Abstract
Objective: Acute atherosis is characterized by subendothelial lipid-filled foam cells, fibrinoid necrosis and perivascular lymphocytic infiltration. This lesion is generally confined to non-transformed spiral arteries and is frequently observed in patients with preeclampsia. However, the frequency of acute atherosis in the great obstetrical syndromes is unknown. The purpose of this study was to determine the frequency and topographic distribution of acute atherosis in placentas and placental bed biopsy samples obtained from women with normal pregnancy and those affected by the “great obstetrical syndromes”. We also examined the relationship between acute atherosis and pregnancy outcome in patients with preeclampsia.
Material and methods: A retrospective cohort study of pregnant women who delivered between July 1998 and July 2014 at Hutzel Women’s Hospital/Detroit Medical Center was conducted to examine 16 345 placentas. Patients were classified into the following groups: (1) uncomplicated pregnancy; (2) spontaneous preterm labor (sPTL) and preterm prelabor rupture of membranes (PPROM); (3) preeclampsia; (4) gestational hypertension; (5) small-for-gestational age (SGA); (6) chronic hypertension; (5) fetal death; (6) spontaneous abortion and (7) others. A subset of patients had placental bed biopsy. The incidence of acute atherosis was compared among the different groups.
Results: (1) The prevalence of acute atherosis in uncomplicated pregnancies was 0.4% (29/6961) based upon examination of nearly 7000 placentas; (2) the frequency of acute atherosis was 10.2% (181/1779) in preeclampsia, 9% (26/292) in fetal death, 2.5% (3/120) in midtrimester spontaneous abortion, 1.7% (22/1,298) in SGA neonates and 1.2% (23/1,841) in sPTL and PPROM; (3) among patients with preeclampsia, those with acute atherosis than in those without the lesion had significantly more severe disease, earlier onset, and a greater frequency of SGA neonates (p < 0.05 all) and (4) the lesion was more frequently observed in the decidua (parietalis or basalis) than in the decidual segment of the spiral arteries in patients with placental bed biopsies.
Conclusions: Acute atherosis is rare in normal pregnancy, and occurs more frequently in patients with pregnancy complications, including preeclampsia, sPTL, preterm PROM, midtrimester spontaneous abortion, fetal death and SGA.
Introduction
Acute atherosis is a unique lesion observed in the spiral arteries characterized by fibrinoid necrosis of the vessel wall, which is often accompanied by the collection of foamy, fat-laden macrophages beneath the intima of the arteries and an inflammatory infiltrate of the vessel wall [Citation1–48]. The similarities with lesions observed in early stages of atherosclerosis and allograft rejection (i.e. kidney transplants) [Citation26,Citation49] suggest that intravascular inflammation and hyperlipidemia might be responsible for the generation of atherosis [Citation44,Citation46,Citation47,Citation50–54].
Although acute atherosis was originally described in the spiral arteries of patients with preeclampsia [Citation2–4,Citation6–12,Citation15–18,Citation20,Citation22,Citation23,Citation25–30,Citation39–41,Citation43–47], this lesion is not specific, and has been reported in normal pregnancy [Citation3,Citation4,Citation21,Citation30,Citation41], as well as those complicated by diabetes mellitus [Citation5,Citation15,Citation24], gestational and chronic hypertension [Citation15,Citation24,Citation27,Citation28], systemic lupus erythematosus, and antiphospholipid antibody syndrome [Citation14,Citation24,Citation31,Citation34,Citation37], as well as intrauterine fetal growth restriction (IUGR) [Citation13,Citation16,Citation19,Citation20,Citation22,Citation32]. The prevalence of acute atherosis in spontaneous midtrimester abortion, spontaneous preterm labor (sPTL), preterm prelabor rupture of membranes (PPROM) and unexplained fetal death, however, is unknown.
Failure of physiologic transformation was originally described in preeclampsia, but is also observed in many of the “great obstetrical syndromes” [Citation55–62]. Therefore, it is possible that acute atherosis may occur in other complications of pregnancy; yet, the frequency of acute atherosis in the great obstetrical syndromes is lacking and its association with adverse pregnancy outcomes remains unclear [Citation3,Citation4,Citation15,Citation23,Citation25,Citation27,Citation41,Citation45]. The purpose of this study was to determine the frequency and the topographic distribution of acute atherosis in the placentas and placental bed biopsy samples obtained from women with normal pregnancies and those affected by the great obstetrical syndromes. We also examined the relationship between acute atherosis and pregnancy outcomes in patients with preeclampsia.
