) [Citation209], angiopoietin-2 [Citation147], Exodus-1 [Citation210], epithelial cell-derived neutrophil-activating peptide-78 (ENA-78) [Citation211], RANTES [Citation212], GRO-α [Citation194,Citation213] and neutrophil attractant/activating peptide-1/interleukin-8 [Citation214]. They have been shown to be elevated in patients with preterm labor and intra-amniotic infection/inflammation with intact or ruptured membranes, or even during the course of spontaneous labor at term. Proteomics is a high-dimensional biology technique, and has been used to find out the biomarkers to be differentially expressed in patients with IAI [Citation138,Citation215–218].
Funisitis and amniotic fluid MMP-8 concentration
Funisitis was excluded in the analysis of this study because it reflects fetal inflammation. While acute HCA reflects a maternal immune response, funisitis is a hallmark of the fetal inflammatory response syndrome and is correlated with the plasma concentration of inflammatory cytokines such as interleukin-6, interleukin-10, or C-reactive protein in umbilical cord blood [Citation163,Citation164,Citation181,Citation219–222]. Funisitis is also a risk factor for cerebral palsy or other neonatal morbidities, such as neonatal sepsis [Citation34,Citation117,Citation155,Citation163,Citation164,Citation222–225]. A previous study [Citation155] indicated that AF MMP-8 concentration is a better predictor of funisitis than AF WBC count or the presence of a positive culture for microorganisms. In this study, the frequency of funisitis increased as a function of the severity of HCA.
In conclusion, we have demonstrated that the higher the AF MMP-8 concentration, the more severe the acute inflammatory process in the placenta. Since advanced stages of acute HCA are associated with worse perinatal outcome, we infer that the magnitude of the elevation of MMP-8 concentrations may have prognostic value.
Declaration of interest
This study was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS, by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C0768) and by grant #03-2009-0250 from the SNUH Research Fund. The authors report no conflicts of interest.
References
- Greig PC, Ernest JM, Teot L, et al. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol 1993;169:1035–44
- Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179:1254–60
- Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–78
- Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol 1990;75:622–26
- Williams MC, O'Brien WF, Nelson RN, et al. Histologic chorioamnionitis is associated with fetal growth restriction in term and preterm infants. Am J Obstet Gynecol 2000;183:1094–99
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–36
- Ustun C, Kocak I, Baris S, et al. Subclinical chorioamnionitis as an etiologic factor in preterm deliveries. Int J Gynaecol Obstet 2001;72:109–15
- Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55
- Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45
- De Paepe ME, Friedman RM, Gundogan F, et al. The histologic fetoplacental inflammatory response in fatal perinatal group B-streptococcus infection. J Perinatol 2004;24:441–45
- Holzman C, Lin X, Senagore P, et al. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol 2007;166:786–94
- Seong HS, Lee SE, Kang JH, et al. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol 2008;199:375
- Park HS, Romero R, Lee SM, et al. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta 2010;31:792–95
- Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis – a complex pathophysiologic syndrome. Placenta 2010;31:113–20
- Lee SM, Lee KA, Kim SM, et al. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta 2011;32:516–21
- Mi Lee S, Romero R, Lee KA, et al. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. J Matern Fetal Neonatal Med 2011;24:37–42
- Bersani I, Thomas W, Speer CP. Chorioamnionitis – the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med 2012;25:12–6
- Martinelli P, Sarno L, Maruotti GM, et al. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med 2012;25:29–31
- Hillier SL, Krohn MA, Kiviat NB, et al. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 1991;165:955–61
- Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28
- Romero R, Salafia CM, Athanassiadis AP, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992;166:1382–88
- Gibbs RS. Chorioamnionitis and bacterial vaginosis. Am J Obstet Gynecol 1993;169:460–62
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–40
- Arias F, Victoria A, Cho K, et al. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol 1997;89:265–71
- Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–30
- Redline RW, Wilson-Costello D, Borawski E, et al. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med 1998;122:1091–98
- Redline RW, Wilson-Costello D, Borawski E, et al. The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr Res 2000;47:721–26
- Dexter SC, Pinar H, Malee MP, et al. Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstet Gynecol 2000;96:172–77
