Abstract
Objective: Preterm birth is associated with 5–18% of pregnancies and is the leading cause of neonatal morbidity and mortality. Amniotic fluid (AF) interleukin-6 (IL-6) is a key cytokine for the identification of intra-amniotic inflammation, and patients with an elevated AF IL-6 are at risk for impending preterm delivery. However, results of the conventional method of measurement (enzyme-linked immunosorbent assay; ELISA) are usually not available in time to inform care. The objective of this study was to determine whether a point of care (POC) test or lateral-flow-based immunoassay for measurement of AF IL-6 concentrations can identify patients with intra-amniotic inflammation and/or infection and those destined to deliver spontaneously before term among women with preterm labor and intact membranes.
Methods: One-hundred thirty-six women with singleton pregnancies who presented with symptoms of preterm labor and underwent amniocentesis were included in this study. Amniocentesis was performed at the time of diagnosis of preterm labor. AF Gram stain and AF white blood cell counts were determined. Microbial invasion of the amniotic cavity (MIAC) was defined according to the results of AF culture (aerobic and anaerobic as well as genital mycoplasmas). AF IL-6 concentrations were determined by both lateral flow-based immunoassay and ELISA. The primary outcome was intra-amniotic inflammation, defined as AF ELISA IL-6 ≥ 2600 pg/ml.
Results: (1) AF IL-6 concentrations determined by a POC test have high sensitivity (93%), specificity (91%) and a positive likelihood ratio of 10 for the identification of intra-amniotic inflammation by using a threshold of 745 pg/ml; (2) the POC test and ELISA for IL-6 perform similarly in the identification of MIAC, acute inflammatory lesions of placenta and patients at risk of impending spontaneous preterm delivery.
Conclusion: A POC AF IL-6 test can identify intra-amniotic inflammation in women who present with preterm labor and intact membranes and those who will subsequently deliver spontaneously before 34 weeks of gestation. Results can be available within 20 min – this has important clinical implications and opens avenues for early diagnosis as well as treatment of intra-amniotic inflammation/infection.
Introduction
Preterm birth affects 5–18% of pregnancies [Citation1–7] and is the leading cause of neonatal morbidity and mortality [Citation8–15]. One of every four women who deliver preterm has an intra-amniotic infection that is largely subclinical [Citation16–48]. Microbial-associated preterm labor is mediated by inflammatory processes that involve the production of cytokines such as interleukin (IL)-1 [Citation49–56], IL-6 [Citation32,Citation53,Citation54,Citation56–74], IL-10 [Citation75,Citation76], tumor necrosis factor-alpha (TNF-α) [Citation53,Citation74,Citation77–80], chemokines [Citation53,Citation54,Citation66,Citation67,Citation81–95], matrix-degrading enzymes [Citation96–106] and other inflammatory-related proteins [Citation56,Citation107–124], which activate the common pathway of parturition [Citation1,Citation3,Citation125–127]. Multiple studies have shown that amniotic fluid (AF) IL-6 concentrations are superior to AF white blood cell (WBC) counts, glucose, Gram stain or equivalent to proteomic markers in identifying intra-amniotic infection and microbial invasion of the amniotic cavity (MIAC) [Citation58,Citation60,Citation71,Citation128–132]. Moreover, even in the absence of demonstrable microorganisms in the amniotic cavity, an elevated AF IL-6 concentrations is associated with an increased risk of adverse pregnancy and neonatal outcomes in the context of preterm labor [Citation46,Citation72,Citation133–136], preterm prelabor rupture of the membranes (preterm PROM) [Citation137,Citation138] and a short cervix [Citation139]. Thus, AF IL-6 concentrations have both diagnostic and prognostic value.
Currently, it usually takes hours to determine AF IL-6 concentrations, and the results are often unavailable in time to inform clinical decisions. A point of care (POC) test (lateral flow-based immunoassay) has been widely used in the settings of adult [Citation140] and neonatal sepsis [Citation141,Citation142], as well as for other inflammation-related conditions [Citation143]. It was not until recently that such tests were used in obstetrics. In a pilot study, our group showed that AF IL-6 concentrations determined using a POC test were strongly correlated with those measured by conventional enzyme-linked immunosorbent assay (ELISA) (Spearman’s ρ = 0.92) [Citation144]. Moreover, POC IL-6 test results can identify patients with preterm PROM who are destined to deliver preterm and/or have acute histological chorioamnionitis [Citation145–147].
In this study, we examined whether AF IL-6 concentrations, determined by a POC test, can identify patients with preterm labor with intact membranes who have intra-amniotic inflammation and/or infection, and/or deliver spontaneously before term, relative to the performance of concentrations determined by conventional ELISA.