Materials and methods
We undertook a retrospective cohort study of pregnant women who delivered between July 1998 and July 2014 at Hutzel Women’s Hospital/Detroit Medical Center and had pathologic examination of the placenta. From this cohort, a subset had placental bed biopsies performed at the time of cesarean delivery. The following groups were excluded from this study: (1) fetal congenital anomaly; (2) multiple gestations; (3) missing clinical data and (4) indicated/elective abortion. All women provided written informed consent prior to the collection of placentas and placental bed biopsy samples. The collection and utilization of the samples were approved by the Human Investigation Committee of Wayne State University and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS).
Clinical definitions
Term delivery without obstetrical complications
Patients without medical or surgical complications of pregnancy who delivered a normal term (≥37 weeks) neonate whose birth weight was between the 10th and 90th percentile for gestational age.
Spontaneous preterm labor (sPTL)
Patients between 20–36 6/7 weeks of gestation who presented with spontaneous labor and intact membranes and delivered prior to 37 weeks of gestation.
Preterm prelabor rupture of membranes (PPROM)
PPROM was diagnosed in the presence of the following criteria: (1) delivery <37 weeks of gestation; (2) history of leaking of fluid from the vagina; and (3) positive pooling of vaginal fluid and positive nitrazine test. A positive ferning test was considered confirmatory, but not necessary, for the diagnosis of PPROM.
Preeclampsia
Defined as new-onset hypertension developing after 20 weeks of gestation (systolic or diastolic blood pressure ≥140 or ≥90 mmHg, respectively, measured at two different time points, 4 h to one week apart) in the presence of proteinuria (≥300 mg in a 24 h urine collection, or two random urine specimens obtained 4 h to one week apart demonstrating ≥1+ protein by dipstick, or one dipstick demonstrating ≥2+ protein) [Citation63]. Severe preeclampsia was defined as previously described [Citation63]. Patients with preeclampsia were also classified as “early” (≤34 weeks) or “late” (>34 weeks) preeclampsia according to the gestational age at delivery. Chronic hypertension with superimposed preeclampsia was diagnosed in women with hypertension documented before 20 weeks of gestation with new-onset proteinuria or in women with hypertension and proteinuria at <20 weeks of gestation with a sudden increase in proteinuria, blood pressure in women whose hypertension was previously well controlled, thrombocytopenia and elevated liver transaminase enzymes [Citation63].
Gestational hypertension
Defined as a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg on at least two determinations 4 h to one week apart without proteinuria (dipstick <1+ or 24 h urine protein <300 mg).
Chronic hypertension
Women with hypertension (systolic or diastolic blood pressure ≥140 or ≥90 mmHg, respectively, measured at two different time points, 4 h to one week apart) before 20 weeks of gestation or those who reported a history of hypertension.
Small-for-gestational age (SGA)
Neonates with birth weight <10th percentile for gestational age, according to the reference range [Citation64,Citation65].
Fetal death
Defined as death of the fetus after 20 weeks of gestation diagnosed by ultrasound examination. Fetuses with known congenital and/or chromosomal abnormalities were excluded. This group was classified according to clinical circumstances into: (1) unexplained fetal death (n = 4); (2) fetal death with preeclampsia (n = 14) and (3) others which included abruptio placentae (n = 8).
Spontaneous abortion
Fetal loss between 10 and 20 completed weeks of gestation.
Others
This group included indicated preterm delivery due to fetal/maternal conditions which were not included in the groups above, such as abruptio placentae, placenta previa, placenta accreta and pregnancy with maternal underlying medical conditions.
Each patient with pregnancy complications was classified according to a mutually exclusive schema which placed priority in the following order: (1) fetal death; (2) pregnancy-associated hypertension (preeclampsia, gestational hypertension, preeclampsia superimposed chronic hypertension and chronic hypertension); (3) spontaneous preterm birth (sPTL and PPROM) and (4) others. The SGA group in the current study included patients with SGA neonates without fetal death, pregnancy-associated hypertension and spontaneous preterm birth. Hence, a pregnancy that was affected by preeclampsia, yet resulted in a fetal death, would be grouped in the fetal death study group rather than in the preeclampsia study group.
Placental specimens
After delivery, placentas were transported to the laboratory and examined by trained personnel according to methods previously described by our group [Citation66]. Tissue samples obtained from each placenta included one roll of chorioamniotic membranes and one of the umbilical cord. Two sections were taken from each the chorionic and basal plate. Tissues were formalin-fixed and embedded in paraffin. Five-micrometer sections of tissue blocks were stained with hematoxylin and eosin (H&E) and the slides were examined by perinatal pathologists masked to clinical outcomes. In a small subset of patients, placental bed biopsy specimens were obtained at the time of cesarean delivery according to techniques previously described [Citation67].
