Abstract
Objective: Intra-amniotic inflammation is a mechanism of disease implicated in preterm labor, preterm prelabor rupture of membrane, cervical insufficiency, a short cervix, and idiopathic vaginal bleeding. Determination of interleukin (IL)-6 with immunoassays has been proven for more than two decades to be an excellent method for the detection of intra-amniotic inflammation. However, assessment of IL-6 for this indication has been based on immunoassays which are not clinically available, and this has been an obstacle for the implementation of this test in clinical practice. It is now possible to obtain results within 20 min with a point of care (POC) test which requires minimal laboratory support. This test is based on lateral flow-based immunoassay. The objective of this study was to compare amniotic fluid (AF) IL-6 and interferon-γ – inducible protein 10 (IP-10 or CXCL-10) concentrations determined using lateral flow-based immunoassay or POC test and standard enzyme-linked immunosorbent assay (ELISA) techniques.
Material and methods: AF samples were collected from patients with singleton gestations and symptoms of preterm labor (n = 20). AF IL-6 and IP-10 concentrations were determined by lateral flow-based immunoassay and ELISA. Intra-amniotic inflammation was defined as AF IL-6 ≥ 2.6 ng/ml. AF IL-6 and IP-10 concentrations between two assays were compared.
Results: (1) Lateral flow-based immunoassay POC AF IL-6 and IP-10 test results were strongly correlated with concentrations of this cytokine/chemokine determined by ELISA (Spearman's ρ = 0.92 and 0.83, respectively, both p < 0.0001); (2) AF IL-6 concentrations determined by the lateral flow-based immunoassay test were, on average, 30% lower than those determined by ELISA, and the median difference was statistically significant (p < 0.0001); and (3) in contrast, AF IP-10 concentrations determined by the lateral flow-based immunoassay test were, on average, only 7% lower than those determined by ELISA, and the median difference was not statistically significant (p = 0.81).
Conclusion: AF IL-6 and IP-10 concentrations determined using a lateral flow-based immunoassay POC are strongly correlated with concentrations determined by conventional ELISA. This justifies further studies about the diagnostic indices and predictive values of this POC test.
Introduction
Intra-amniotic infection and inflammation are causally linked to preterm birth [Citation1–13], which is the leading cause of perinatal morbidity and mortality [Citation14–35]. The gold standard for the diagnosis of intra-amniotic infection has relied on cultivation of bacteria in the amniotic fluid (AF) [Citation36–61]. AF culture detects microbial invasion of the amniotic cavity (MIAC) in approximately 10% of patients with preterm labor and intact membrane [Citation37,Citation38,Citation41,Citation43,Citation47,Citation50,Citation62–80], 30% of patients with preterm prelabor rupture of membrane (PROM) [Citation40,Citation46,Citation49,Citation53,Citation56,Citation60,Citation81–85] and about 50% of pregnant women with acute cervical insufficiency [Citation86–89]. Patients with a short cervix [Citation90,Citation91] or those with idiopathic vaginal bleeding [Citation92] have a frequency of MIAC of approximately 9%.
Several studies have now shown that patients with intra-amniotic inflammation have adverse pregnancy and neonatal outcome, even if there is no evidence of microorganisms in the amniotic cavity, suggesting that the diagnosis of intra-amniotic inflammation is key [Citation57,Citation58,Citation91,Citation93,Citation94].
Multiple biomarkers for the diagnosis of intra-amniotic inflammation have been used, including determinations of cytokines [Citation68,Citation73,Citation94–114], chemokines [Citation115–121], matrix-degrading enzymes [Citation122–129], and other inflammatory mediators [Citation130–140]. Parameters have ranged from a simple AF white blood cell (WBC) count [Citation49,Citation70,Citation71,Citation141] to the determination of analytes with immunoassays or bioassays [interleukin-8 (IL-8), chemotaxis] [Citation115,Citation116,Citation142,Citation143]. AF concentrations of IL-6 have been reported by several investigators to be informative and reliable indicators of the prognosis of patients at risk for preterm delivery [Citation49,Citation61,Citation100,Citation144–148]. IL-6 determinations have been performed in most studies, using research immunoassays not available for clinical management. Often, clinical laboratories in hospitals send samples to reference laboratories, and results are not available for days. What is necessary is a rapid test that can inform clinical decision-making. Several investigators have reported a rapid IL-6 test for the diagnosis of adult [Citation149] and neonatal sepsis [Citation150,Citation151], as well as inflammation of the cerebrospinal fluid [Citation152]. Kacerovsky et al. have recently reported the use of a point of care (POC) test for IL-6 in preterm prelabor rupture of membranes (PROM), which uses lateral-flow immunoassays [Citation113]. Other investigators have used determination of IL-6 in vaginal fluid [Citation153,Citation154] as a POC test.