- Elimian A, Verma U, Beneck D, et al. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol 2000;96:333–36
- Wu YW, Colford JM, Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000;284:1417–24
- Gaudet LM, Smith GN. Cerebral palsy and chorioamnionitis: the inflammatory cytokine link. Obstet Gynecol Surv 2001;56:433–36
- Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81
- Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol 2001;6:153–63
- Holcroft CJ, Askin FB, Patra A, et al. Are histopathologic chorioamnionitis and funisitis associated with metabolic acidosis in the preterm fetus? Am J Obstet Gynecol 2004;191:2010–15
- Choi CW, Kim BI, Park JD, et al. Risk factors for the different types of chronic lung diseases of prematurity according to the preceding respiratory distress syndrome. Pediatr Int 2005;47:417–23
- Dempsey E, Chen MF, Kokottis T, et al. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol 2005;22:155–59
- Andrews WW, Goldenberg RL, Faye-Petersen O, et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–8
- Rocha G, Proenca E, Quintas C, et al. Chorioamnionitis and brain damage in the preterm newborn. J Matern Fetal Neonatal Med 2007;20:745–49
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83
- Kallapur SG, Nitsos I, Moss TJ, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 2009;179:955–61
- Hendson L, Russell L, Robertson CM, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr 2011;158:397–402
- Trevisanuto D, Peruzzetto C, Cavallin F, et al. Fetal placental inflammation is associated with poor neonatal growth of preterm infants: a case-control study. J Matern Fetal Neonatal Med 2013;26:1484–90
- Tsiartas P, Kacerovsky M, Musilova I, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:1332–36
- Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:795–801
- Vedovato S, Lo Iacono A, Morando C, et al. Sensorineural hearing loss in very low birth weight infants with histological chorioamnionitis. J Matern Fetal Neonatal Med 2014;11:1–5
- Arayici S, Simsek KG, Oncel MY, et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants ≤32 weeks: a single-center study. J Matern Fetal Neonatal Med 2014;27:1129–33
- Collins JJ, Kuypers E, Nitsos I, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2012;303:L778–87
- Jobe AH. Effects of chorioamnionitis on the fetal lung. Clin Perinatol 2012;39:441–57
- Snyder CC, Wolfe KB, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol 2013;208:399.e1–8
- Wolfe KB, Snyder CC, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reprod Sci 2013;20:1447–54
- Kallapur SG, Presicce P, Rueda CM, et al. Fetal immune response to chorioamnionitis. Semin Reprod Med 2014;32:56–67
- Park CW, Moon KC, Park JS, et al. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta 2009;30:56–61
- Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med 1977;19:8–12
- Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol 1978;51:56–62
- Miller JM, Jr, Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol 1980;136:796–804
- Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52
- Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol 1981;57:483–86
- Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Watts DH, Krohn MA, Hillier SL, et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–57
- Wahbeh CJ, Hill GB, Eden RD, et al. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43
- Hameed C, Tejani N, Verma UL, et al. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol 1984;149:726–30
- Gravett MG, Hummel D, Eschenbach DA, et al. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37
- Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol 1986;67:500–6
- Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–19
- Kara M, Ozden S, Arioglu P, et al. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust N Z J Obstet Gynaecol 1998;38:403–6
- Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 1988;5:64–9
- Romero R, Scharf K, Mazor M, et al. The clinical value of gas-liquid chromatography in the detection of intra-amniotic microbial invasion. Obstet Gynecol 1988;72:44–50
- Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79
- Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24
- Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol 1989;161:813–16
- Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74
- Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–30
- Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 1991;165:1105–10
- Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1992;167:1231–42
- Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12
- Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82
- Hussey MJ, Levy ES, Pombar X, et al. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol 1998;179:650–56
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Rizzo G, Capponi A, Vlachopoulou A, et al. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol 1998;12:86–92
- Oyarzun E, Yamamoto M, Kato S, et al. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol 1998;179:1115–19