Criteria for histopathologic diagnosis
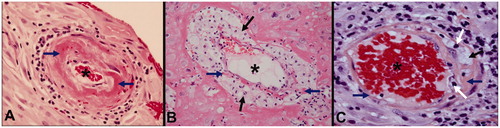
Atherosis was diagnosed by the presence of fibrinoid necrosis of the spiral artery wall with the presence of lipid laden macrophages in the lumen and a perivascular lymphocytic infiltrate [Citation28]. shows a normal spiral artery and several examples of acute atherosis with fibrinoid necrosis, foamy macrophages and inflammatory infiltration of the vessel wall.
Figure 1. Acute atherosis in the decidual segment of spiral arteries (Hematoxylin and Eosin, ×400). (A) Fibrinoid necrosis (blue arrow) and few chronic inflammatory cells within the vessel wall but no macrophages; (B) mainly lipid-laden macrophages (black arrows) with minor fibrinoid necrosis (blue arrow) in the vessel wall and (C) fibrinoid necrosis (blue arrow) with lipid-laden macrophages (black arrow) as well as chronic inflammatory cells (white arrows) in the vessel wall and perivascular areas. * Lumen of spiral artery.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the distributions of arithmetic data. The Kruskal–Wallis test and the Mann–Whitney U test were used to make comparisons among and between groups for arithmetic variables. Chi-square or McNemar–Bowker tests were used for comparisons of categorical variables. Statistical analysis was performed using SPSS 19 (IBM Corp, Armonk, NY) and SAS 9.3 (Cary, NC). A p value <0.05 was considered statistically significant.
Results
Among 16 345 women who were enrolled and delivered at Hutzel Women’s Hospital between July 1998 and July 2014, 9.5% (1559/16 345) were excluded from this study due to the following: clinical information was incomplete [4.2% (694/16 345)], fetal anomalies [1.9% (304/16 345)] or multiple pregnancies [3.4% (561/16 345)], leaving 14 786 cases for analysis. Among these, 47.1% (6961/14 786) had normal term delivery, 12.5% (1841/14 786) had spontaneous preterm delivery or PPROM, 12% (1779/14 786) were diagnosed with preeclampsia, 10.8% (1597/14 786) had gestational hypertension, 8.8% (1298/14 786) had SGA neonates and 4.7% (698/14 786) had chronic hypertension. The frequency of pregnancy complications in this study is described in . A total of 543 placental bed biopsies were available for examination.
Table 1. Clinical diagnosis of patients included in the study.
Frequency of atherosis according to pregnancy outcome
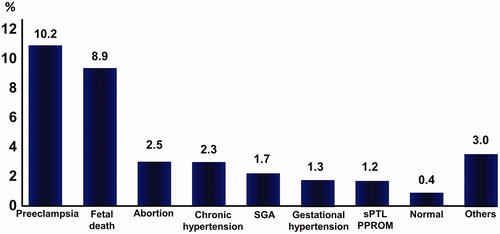
Acute atherosis was more frequently identified in patients with preeclampsia [10.2% (181/1779), fetal death [8.9% (26/292)], midtrimester spontaneous abortion [2.5% (3/120)], chronic hypertension without preeclampsia [2.3% (16/698)], SGA alone [1.7% (22/1298)], gestational hypertension [1.3% (20/1597)], sPTL and PPROM [1.2% (23/1841)] and others [3% (6/200)] than in those with uncomplicated pregnancies [0.4% (29/6961)] (p < 0.001 for all) ().
Figure 2. Frequency of acute atherosis according to pregnancy outcomes. Each patient with pregnancy complications was classified according to a mutually exclusive schema. Abortion: midtrimester abortion, SGA: small-for-gestational age, sPTL: spontaneous preterm labor, PPROM: preterm prelabor rupture of membranes. SGA group included patients with SGA neonates without fetal death, pregnancy associated hypertension and spontaneous preterm birth. Comparison between each pregnancy complication and term delivery: p < 0.001 for all.
Among patients with preeclampsia (n = 1779), those with acute atherosis had a higher frequency of preterm delivery, a lower median birth weight, higher frequencies of SGA, severe preeclampsia and early preeclampsia than those without this lesion ().
Table 2. Clinical characteristics and pregnancy outcome of patients with preeclampsia according to the presence or absence of acute atherosis.