We have described a new form of intra-amniotic inflammation that occurs in patients with preterm labor, and which is characterized by an elevation of AF IP-10, rather than IL-6 [Citation155]. This form of intra-amniotic inflammation is associated with chronic chorioamnionitis, anti-fetal HLA maternal sensitization [Citation156], and is thought to represent maternal anti-fetal rejection [Citation157]. Therefore, it may be important to have a rapid test to identify an elevation of IP-10 in AF.
The objective of this study was to determine the relationship between the AF concentrations of IL-6 and IP-10 (different markers of intra-amniotic inflammation) determined by lateral-flow immunoassays and conventional enzyme-linked immunosorbent assay (ELISA).
Methods
Study design and participants
A cross-sectional study was conducted by searching the clinical database and bank of biologic samples of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD) (Detroit, MI), to identify patients with a diagnosis of spontaneous preterm labor. Patients were included if they met the following criteria: (1) had a singleton gestation; (2) presented with preterm labor; and (3) had a transabdominal amniocentesis performed between 20 and 35 weeks of gestation with microbiologic studies. Patients were excluded from the study if they had: (1) rupture of the chorioamniotic membranes occurred before AF collection; (2) a chromosomal or structural fetal anomaly. In order to have a wide range of IL-6 for the assay comparison, we selected three groups of patients in which: (1) preterm labor delivered at term; (2) preterm labor delivered preterm without intra-amniotic infection; and (3) preterm labor and delivered preterm with intra-amniotic infection. All patients provided written informed consent; the use of biologic specimens and clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
Clinical definitions
Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 min in associated with cervical changes in patients with a gestational age between 20 and 36 6/7 weeks which led to preterm delivery (defined as birth prior to the 37th week of gestation). Acute histologic chorioamnionitis was diagnosed based on the presence of neutrophils in the chorionic plate and/or chorioamniotic membranes [Citation158–160]. Intra-amniotic inflammation was diagnosed when IL-6 AF concentration was ≥ 2.6 ng/ml [Citation57]. MIAC was defined according to the results of AF culture. Intra-amniotic infection was defined as a combination of MIAC with intra-amniotic inflammation.
Biological samples and analysis
Patients with preterm labor and intact membranes who underwent transabdominal ultrasound-guided amniocentesis for evaluating possible MIAC (within the standard of care at Hutzel Women's Hospital, Detroit, MI) were eligible for the study. AF was transported in a capped sterile syringe to the clinical laboratory where it was cultured for aerobic and anaerobic bacteria, including genital mycoplasmas. Evaluation of WBC count, glucose concentration, and Gram stain of AF were also performed shortly after collection. AF not required for clinical assessment was centrifuged for 10 min at 4 °C shortly after and stored at −70 °C until analysis. The presence of intra-amniotic infection inflammation was assessed by determination of AF IL-6 concentration by ELISA. AF IL-6 concentrations were determined for research purposes, and such results were not used in patient management.
Analysis of amniotic fluid samples for IL-6 and IP-10 concentrations
AF IL-6 and IP-10 concentrations (ng/ml) were determined both by ELISA and lateral flow-based immunoassay POC test. For ELISA, AF concentrations of IL-6 and IP-10 were determined by immunoassays obtained from R&D Systems (Minneapolis, MN). The details of these immunoassays and their performance have been previously described [Citation49,Citation57,Citation96,Citation101,Citation142,Citation161–170]. The POC determination of AF IL-6 and IP-10 concentrations (ng/ml) was performed using a lateral flow-based immunoassay POC test (Milenia QuickLine® IL-6; Milenia Biotec, Bad Nauheim, Germany). Briefly, 100 μl of AF were transferred to the test unit and after 15 min of incubation, 50 μl of buffer was added. In the first step, IL-6 present in the sample binds to a monoclonal anti-human IL-6 antibody (Ab) conjugated to gold particles. The complex of IL-6 and the conjugate continues to migrate through the analytical membrane. A test line and a control line are printed on this membrane. The test line is coated with a second anti-IL-6 Ab directed against a different epitope than the conjugate Ab. Once the complex of IL-6 and the conjugate passes the test line, it forms a sandwich with the coated IL-6 Ab. A band becomes visible PicoScan® densitometry system (Milenia Biotec, Bad Nauheim, Germany). This system measures the intensity of the test band and calculates concentrations according to standard curve. Results are typically available after 20 min and can be interpreted both visually and by densitometry. The assay time, volume and other assay characteristics for each method are shown in .