- Elimian A, Figueroa R, Canterino J, et al. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol 1998;92:72–6
- Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999;8:155–8
- Locksmith GJ, Clark P, Duff P, et al. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol 1999;94:1–6
- Ovalle A, Martinez MA, Gomez R, et al. Premature labor with intact membranes: microbiology of the amniotic fluid and lower genital tract and its relation with maternal and neonatal outcome. Rev Med Chil 2000;128:985–95
- Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–37
- Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56
- Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003;82:120–28
- Romero R, Lockwood CJ. Pathogenesis of spontaneous preterm labor. In: Creasy RK, Resnik R, Iams J, eds. Creasy and resnik's maternal-fetal medicine: principles and practice. 6th ed. Philadelphia (PA): Elsevier; 2009:521–43
- Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 2012;17:12–9
- Park CW, Kim SM, Park JS, et al. Fetal, amniotic and maternal inflammatory responses in early stage of ascending intrauterine infection, inflammation restricted to chorio-decidua, in preterm gestation. J Matern Fetal Neonatal Med 2014;27:98–105
- Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–95
- Font GE, Gauthier DW, Meyer WJ, et al. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol 1995;85:656–58
- Carroll SG, Papaioannou S, Ntumazah IL, et al. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol 1996;103:54–9
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol 1999;11:541–47
- Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292.e1–5
- DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57
- Lee SE, Romero R, Lee SM, et al. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med 2010;38:39–44
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325.e1–10
- Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol 1982;9:593–607
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–66
- McGregor JA, French JI, Lawellin D, et al. Preterm birth and infection: pathogenic possibilities. Am J Reprod Immunol Microbiol 1988;16:123–32
- Ledger WJ. Infection and premature labor. Am J Perinatol 1989;6:234–36
- Gray DJ, Robinson HB, Malone J, et al. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn 1992;12:111–17
- Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Romero R, Gomez R, Galasso M, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol 1994;171:912–21
- Horowitz S, Mazor M, Horowitz J, et al. Antibodies to Ureaplasma urealyticum in women with intraamniotic infection and adverse pregnancy outcome. Acta Obstet Gynecol Scand 1995;74:132–36
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8
- Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–76
- Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10
- Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–78
- Alexander JM, Gilstrap LC, Cox SM, et al. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 1998;91:725–29
- Wenstrom KD, Andrews WW, Hauth JC, et al. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–50
- Smulian JC, Vintzileos AM, Lai YL, et al. Maternal chorioamnionitis and umbilical vein interleukin-6 levels for identifying early neonatal sepsis. J Matern Fetal Med 1999;8:88–94
- Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–79
- Athayde N, Romero R, Maymon E, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000;182:135–41
- Vigneswaran R. Infection and preterm birth: evidence of a common causal relationship with bronchopulmonary dysplasia and cerebral palsy. J Paediatr Child Health 2000;36:293–96
- Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1149–55
- Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–6
- Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–67
- Moss TJ, Newnham JP, Willett KE, et al. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002;165:805–11
- Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21
- Berger A, Witt A, Haiden N, et al. Microbial invasion of the amniotic cavity at birth is associated with adverse short-term outcome of preterm infants. J Perinat Med 2003;31:115–21
- Ramsey PS, Lieman JM, Brumfield CG, et al. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol 2005;192:1162–66
- Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol 2006;195:1025–30
- Chaiworapongsa T, Hong JS, Hull WM, et al. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med 2008;21:663–70
- Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–16
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge' in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol 2007;30:706–14
- Soto E, Espinoza J, Nien JK, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22
- Kusanovic JP, Romero R, Mazaki-Tovi S, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med 2008;21:902–16
- Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–96
- Nhan-Chang CL, Romero R, Kusanovic JP, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2008;21:763–75