The topographic distribution of acute atherosis
Acute atherosis was observed more frequently in the decidua parietalis (chorioamniotic membranes) and basal plate of the placenta (). There was a significantly higher frequency of acute atherosis lesions in the placenta (both basal plate and chorioamnion) than in placental bed biopsies (both decidua and myometrial segment) (p < 0.001) (). Among women with preeclampsia, patients with acute atherosis lesions in the myometrial segment from placental bed biopsy (n = 19/71) had a significantly lower median (IQR) gestational age at delivery (weeks) than those without this lesion in the myometrial segment (n = 53/71) [27.4 (25.3–30.7) versus 31.9 (28.6-35.6); p = 0.005], indicating that the depth of the lesion is associated with the severity of preeclampsia.
Table 3. Distribution of acute atherosis according to histologic sections examined in the placenta and placental bed biopsies.
Discussion
Principal findings
(1) The prevalence of acute atherosis in uncomplicated pregnancies was 0.4% based upon examination of nearly 7000 placentas; (2) the frequency of acute atherosis varied with the specific obstetrical syndrome – preeclampsia, 10%; fetal death, 9%; midtrimester spontaneous abortion, 2.5%; SGA neonates (without preeclampsia), 1.7%; sPTL, 1.2%; (3) among patients with preeclampsia, those with acute atherosis had a more severe form, earlier onset and a greater frequency of SGA neonates; and (4) the lesion was more frequently observed in the decidua (parietalis or basalis) of placental specimens than in the decidual segment of the spiral arteries in patients with placental bed biopsies.
Relationship between acute atherosis and clinical severity of preeclampsia
We report herein that the presence of acute atherosis is associated with severe and early preeclampsia, an observation that is consistent with other reports [Citation4,Citation45]. The link probably reflects the association among severe intravascular inflammation [Citation68–77], immune dysregulation [Citation78–80] in patients with early-onset and severe disease and the degree of defective deep placentation of spiral arteries [Citation61] in this subpopulation of patients.
The distribution of acute atherosis and depth of the process in the spiral arteries
Topographically, acute atherosis is typically present in spiral arteries with failure of physiologic transformation, usually in the decidual segment [Citation11,Citation28,Citation29,Citation48]. However, the lesion has also been observed in the myometrial section of the spiral arteries. The observations reported in this study are in keeping with those in the literature indicating that acute atherosis affects the distal segment of the spiral arteries. It is possible that the presence of atherosis in the myometrial segment is indicative of more extensive disease, which would be clinically manifested as early and severe preeclampsia.
Possible mechanisms implicated in the genesis of acute atherosis
The interested reader is referred to recent reviews on the pathophysiology of acute atherosis [Citation44,Citation46–48]. Briefly, the mechanisms implicated include: (1) shear flow stress caused by abnormal blood flow in inadequate remodeled spiral arteries; (2) decidual inflammation induced by an immune response to trophoblasts or danger signals in the decidua; (3) an exaggerated systemic maternal inflammatory response which creates conditions similar to those observed in atherosclerosis and favors the generation of arterial wall lesions; and (4) maternal genetic predisposition, given that a polymorphism in RGS2 (regulator of G protein signaling) increases the risk of preeclampsia and acute atherosis [Citation81]. The relative contributions of each of these mechanisms have not been determined.
We report herein that acute atherosis can be observed in unexplained fetal death, spontaneous midtrimester abortion, spontaneous preterm birth and PPROM. Normal pregnancy is characterized by physiologic intravascular inflammation, demonstrated by a change in the immunophenotype of granulocytes and monocytes, and increased production of reactive oxygen species [Citation82,Citation83], as well as an increase in acute phase reactants during normal pregnancy (fibrinogen [Citation84], C-reactive protein [Citation85], etc.). In complications of pregnancy such as sPTL with intact membranes, [Citation86–92], PPROM [Citation93–98], preeclampsia [Citation99–128], SGA [Citation103,Citation118,Citation120,Citation122,Citation129–138] and pyelonephritis [Citation83,Citation139–141], intravascular inflammation is increased compared to normal pregnancy. These observations support the hypothesis that an exaggerated intravascular inflammatory response may play a role in the genesis of acute atherosis in the susceptible patient.
The frequency of atherosis in preeclampsia reported in the present study is lower than that typically observed in other studies [Citation4,Citation6,Citation7,Citation9–12,Citation15–18,Citation22,Citation23,Citation25–30,Citation39,Citation41,Citation44,Citation45], yet prevalence ranging from 5% to 40% has been reported. The lower prevalence reported herein might be attributable to differences in case definition, or since most prior studies performed a deliberate search for acute atherosis, and oursrsquo; involved data collected during the course of ongoing research and clinical care, ascertainment bias may have inflated prior estimates.