Table 1. Comparison of assay characteristics between ELISA and lateral flow-based immunoassay point of care test (POC).
Statistical analysis
The Kolmogorov–Smirnov test was used to assess normality of arithmetic data distributions. The Kruskal–Wallis test for comparisons and the Mann–Whitney U test were used to make comparisons among and between groups for arithmetic variables. Chi-square was used for comparisons of categorical variables. Correlation between AF IL-6 concentrations determined by lateral flow-based immunoassay POC and ELISA was assessed using Spearman's correlation coefficients. Bland–Altman plots were constructed to assess between-assay agreement. General linear models were fit to determine coefficients of determination (r2). Statistical analysis was performed using SPSS 19 (IBM Corp, Armonk, NY) and SAS 9.4 (Cary, NC). A p value < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the study population are displayed in . Ten of the 20 patients with preterm labor delivered at term. Three of the remaining ten patients who delivered preterm had intra-amniotic infection, and each was delivered at < 32 weeks of gestation. Organisms identified in the AF of these three patients included Gram-negative bacilli, Ureaplasma urealyticum and Gemella Morbillorum.
Table 2. Characteristics of study populations.
Correlation among amniotic fluid IL-6 concentrations determined by point of care and standard ELISA techniques
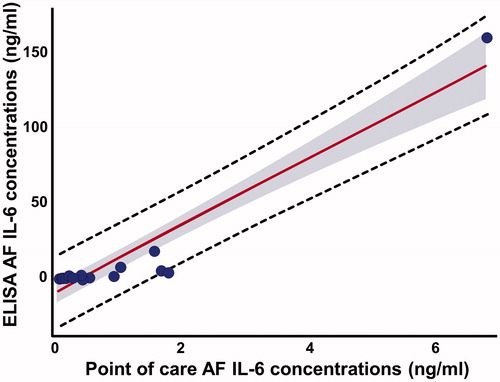
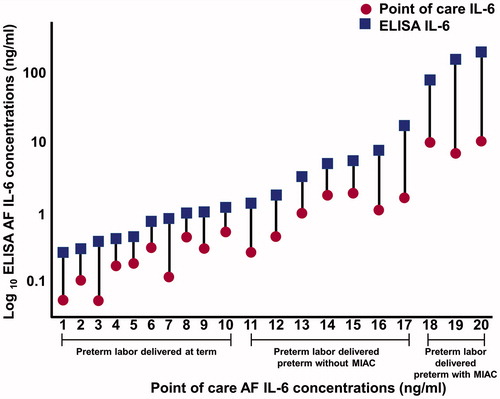
All of the samples had IL-6 concentrations above the lowest limit of detection (sensitivity) for both assays. Two samples from patients with microorganisms in the amniotic cavity had AF IL-6 concentrations that were above the standard threshold of the lateral flow-based immunoassay POC test (concentrations were high and precise calculation would have needed dilution of the sample and a new assay). Excluding these two patients, AF IL-6 concentrations determined using ELISA and the lateral flow-based immunoassay POC test were strongly correlated (Spearman ρ = 0.92; p = < 0.0001). The Bland–Altman plot analysis () shows that AF IL-6 concentrations determined by the POC test were, on average, approximately 30% lower than those determined by ELISA (Sign rank test for median difference, p < 0.0001). shows that this was a systematic observation, as it was consistent among patients. Overall, 92% of the variability in AF IL-6 as determined by ELISA was explained by the lateral flow-based immunoassay POC test results (r2 = 0.92, β = 21.9, standard error = 1.66, p < 0.0001) ().
Figure 1. Bland–Altman plot: direct comparison between enzyme-linked immunosorbent assay (ELISA) and lateral flow-based immunoassay point of care (POC) amniotic fluid (AF) interleukin-6 (IL-6) techniques. AF IL-6 concentrations from three patients with preterm labor with microbial invasion of the amniotic cavity (MIAC) were excluded. SD, standard deviation.