- Park CW, Lee SM, Park JS, et al. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008;36:497–502
- Romero R, Espinoza J, Rogers WT, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med 2008;21:367–88
- Vaisbuch E, Romero R, Erez O, et al. Total hemoglobin concentration in amniotic fluid is increased in intraamniotic infection/inflammation. Am J Obstet Gynecol 2008;199:426.e1–7
- Kusanovic JP, Romero R, Jodicke C, et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:1151–66
- Lee J, Oh KJ, Yang HJ, et al. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23
- Massaro G, Scaravilli G, Simeone S, et al. Interleukin-6 and Mycoplasma hominis as markers of preterm birth and related brain damage: our experience. J Matern Fetal Neonatal Med 2009;22:1063–67
- Snegovskikh VV, Schatz F, Arcuri F, et al. Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and -2 expression: implications for infection-related preterm birth. Reprod Sci 2009;16:767–80
- Soto E, Romero R, Richani K, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med 2009;22:983–92
- Vaisbuch E, Romero R, Erez O, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–16
- Gavilanes AW, Strackx E, Kramer BW, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol 2009;200:437.e1–8
- Pacora P, Romero R, Chaiworapongsa T, et al. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med 2009;37:503–11
- Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med 2010;23:1344–59
- Cruciani L, Romero R, Vaisbuch E, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med 2010;38:161–71
- Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol 2010;202:433.e1–8
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:34–47
- Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:120–30
- Balbin M, Fueyo A, Knauper V, et al. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem 1998;273:23959–68
- Angus SR, Segel SY, Hsu CD, et al. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol 2001;185:1232–38
- Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol 2001;185:1156–61
- Shim SS, Romero R, Jun JK, et al. C-reactive protein concentration in vaginal fluid as a marker for intra-amniotic inflammation/infection in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2005;18:417–22
- Biggio JR, Jr, Ramsey PS, Cliver SP, et al. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol 2005;192:109–13
- Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633
- Lee SE, Park IS, Romero R, et al. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2009;22:880–86
- Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med 2010;38:261–68
- Kim BJ, Romero R, Mi Lee S, et al. Clinical significance of oligohydramnios in patients with preterm labor and intact membranes. J Perinat Med 2011;39:131–6
- Lee J, Lee SM, Oh KJ, et al. Fragmented forms of insulin-like growth factor binding protein-1 in amniotic fluid of patients with preterm labor and intact membranes. Reprod Sci 2011;18:842–49
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–29
- Yoon BH, Romero R, Shim JY, et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90
- Blanc WA. Pathways of fetal and early neonatal infection. Viral placentitis, bacterial and fungal chorioamnionitis. J Pediatr 1961;59:473–96
- Maudsley RF, Brix GA, Hinton NA, et al. Placental inflammation and infection. A prospective bacteriologic and histologic study. Am J Obstet Gynecol 1966;95:648–59
- Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–48
- Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–37
- Yoon BH, Romero R, Moon JB, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–42
- Lee SE, Romero R, Jung H, et al. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294 e291–6
- DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056
- Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–61
- Erez O, Romero R, Tarca AL, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2009;22:1103–15
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20 (discussion 220–203)
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Allbert JR, Naef RW III, Perry KG, Jr, et al. Amniotic fluid interleukin-6 and interleukin-8 levels predict the success of tocolysis in patients with preterm labor. J Soc Gynecol Investig 1994;1:264–8
- Stallmach T, Hebisch G, Joller H, et al. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod Fertil Dev 1995;7:1573–80
- Arntzen KJ, Kjollesdal AM, Halgunset J, et al. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998;26:17–26
- Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol 1998;179:1267–70
- Rogers BB, Alexander JM, Head J, et al. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol 2002;33:335–40
- Mazzucchelli I, Avanzini MA, Ciardelli L, et al. Human amniotic fluid cells are able to produce IL-6 and IL-8. Am J Reprod Immunol 2004;51:198–203
- Holst RM, Mattsby-Baltzer I, Wennerholm UB, et al. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand 2005;84:551–57
- Jacobsson B, Mattsby-Baltzer I, Hagberg H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG 2005;112:719–24