Strengths and limitations
The frequency of any lesion in histopathologic studies is a function of sampling of the organ, the definition of the lesion, the methods used for staining of tissues and recognizing specific features (such as macrophages, fibrinoid necrosis, the deposition of lipids, etc.). The findings of this study reflect the practice of placental pathology worldwide. We used H&E, as this is the standard immunohistochemical staining. In previous reports in which we have focused on the study of the placental bed, we used Periodic Acid Schiff (PAS) to detect fibrinoid necrosis and cytokeratin to identify interstitial trophoblast [Citation57,Citation58,Citation67]. Neither of these methods was used in this study. The original description of atherosis included the presence of lipid-laden macrophages within the spiral arteries. Macrophage markers such as CD-68, as well as immunohistochemistry staining to identify lipids (oil-red O, sudan black B, etc.), [142] could be used to identify macrophages and lipid deposition in future studies. Staining for smooth-muscle actin could be used to determine whether there is loss of the smooth muscle in the spiral arteries. Atherosis typically occurs in physiologically non-transformed spiral arteries in which the smooth muscle in the media has not been replaced by fibrinoid. As studies of the Human Placenta Project move forward, a more in-depth characterization of acute atherosis and other lesions can be undertaken by increasing the number of sections - particularly those of the basal plate of the placenta. Alnaes-Katjavivi et al. emphasized that the standard definition of acute atherosis lacks quantitative criteria, and could be subject to observer bias, and proposed new diagnostic criteria using quantitative methods [143]. We agree that such an approach would be useful in assessing the frequency and clinical significance of acute atherosis in future studies.
Conclusion
Acute atherosis is rare in normal pregnancy, occurs more frequently in patients with pregnancy complications including preeclampsia, sPTL, preterm PROM, midtrimester spontaneous abortion, fetal death and SGA, and is associated with poorer pregnancy outcomes in women with preeclampsia.
Declaration of interest
The authors report no conflicts of interest.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
Notes
* Presented at the Twenty-third Annual Meeting of the Society for Maternal-Fetal Medicine, San Francisco, 4–8 February 2003.
References
- Hertig AT. Vascular pathology in the hypertensive albuminuric toxemias of pregnancy. Clinics 1945;4:602–14
- Sexton LI, Hertig AT, Reid DE, et al. Premature separation of the normally im planted placenta; a clinicopathological study of 476 cases. Am J Obstet Gynecol 1950;59:13–24
- Zeek PM, Assali NS. Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy. Am J Clin Pathol 1950;20:1099–109
- Maqueo M, Chavezazuela J, Dosaldelavega M. Placental pathology in eclampsia and preeclampsia. Obstet Gynecol 1964;24:350–6
- Driscoll SG. The pathology of pregnancy complicated by diabetes mellitus. Med Clin North Am 1965;49:1053–67
- Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol 1967;93:581–92
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972;1:177–91
- Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw 1972;79:794–9
- Emmrich P, Birke R, Godel E. Morphology of myometrial and decidual arteries in normal pregnancy, in toxemia of pregnancy and in maternal diabetes (author's transl). Pathol Microbiol (Basel) 1975;43:38–61
- De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol 1975;123:164–74
- Robertson WB, Brosens I, Dixon G. Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol 1975;5:47–65
- Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens 1976;5:115–27
- De Wolf F, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol 1980;87:678–85
- Abramowsky CR, Vegas ME, Swinehart G, et al. Decidual vasculopathy of the placenta in lupus erythematosus. N Engl J Med 1980;303:668–72
- Kitzmiller JL, Watt N, Driscoll SG. Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. Am J Obstet Gynecol 1981;141:773–9
- Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 1981;88:695–705
- De Wolf F, Carreras LO, Moerman P, et al. Decidual vasculopathy and extensive placental infarction in a patient with repeated thromboembolic accidents, recurrent fetal loss and a lupus anticoagulant. Am J Obstet Gynecol 1982;142:829–34
- Hustin J, Foidart JM, Lambotte R. Maternal vascular lesions in pre-eclampsia and intrauterine growth retardation: light microscopy and immunofluorescence. Placenta 1983;4::489–98
- Althabe O, Labarrere C, Telenta M. Maternal vascular lesions in placentae of small-for-gestational-age infants. Placenta 1985;6:265–76
- Labarrere C, Alonso J, Manni J, et al. Immunohistochemical findings in acute atherosis associated with intrauterine growth retardation. Am J Reprod Immunol Microbiol 1985;7:149–55