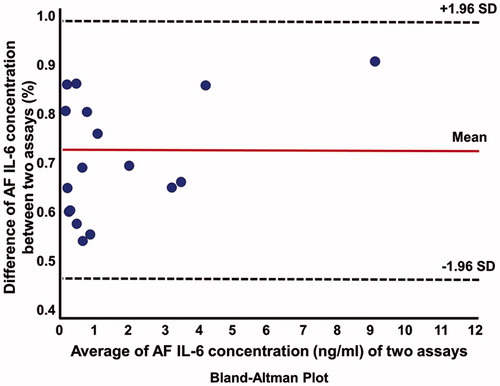
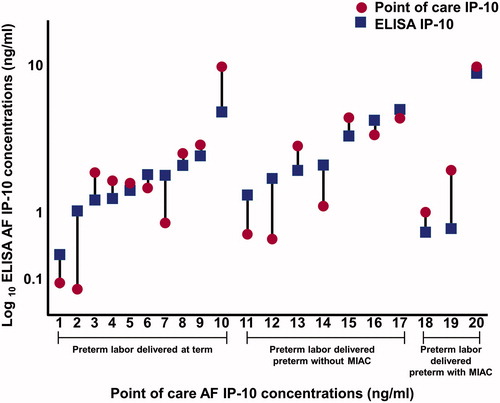
Figure 2. Amniotic fluid (AF) concentrations of interleukin-6 (IL-6) determined by enzyme-linked immunosorbent assay (ELISA) (square) and a lateral flow-based immunoassay point of care (POC) test (circle) of patients with preterm labor. Lateral flow-based immunoassay POC AF IL-6 concentrations were significantly lower than those of ELISA in every pair samples.
Correlation among amniotic fluid IP-10 concentrations determined by point of care and standard ELISA techniques
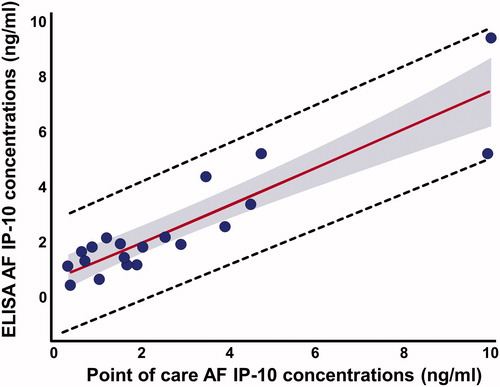
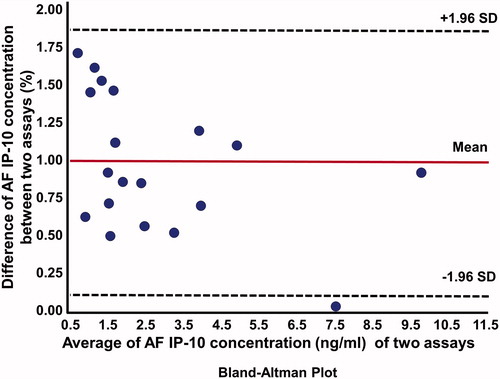
AF IP-10 concentrations determined using the POC test were strongly correlated with those determined by ELISA (Spearman ρ = 0.83; p = < 0.0001). The Bland–Altman plot for these two assays () shows that IP-10 concentrations were only approximately 7% lower, on average, than those determined by ELISA (Sign rank test for median difference, p = 0.81). demonstrated the actual concentrations in all patients between these two techniques. Overall, 80% of the variation in AF IP-10 concentrations determined by ELISA was explained by the lateral flow-based immunoassay POC test results (r2 = 0.80, β = 0.67, standard error = 0.08, p < 0.0001, ).
Figure 4. Bland–Altman plot: direct comparison between enzyme-linked immunosorbent assay (ELISA) and lateral flow-based immunoassay point of care (POC) amniotic fluid (AF) interferon-γ – inducible protein 10 (IP-10 or CXCL 10) techniques. SD, standard deviation.
Discussion
Principal findings of the study
1) The lateral flow-based immunoassay POC AF IL-6 and IP-10 test results were strongly correlated with concentrations determined by ELISA; 2) AF IL-6 concentrations determined by the lateral flow-based immunoassay were, on average, 30% lower than those determined by ELISA, and the median difference between assays was statistically significant (p < 0.0001); and 3) in contrast, AF IP-10 concentrations determined by the lateral flow-based immunoassay POC test were, on average, only 7% lower than those determined by ELISA, and the median difference between assays was not statistically significant (p = 0.81). These findings justify the need for further studies about the diagnostic indices and predictive values of these POC tests.