- Holst RM, Laurini R, Jacobsson B, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med 2007;20:885–93
- Bryant-Greenwood GD, Yamamoto SY, Sadowsky DW, et al. Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta 2009;30:599–606
- Bamberg C, Fotopoulou C, Linder M, et al. Mid-trimester amniotic fluid concentrations of the proinflammatory cytokines IL-6, IL-8, TNF-alpha, and lipopolysaccharide binding protein in normal pregnancies: a prospective evaluation according to parity, gestational age, and fetal gender. J Perinat Med 2011;39:403–9
- Ogge G, Romero R, Lee DC, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol 2011;223:553–65
- Park JC, Kim DJ, Kwak-Kim J. Upregulated amniotic fluid cytokines and chemokines in emergency cerclage with protruding membranes. Am J Reprod Immunol 2011;66:310–19
- Yoneda S, Shiozaki A, Yoneda N, et al. Prediction of exact delivery time in patients with preterm labor and intact membranes at admission by amniotic fluid interleukin-8 level and preterm labor index. J Obstet Gynaecol Res 2011;37:861–66
- Marconi C, de Andrade Ramos BR, Peracoli JC, et al. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol 2011;65:549–56
- Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014;27:757–69
- Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014. [Epub ahead of print]
- Hsu CD, Meaddough E, Aversa K, et al. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol 1998;178:428–32
- Yamada T, Matsubara S, Minakami H, et al. Chemotactic activity for polymorphonuclear leukocytes: meconium versus meconium-stained amniotic fluid. Am J Reprod Immunol 2000;44:275–78
- Yamada T, Minakami H, Matsubara S, et al. Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 2000;46:21–30
- Kim SM, Romero R, Lee J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med 2012;25:1212–21
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Keelan JA, Wang K, Chaiworapongsa T, et al. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod 2003;9:535–40
- Chaiworapongsa T, Romero R, Tolosa JE, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med 2002;12:159–64
- Jacobsson B, Holst RM, Wennerholm UB, et al. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol 2003;189:1161–67
- Esplin MS, Romero R, Chaiworapongsa T, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med 2003;14:51–6
- Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73
- Miller JD, Benjamin JT, Kelly DR, et al. Chorioamnionitis stimulates angiogenesis in saccular stage fetal lungs via CC chemokines. Am J Physiol Lung Cell Mol Physiol 2010;298:L637–45
- Jacobsson B, Holst RM, Andersson B, et al. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand 2005;84:566–71
- Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994;32:108–13
- Dudley DJ, Hunter C, Mitchell MD, et al. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol 1996;87:94–8
- Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 2008;60:246–57
- Romero R, Espinoza J, Hassan S, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008;36:388–98
- Hamill N, Romero R, Gotsch F, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008;36:217–27
- Keelan JA, Yang J, Romero RJ, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod 2004;70:253–9
- Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999;181:989–94
- Cohen J, Ghezzi F, Romero R, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol 1996;35:23–9
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Bujold E, Romero R, Kusanovic JP, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med 2008;21:697–713
- Cobo T, Palacio M, Navarro-Sastre A, et al. Predictive value of combined amniotic fluid proteomic biomarkers and interleukin-6 in preterm labor with intact membranes. Am J Obstet Gynecol 2009;200:499.e1–6
- Romero R, Kusanovic JP, Gotsch F, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:261–80
- Hitti J, Lapidus JA, Lu X, et al. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol 2010;203:32.e1–8
- Kashlan F, Smulian J, Shen-Schwarz S, et al. Umbilical vein interleukin 6 and tumor necrosis factor alpha plasma concentrations in the very preterm infant. Pediatr Infect Dis J 2000;19:238–43
- Naccasha N, Hinson R, Montag A, et al. Association between funisitis and elevated interleukin-6 in cord blood. Obstet Gynecol 2001;97:220–24
- Kim CJ, Yoon BH, Park SS, et al. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol 2001;32:623–29
- Wirbelauer J, Seidenspinner S, Thomas W, et al. Funisitis is associated with increased interleukin-10 gene expression in cord blood mononuclear cells in preterm infants ≤32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol 2011;155:31–35
- Overbach AM, Daniel SJ, Cassady G. The value of umbilical cord histology in the management of potential perinatal infection. J Pediatr 1970;76:22–31
- Kim CJ, Yoon BH, Romero R, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol 2001;185:496–500
- Goepfert AR, Andrews WW, Carlo W, et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol 2004;191:1375–81