- Labarrere C, Althabe O. Chronic villitis of unknown etiology and maternal arterial lesions in preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol 1985;20:1–11
- Khong TY, De Wolf F, Robertson WB, et al. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049–59
- McFadyen IR, Price AB, Geirsson RT. The relation of birthweight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol 1986;93:476–81
- Labarrere CA, Catoggio LJ, Mullen EG, et al. Placental lesions in maternal autoimmune diseases. Am J Reprod Immunol Microbiol 1986;12:78–86
- Khong TY, Pearce JM, Robertson WB. Acute atherosis in preeclampsia: maternal determinants and fetal outcome in the presence of the lesion. Am J Obstet Gynecol 1987;157:360–3
- Labarrere CA. Acute atherosis. A histopathological hallmark of immune aggression? Placenta 1988;9:95–108
- Frusca T, Morassi L, Pecorelli S, et al. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br J Obstet Gynaecol 1989;96:835–9
- Khong TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants and diabetes mellitus. Arch Pathol Lab Med 1991;115:722–5
- Meekins JW, Pijnenborg R, Hanssens M, et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–74
- Meekins JW, Pijnenborg R, Hanssens M, et al. Immunohistochemical detection of lipoprotein(a) in the wall of placental bed spiral arteries in normal and severe preeclamptic pregnancies. Placenta 1994;15:511–24
- Salafia CM, Parke AL. Placental pathology in systemic lupus erythematosus and phospholipid antibody syndrome. Rheum Dis Clin North Am 1997;23:85–97
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 1977;84:656–63
- Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol 1981;88:876–81
- Nayar R, Lage JM. Placental changes in a first trimester missed abortion in maternal systemic lupus erythematosus with antiphospholipid syndrome; a case report and review of the literature. Hum Pathol 1996;27:201–6
- Hayati AR, Azizah A, Wahidah A. Incidence of acute atherosis in complete molar pregnancy. Malays J Pathol 1998;20:113–4
- Khong TY, Hague WM. The placenta in maternal hyperhomocysteinaemia. Br J Obstet Gynaecol 1999;106:273–8
- Ogishima D, Matsumoto T, Nakamura Y, et al. Placental pathology in systemic lupus erythematosus with antiphospholipid antibodies. Pathol Int 2000;50:224–9
- Sebire NJ, Rees H, Paradinas F, et al. Extravillus endovascular implantation site trophoblast invasion is abnormal in complete versus partial molar pregnancies. Placenta 2001;22:725–8
- Moldenhauer JS, Stanek J, Warshak C, et al. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol 2003;189:1173–7
- Faye-Petersen O, Heller DS, Joshi VV. Histologic lesions of the placenta: villi, fetal stem arteries, intervillous space and maternal arteries in decidua. In: Faye-Petersen O, Heller DS, Joshi VV, eds. Handbook of placental pathology. 2nd ed. United Kingdom: Taylor & Francis; 2006:53–78
- Harsem NK, Roald B, Braekke K, et al. Acute atherosis in decidual tissue: not associated with systemic oxidative stress in preeclampsia. Placenta 2007;28:958–64
- Fox H, Sebire NJ. Histological abnormalities of the placenta. In: Fox H, Sebire NJ, eds. Pathology of the placenta. 3rd ed. China: ELSEVIER; 2007:147–86
- Brosens I, Khong TY. Defective spiral artery remodeling. In: Pijnenborg R, Brosens I, Romero R, eds. Placental bed disorders. Cambridge, UK: Cambridge University Press; 2010:11
- Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010;56:1026–34
- Stevens DU, Al-Nasiry S, Bulten J, et al. Decidual vasculopathy in preeclampsia: lesion characteristics relate to disease severity and perinatal outcome. Placenta 2013;34:805–9
- Staff AC, Dechend R, Redman CW. Review: preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta 2013;34:S73–8
- Staff AC, Johnsen GM, Dechend R, et al. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol 2014;101–102:120–6
- Staff AC, Redman CW. IFPA award in placentology lecture: preeclampsia, the decidual battleground and future maternal cardiovascular disease. Placenta 2014;35:S26–31
- Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol 2009;4:19–47
- Danesh J, Collins R, Appleby P, et al. Association of fibrinogen, C-reactive protein, albumin or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477–82
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999;340:115–26
- Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000;148:209–14
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol 2009;27:165–97
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–51
- Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol 1987;94:649–55
- Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol 1992;99:651–4
- Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–42
- Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189:1063–9
- Ball E, Bulmer JN, Ayis S, et al. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol 2006;208:535–42
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;27:939–58