Point of care test for amniotic fluid IL-6
A large body of evidence indicates that patients with intra-amniotic inflammation are at greater risk for impending preterm delivery and other adverse outcomes, even when the results of AF culture are negative for microorganisms [Citation57,Citation58,Citation91,Citation93,Citation94]. Our group has previously shown that an AF IL-6 concentration > 11.3 ng/ml has a sensitivity of 93% and a specificity of 92% for the identification of positive AF culture, and also that it is predictive of preterm delivery, amniocentesis-to-delivery interval and neonatal morbidity and mortality [Citation50]. Subsequently, the AF IL-6 cut-off of 2.6 ng/ml was proposed to diagnose intra-amniotic inflammation that is associated with a shorter amniocentesis-to-delivery interval, and also with significantly higher risks of adverse neonatal outcomes, even among patients with a negative AF culture [Citation57]. The cut-off of 2.6 ng/ml has been extensively used in the literature by other investigators to identify those at risk for adverse pregnancy outcome in patients with preterm labor and intact membranes [Citation129,Citation171–173].
Patients with an elevated IL-6 but no evidence of microorganisms with cultivation methods and molecular microbiologic techniques are considered to have sterile intra-amniotic inflammation, and are at high risk for adverse pregnancy outcome in the context of preterm labor with intact membranes [Citation58,Citation59], preterm PROM [Citation60], and a short cervix [Citation91]. Therefore, the detection of an elevated IL-6 seems to be clinically important.
In the present study, we demonstrated that AF IL-6 concentrations measured using a lateral flow-based immunoassay POC test were strongly correlated with those determined using the conventional ELISA technique. These observations are consistent with findings of studies involving adult [Citation149]/neonatal sepsis [Citation150,Citation151] and sub-arachnoid hemorrhage [Citation152], which also reported that IL-6 concentrations measured with a POC test correlated strongly with those measured by ELISA.
In addition, prior studies with this POC test for IL-6 have shown that results correlate with the microbial burden of Ureaplasma sp in AF from patients with preterm PROM [Citation113], and that a cut-off of 1000 pg/ml had a 50% sensitivity and a 95% specificity for the identification of MIAC or both MIAC and acute histological chorioamnionitis in these patients [Citation113]. A high negative predictive value (97%) has also been reported for a POC vaginal fluid IL-6 concentration in identifying intra-amniotic inflammation in patients with rupture of membranes [Citation154].
The ELISA method was chosen for the comparison since this technique is currently considered the gold standard for IL-6 determination. However, ELISA results are not practical, because they are not available in time for clinical decision-making. In contrast, the POC IL-6 assay allows IL-6 determinations within 20 min and might be useful in some institutions that are not able to perform IL-6 ELISA rapidly. The assay characteristics are different; the POC test can measure IL-6 between 0.05 and 10 ng/ml for AF IL-6, while the IL-6 ELISA has a range between 0.003 ng/ml and 0.3 ng/ml. Although the assay sensitivity of the latter is much higher, repeated assays with further sample dilutions would take more time to obtain valid assay values. The standard threshold of IL-6 POC test is 10 ng/ml, however, from a clinical perspective this threshold is acceptable, since most patients with intra-amniotic inflammation and adverse perinatal outcomes have AF IL-6 concentrations > 2.6 ng/ml. Moreover, there would be no difference in the clinical management of patients with an IL-6 ± 10.
Amniotic fluid IP-10 and preterm labor: a point of care test for the identification of intra-amniotic inflammation
We have previously reported that a subset of patients with preterm labor have a different form of intra-amniotic inflammation that is associated with elevated AF IP-10 concentrations [Citation155] and a higher prevalence of chronic chorioamnionitis, the most common placental lesion in late spontaneous preterm birth. This lesion is characterized by maternal T-cell infiltration of the chorion laeve and amnion, and resembles allograft rejection [Citation157]. We have reported that chronic chorioamnionitis is associated with anti-fetal HLA maternal sensitization [Citation156] and complement deposition in umbilical vein endothelium [Citation155], which is associated with a novel form of fetal systemic inflammation characterized by overexpression of T cell chemokines, such as IP-10 or CXCL-10 [Citation174]. An elevation of mid-trimester AF IP-10 concentration is associated with late (but not early) spontaneous preterm delivery [Citation170]. Thus, it is possible that patients with an elevated CXCL-10/IP-10 are at increased risk for spontaneous preterm delivery. Similar to IL-6, ELISA is currently the gold standard for assessment of AF IP-10. Our observations in this study support that AF IP-10 determined by the POC test is strongly correlated with that determined by ELISA. Further studies are warranted to determine the diagnostic performance of IP-10 by the POC test in the identification of adverse obstetrical outcomes.