- Brosens I, Pijnenborg R, Vercruysse L, et al. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201
- Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol 2011;25:273–85
- Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The calcium for preeclampsia prevention (CPEP) study group. Am J Obstet Gynecol 1997;177:1003–10
- Seeds JW. Impaired fetal growth: definition and clinical diagnosis. Obstet Gynecol 1984;64:303–10
- Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–8
- Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med 2011;39:641–52
- Espinoza J, Romero R, Mee Kim Y, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med 2006;34:447–58
- Ustun Y, Engin-Ustun Y, Kamaci M. Association of fibrinogen and C-reactive protein with severity of preeclampsia. Eur J Obstet Gynecol Reprod Biol 2005;121:154–8
- Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–9
- Hirashima C, Ohkuchi A, Matsubara S, et al. Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens Res 2008;31:1541–8
- Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 2010;63:534–43
- Hertig A, Fort J, Lefevre G, et al. Soluble endoglin in preeclamptic patients with or without HELLP syndrome. Am J Obstet Gynecol 2010;202:594.e1–4
- Boij R, Svensson J, Nilsson-Ekdahl K, et al. Biomarkers of coagulation, inflammation and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol 2012;68:258–70
- Ozler A, Turgut A, Sak ME, et al. Serum levels of neopterin, tumor necrosis factor-alpha and Interleukin-6 in preeclampsia: relationship with disease severity. Eur Rev Med Pharmacol Sci 2012;16:1707–12
- Peracoli JC, Bannwart-Castro CF, Romao M, et al. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol 2013;100:129–34
- Pinheiro MB, Martins-Filho OA, Mota AP, et al. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine 2013;62:165–73
- Xiao JP, Yin YX, Gao YF, et al. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine 2012;60:856–60
- Schonkeren D, van der Hoorn ML, Khedoe P, et al. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol 2011;178:709–17
- Quinn KH, Lacoursiere DY, Cui L, et al. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol 2011;91:76–82
- Kimura C, Watanabe K, Iwasaki A, et al. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med 2013;26:491–6
- Kvehaugen AS, Melien O, Holmen OL, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension 2013;61:655–61
- Sacks GP, Studena K, Sargent K, et al. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–6
- Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001;185:1118–23
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14
- Watts DH, Krohn MA, Wener MH, et al. C-reactive protein in normal pregnancy. Obstet Gynecol 1991;77:176–80
- Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1124–9
- Pitiphat W, Gillman MW, Joshipura KJ, et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol 2005;162:1108–13
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Kim MA, Lee BS, Park YW, et al. Serum markers for prediction of spontaneous preterm delivery in preterm labour. Eur J Clin Invest 2011;41:773–80
- Laudanski P, Raba G, Kuc P, et al. Assessment of the selected biochemical markers in predicting preterm labour. J Matern Fetal Neonatal Med 2012;25:2696–9
- Cruciani L, Romero R, Vaisbuch E, et al. Pentraxin 3 in maternal circulation: an association with preterm labor and preterm PROM, but not with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:1097–105
- Stampalija T, Chaiworapongsa T, Romero R, et al. Soluble ST2, a modulator of the inflammatory response, in preterm and term labor. J Matern Fetal Neonatal Med 2014;27:111–21
- Ismail MA, Zinaman MJ, Lowensohn RI, et al. The significance of C-reactive protein levels in women with premature rupture of membranes. Am J Obstet Gynecol 1985;151:541–4
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:171–5
- Loukovaara MJ, Alfthan HV, Kurki MT, et al. Serum highly sensitive C-reactive protein in preterm premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol 2003;110:26–8
- Moghaddam Banaem L, Mohamadi B, Asghari Jaafarabadi M, et al. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J Obstet Gynaecol Res 2012;38:780–6
- Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med 2012;25:1428–32
- Vince GS, Starkey PM, Austgulen R, et al. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol 1995;102:20–5
- Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol 1997;38:89–93
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 1998;40:102–11
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506
- Sabatier F, Bretelle F, D'Ercole C, et al. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol 2000;183:1558–63
- Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–7
- Teran E, Escudero C, Moya W, et al. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet 2001;75:243–9
- Chaiworapongsa T, Romero R, Yoshimatsu J, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002;12:19–27