A point of care test for the identification of intra-amniotic inflammation
A simple definition for POC testing is: “Diagnostic testing performed at or near the site of patients care” [Citation175]. This testing is designed to be quick, readily available, accurate, and useful for clinical decision making and considered valuable to increase clinical efficiency and improve medical and economic outcomes [Citation175–178]. The AF lateral flow-based immunoassay POC fulfills most of the criteria proposed to assess an optimal POC, namely: 1) simple testing methodology; 2) rapid availability of the results (up to 20 min); 3) easy interpretation of the results; 4) low maintenance, because the kit can be stored at room temperature; 5) strong correlation with standard laboratory procedures (ELISA); and 6) low cost, because there is no need for capital equipment and the market price can be driven by need. Larger studies are needed to determine whether other criteria pertaining to clinical utility and cost-benefit are satisfied.
Conclusions
A POC test using lateral-flow based immunoassay for the determination of IL-6 and IP-10 concentrations in AF yields results that are strongly correlated with those determined by ELISA. Further studies are warranted to determine the diagnostic and prognostic performance of these tests in clinical obstetrics.
Declaration of interest
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C. Authors declare no conflict of interest.
References
- Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–84
- Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29
- Romero R, Gomez R, Mazor M, et al. The preterm labor syndrome. In: Elder MG, Romero R, Lamont RF, eds. Preterm labor. New York: Churchill Livingstone; 1997:29–49
- Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113:17–42
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84
- Romero R, Lockwood CJ. Pathogenesis of spontaneous preterm Labor. In: Creasy RK, Resnik R, Iams J, eds. Creasy and Resnik's maternal-fetal medicine: principles and practice, 6th ed. Philadelphia (PA): Elsevier; 2009:521–43
- Kemp MW, Saito M, Newnham JP, et al. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci 2010;17:619–28
- Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol 2011;38:385–406
- Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 2012;17:12–19
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–65
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet 2005;365:891–900
- March of dimes. Premature birth. White Plains, NY: March of dimes; 2009
- O'Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 2009;85:719–25
- Deutsch A, Deutsch E, Totten C, et al. Maternal and neonatal outcomes based on the gestational age of midtrimester preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2010;23:1429–34
- Picone S, Paolillo P. Neonatal outcomes in a population of late-preterm infants. J Matern Fetal Neonatal Med 2010;23:116–20
- World Health Organization. Born too soon: the global action report in preterm birth. WHO Library Cataloguing-in-Publication: World Health Organization; 2012
- Bastek JA, Sammel MD, Rebele EC, et al. The effects of a preterm labor episode prior to 34 weeks are evident in late preterm outcomes, despite the administration of betamethasone. Am J Obstet Gynecol 2010;203:140 e141–7
- Teune MJ, van Wassenaer AG, van Dommelen P, et al. Perinatal risk indicators for long-term neurological morbidity among preterm neonates. Am J Obstet Gynecol 2011;204:396 e391–6 e314
- Kamath BD, Marcotte MP, DeFranco EA. Neonatal morbidity after documented fetal lung maturity in late preterm and early term infants. Am J Obstet Gynecol 2011;204:518 e511–18
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72
- Keunen K, Kersbergen KJ, Groenendaal F, et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J Matern Fetal Neonatal Med 2012;25:89–100
- Ferrari F, Gallo C, Pugliese M, et al. Preterm birth and developmental problems in the preschool age. Part I: minor motor problems. J Matern Fetal Neonatal Med 2012;25:2154–59
- Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med 2012;25:1428–32
- Grobman WA, Lai Y, Rouse DJ, et al. The association of cerebral palsy and death with small-for-gestational-age birthweight in preterm neonates by individualized and population-based percentiles. Am J Obstet Gynecol 2013;209:340 e341–5
- Hamilton BE, Hoyert DL, Martin JA, et al. Annual summary of vital statistics: 2010–2011. Pediatrics 2013;131:548–58
- Sun L, Yue H, Sun B, et al. Estimation of birth population-based perinatal-neonatal mortality and preterm rate in China from a regional survey in 2010. J Matern Fetal Neonatal Med 2013;26:1641–8
- Acaia B, Crovetto F, Ossola MW, et al. Predictive factors for neonatal survival in women with periviable preterm rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:1628–34
- Strunk T, Inder T, Wang X, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62
- Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet 2014;384:189–205
- Herber-Jonat S, Streiftau S, Knauss E, et al. Long-term outcome at age 7-10 years after extreme prematurity – a prospective, two centre cohort study of children born before 25 completed weeks of gestation (1999–2003). J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Bastek JA, Weber AL, McShea MA, et al. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol 2014;210:450.e1–450.e10
- Manuck TA, Sheng X, Yoder BA, et al. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol 2014;210:426 e421–29
- Garite TJ, Freeman RK, Linzey EM, et al. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol 1979;54:226–30
- Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52
- Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol 1981;57:483–86
- Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol 1983;62:137–44
- Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol 1984;63:38–43
- Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol 1986;67:500–6
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–66
- Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–19
- Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79
- Asrat T, Nageotte MP, Garite TJ, et al. Gram stain results from amniocentesis in patients with preterm premature rupture of membranes--comparison of maternal and fetal characteristics. Am J Obstet Gynecol 1990;163:887–89
- Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol 1991;31:331–36
- Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–30
- Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–33
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med 1993;38:543–48
- Gomez R, Romero R, Galasso M, et al. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–10
- Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol 1999;11:541–7
- Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003;82:120–8
- Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2003;82:423–31
- Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66:316–21
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6
- Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation is more frequent than microbial-associated intra-amniotic inflammation in preterm labor with intact membranes. Am J Reprod Immunol (submitted). 2014. [Epub ahead of print]
- Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58
- Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med (submitted). 2014. [Epub ahead of print]
- Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014;27:757–69
- Miller JM Jr, Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol 1980;136:796–804
- Wahbeh CJ, Hill GB, Eden RD, et al. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43
- Hameed C, Tejani N, Verma UL, et al. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol 1984;149:726–30
- Gravett MG, Hummel D, Eschenbach DA, et al. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37
- Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24
- Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol 1989;161:813–16
- Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74
- Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 1991;165:1105–10
- Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1992;167:1231–42
- Watts DH, Krohn MA, Hillier SL, et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–57
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Hussey MJ, Levy ES, Pombar X, et al. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol 1998;179:650–56
- Rizzo G, Capponi A, Vlachopoulou A, et al. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol 1998;12:86–92
- Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82
- Elimian A, Figueroa R, Canterino J, et al. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol 1998;92:72–6
- Oyarzun E, Yamamoto M, Kato S, et al. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol 1998;179:1115–19
- Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999;8:155–8
- Locksmith GJ, Clark P, Duff P, et al. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol 1999;94:1–6
- Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol 1982;59:539–45
- Zlatnik FJ, Cruikshank DP, Petzold CR, et al. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med 1984;29:656–60
- Feinstein SJ, Vintzileos AM, Lodeiro JG, et al. Amniocentesis with premature rupture of membranes. Obstet Gynecol 1986;68:147–52
- Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 1988;5:64–69
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–91
- Mays JK, Figueroa R, Shah J, et al. Amniocentesis for selection before rescue cerclage. Obstet Gynecol 2000;95:652–55
- Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633 e631–38
- Bujold E, Morency AM, Rallu F, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can 2008;30:882–87
- Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–9
- Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalance and clinical significance. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–7
- Lee SE, Romero R, Jung H, et al. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294 e291–6
- Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e121–5 e115
- Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20 (discussion 220–3)
- Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–87
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–8
- Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Athayde N, Romero R, Maymon E, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000;182:135–41
- Leslie KK, Lee SL, Woodcock SM, et al. Acute intrauterine infection results in an imbalance between pro- and anti-inflammatory cytokines in the pregnant rabbit. Am J Reprod Immunol 2000;43:305–11
- Blank V, Hirsch E, Challis JR, et al. Cytokine signaling, inflammation, innate immunity and preterm labour – a workshop report. Placenta 2008;29:S102–4
- Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod 2010;83:767–73
- Cobo T, Kacerovsky M, Palacio M, et al. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS One 2012;7:e43677
- Cobo T, Kacerovsky M, Holst RM, et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012;91:930–5
- Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical and vaginal fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014 . [Epub ahead of print]
- Cobo T, Jacobsson B, Kacerovsky M, et al. Systemic and local inflammatory response in women with preterm prelabor rupture of membranes. PLoS One 2014;9:e85277
- Kacerovsky M, Musilova I, Jacobsson B, et al. Vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor membrane ruptures. J Matern Fetal Neonatal Med 2014 . [Epub ahead of print]
- Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014 . [Epub ahead of print]
- Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014 . [Epub ahead of print]
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325 e321–25 e310
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 1993;169:1299–303
- Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994;32:108–13
- Cohen J, Ghezzi F, Romero R, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol 1996;35:23–9
- Hsu CD, Meaddough E, Aversa K, et al. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol 1998;178:428–32
- Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–53
- Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–20
- Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–9
- Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–53
- Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–16
- Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002;12:237–46
- Park KH, Chaiworapongsa T, Kim YM, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med 2003;31:12–22
- Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292 e291–95
- Romero R, Quintero R, Emamian M, et al. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454–60
- Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–7
- Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–61
- Mazor M, Wiznitzer A, Maymon E, et al. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci. 1990;26:425–8
- Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–96
- Soto E, Romero R, Richani K, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med 2009;22:983–92
- Vaisbuch E, Romero R, Erez O, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–16
- Cobo T, Palacio M, Martinez-Terron M, et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol 2011;205:126 e121–28
- Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–19
- Kacerovsky M, Drahosova M, Krejsek J, et al. Amniotic fluid CD200 levels in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:1416–24
- Andrys C, Kacerovsky M, Drahosova M, et al. Amniotic fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:520–7
- Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–5
- Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–30
- Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10
- Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11
- Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–8
- El-Bastawissi AY, Williams MA, Riley DE, et al. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol 2000;95:1056–64
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010;116:393–401
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, et al. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG 2011;118:1042–54
- Schefold JC, Hasper D, von Haehling S, et al. Interleukin-6 serum level assessment using a new qualitative point-of-care test in sepsis: a comparison with ELISA measurements. Clin Biochem 2008;41:893–8
- Meem M, Modak JK, Mortuza R, et al. Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. J Glob Health 2011;1:201–9
- Batfalsky A, Lohr A, Heussen N, et al. Diagnostic value of an interleukin-6 bedside test in term and preterm neonates at the time of clinical suspicion of early- and late-onset bacterial infection. Neonatology 2012;102:37–44
- Dengler J, Schefold JC, Graetz D, et al. Point-of-care testing for interleukin-6 in cerebro spinal fluid (CSF) after subarachnoid haemorrhage. Med Sci Monit 2008;14:BR265–68
- Vousden N, Chandiramani M, Seed P, et al. Interleukin-6 bedside testing in women at high risk of preterm birth. J Matern Fetal Neonatal Med 2011;24:1301–4
- Berthiaume M, Rousseau E, Rola-Pleszczynski M, et al. Rapid evaluation of the absence of inflammation after rupture of membranes. J Matern Fetal Neonatal Med 2014;27:865–9
- Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010;23:1000–11
- Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol 2011;66:510–26
- Lee J, Kim JS, Park JW, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta 2013;34:681–9
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Redline RW, Heller D, Keating S, et al. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta 2005;26:S114–17
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine 1991;3:155–63
- Romero R, Galasso M, Gomez R, et al. A comparative study of the value of amniotic fluid interleukin-6, white blood cell count and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients with spontaneous labor at term. Annual Meeting of the Society of Perinatal Obstetricians; 1998; Las Vegas, NV, A250
- Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12
- Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179:1254–60
- Yoon BH, Romero R, Moon JB, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–42
- Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24
- DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056
- DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–13
- Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med 2010;38:275–9
- Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med 2012;40:329–43
- Gomez R, Ghezzi F, Romero R, et al. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995;22:281–342
- Lee J, Oh KJ, Yang HJ, et al. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23
- Kim SM, Romero R, Lee J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med 2012;25:1212–21
- Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–84
- Gutierres SL, Welty TE. Point-of-care testing: an introduction. Ann Pharmacother 2004;38:119–25
- Kost GJ, Tran NK. Point-of-care testing and cardiac biomarkers: the standard of care and vision for chest pain centers. Cardiol Clin 2005;23:467–90, vi
- Lewandrowski K. Point-of-care testing: an overview and a look to the future (circa 2009, United States). Clin Lab Med 2009;29:421–32
- Pfafflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem 2009;393:1473–80