- Chaiworapongsa T, Gervasi MT, Refuerzo J, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol 2002;187:889–93
- Velzing-Aarts FV, Muskiet FA, van der Dijs FP, et al. High serum interleukin-8 levels in afro-caribbean women with pre-eclampsia. Relations with tumor necrosis factor-alpha, duffy negative phenotype and von Willebrand factor. Am J Reprod Immunol 2002;48:319–22
- Serin IS, Ozcelik B, Basbug M, et al. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2002;100:143–5
- Belo L, Santos-Silva A, Caslake M, et al. Neutrophil activation and C-reactive protein concentration in preeclampsia. Hypertens Pregnancy 2003;22:129–41
- Levine RJ, Qian C, Leshane ES, et al. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol 2004;190:707–13
- Kocyigit Y, Atamer Y, Atamer A, et al. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol 2004;19:267–73
- Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004;44:708–14
- Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system and preeclampsia? Am J Obstet Gynecol 2005;193:921–7
- Braekke K, Holthe MR, Harsem NK, et al. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am J Obstet Gynecol 2005;193:227–33
- Enquobahrie DA, Williams MA, Qiu C, et al. Maternal plasma transforming growth factor-beta1 concentrations in preeclamptic and normotensive pregnant Zimbabwean women. J Matern Fetal Neonatal Med 2005;17:343–8
- Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol 2006;70:83–91
- Kusanovic JP, Romero R, Hassan SS, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med 2007;20:867–78
- Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol 2007;58:21–30
- Laskowska M, Laskowska K, Leszczynska-Gorzelak B, et al. Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med 2007;20:527–32
- Sibai B, Romero R, Klebanoff MA, et al. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. Am J Obstet Gynecol 2009;200:630.e1–8
- Ogge G, Romero R, Chaiworapongsa T, et al. Leukocytes of pregnant women with small-for-gestational age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Fetal Neonatal Med 2010;23:476–87
- Soto E, Romero R, Richani K, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med 2010;23:646–57
- Szarka A, Rigo J Jr, Lazar L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 2010;11:59
- Xie C, Yao MZ, Liu JB, et al. A meta-analysis of tumor necrosis factor-alpha, interleukin-6 and interleukin-10 in preeclampsia. Cytokine 2011;56:550–9
- Twina G, Sheiner E, Shahaf G, et al. Lower circulation levels and activity of alpha-1 antitrypsin in pregnant women with severe preeclampsia. J Matern Fetal Neonatal Med 2012;25:2667–70
- Stampalija T, Chaiworapongsa T, Romero R, et al. Maternal plasma concentrations of sST2 and angiogenic/anti-angiogenic factors in preeclampsia. J Matern Fetal Neonatal Med 2013;26:1359–70
- Sahin S, Ozakpinar OB, Eroglu M, et al. The impact of platelet functions and inflammatory status on the severity of preeclampsia. J Matern Fetal Neonatal Med 2014 . [Epub ahead of print]
- Labarrere C, Manni J, Salas P, et al. Intrauterine growth retardation of unknown etiology. I. Serum complement and circulating immune complexes in mothers and infants. Am J Reprod Immunol Microbiol 1985;8:87–93
- Labarrere CA, Althabe OH. Intrauterine growth retardation of unknown etiology: II. Serum complement and circulating immune complexes in maternal sera and their relationship with parity and chronic villitis. Am J Reprod Immunol Microbiol 1986;12:4–6
- Johnston TA, Greer IA, Dawes J, et al. Neutrophil activation in small for gestational age pregnancies. Br J Obstet Gynaecol 1991;98:105–6
- Johnson MR, Anim-Nyame N, Johnson P, et al. Does endothelial cell activation occur with intrauterine growth restriction? BJOG 2002;109:836–9
- Coata G, Pennacchi L, Bini V, et al. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med 2002;12:28–34
- Tjoa ML, van Vugt JM, Go AT, et al. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol 2003;59:29–37
- Cetin I, Cozzi V, Pasqualini F, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 2006;194:1347–53
- Girardi G, Yarilin D, Thurman JM, et al. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006;203:2165–75
- Laskowska M, Laskowska K, Leszczynska-Gorzelak B, et al. Maternal and umbilical sTNF-R1 in preeclamptic pregnancies with intrauterine normal and growth retarded fetus. Hypertens Pregnancy 2007;26:13–21
- Erez O, Romero R, Hoppensteadt D, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med 2008;21:855–69
- Duff P. Pyelonephritis in pregnancy. Clin Obstet Gynecol 1984;27:17–31
- Gilstrap LC 3rd, Lucas MJ. Urinary tract infections in women. Curr Opin Obstet Gynecol 1990;2:643–8
- Soto E, Richani K, Romero R, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med 2005;17:247–52
- Kim YM, Romero R. Cover image. Am J Obstet Gynecol 2014;212
- Alnaes-Katjavivi P, Lyall F, Roald B, et al. Placenta 2013;34:A11 (abstract P1.1)