Material and methods
Study population
A retrospective cohort study was conducted by searching the clinical database and bank of biological samples of Wayne State University, the Detroit Medical Center and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD; Detroit, MI) to identify patients with a diagnosis of spontaneous preterm labor with intact membranes. Patients were included if they met the following criteria: (1) singleton gestation; (2) trans-abdominal amniocentesis performed between 20 and 35 weeks of gestation with microbiological studies; (3) available AF for the performance of microbiologic studies; and (4) neonatal outcomes were known. Patients were excluded from this study if they had placenta previa or if their fetus had a chromosomal or structural anomaly.
Patients with the diagnosis of preterm labor with intact membranes were counseled by their treating physicians about the potential value of identifying microorganisms in AF. Women who agreed to undergo an amniocentesis were asked to donate additional AF other than that required for clinical studies, and to allow collection of clinical information for research purposes. Further management of these patients was at the discretion of the attending physician. All patients provided written informed consent, and the use of biological specimens and clinical data for research purposes were approved by the Institutional Review Boards of NICHD and Wayne State University.
Biological samples and analysis
AF was transported in a capped sterile syringe to the clinical laboratory where it was cultured for aerobic and anaerobic bacteria, including genital mycoplasmas. AF not required for clinical assessment was centrifuged for 10 min at 4 °C and stored at −70 °C until analysis. Evaluation of WBC count, glucose concentration and Gram stain of AF were also performed shortly after collection. The presence of intra-amniotic infection/inflammation was assessed by determination of AF IL-6 concentration by ELISA.
Clinical definitions
Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 min associated with cervical changes in patients with a gestational age between 20 and 36 6/7 weeks. Acute histologic chorioamnionitis was diagnosed according to previously described criteria [Citation148,Citation149]. Funisitis was diagnosed when neutrophil infiltration was detected into the umbilical vessel walls or Wharton’s jelly using previously reported criteria [Citation150–152]. Intra-amniotic inflammation was diagnosed when the AF IL-6 concentration was ≥2600 pg/ml (≥2.6 ng/ml), as determined by ELISA [Citation46,Citation87,Citation117,Citation153]. MIAC was defined according to the results of AF culture. Intra-amniotic infection was defined as a combination of MIAC with intra-amniotic inflammation.
Analysis of AF samples for IL-6 concentrations
AF IL-6 concentrations (pg/ml) were determined both by ELISA and the lateral flow-based immunoassay POC test. For ELISA, AF IL-6 concentrations were determined by immunoassays obtained from R&D Systems (Minneapolis, MN). The POC determination of AF IL-6 concentrations (pg/ml) was performed using a lateral flow-based immunoassay POC test (Milenia QuickLine® IL-6; Milenia Biotec, Bad Nauheim, Germany). The details and performance of ELISA [Citation37,Citation46,Citation53,Citation57,Citation60,Citation153–157] and POC immunoassays have been previously described [Citation144]. The IL-6 POC test inter- and intra-assay coefficients of variations are 15.5% and 12.1%, respectively.
Study outcomes
The primary outcomes in this study are intra-amniotic inflammation and positive AF culture. Secondary outcomes include the occurrence of spontaneous preterm delivery (within 24 h, 48 d and 7 d of admission), spontaneous preterm delivery (<28 and <34 weeks of gestation) and the presence of placental lesions consistent with acute inflammation (acute histologic chorioamnionitis and/or acute funisitis). The relationships between acute histologic chorioamnionitis and AF IL-6 concentrations were examined in 55 patients who delivered within three days of amniocentesis. This interval was chosen to preserve a meaningful temporal relationship between the results of amniocentesis and placental pathology.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess normality of arithmetic data distributions. The Kruskal–Wallis and Mann–Whitney U tests were used to make comparisons among and between groups for arithmetic variables. Chi-square or Fisher’s exact test were used for comparisons of categorical variables. Statistical analysis was performed using SPSS 19 (IBM Corp, Armonk, NY) and SAS 9.4 (Cary, NC). A p value <0.05 was considered statistically significant.
Results
Characteristics of the study population
A total of 136 women with preterm labor with intact membranes were included in this study. Their clinical characteristics are listed in . The prevalence of MIAC and intra-amniotic inflammation was 16.2% (22/136) and 44.1% (60/136), respectively. Most of the participants had spontaneous preterm deliveries, specifically 22.8% (31/136) at <28 weeks, 54% (74/136) at <34 weeks and 64% (87/136) at <37 weeks. The rates of spontaneous preterm delivery within 24 h, 48 h and 7 d were 33.8% (46/136), 43.4% (59/136) and 47.8% (65/136), respectively. The median (interquartile range) gestational age at amniocentesis was 30.9 (27–32.4) weeks. Of the 54 women who delivered within three days of the amniocentesis, and had placenta pathologic reports, 57.4% (31/54) had acute histologic chorioamnionitis, and most of their offspring were diagnosed with funisitis [67.7% (21/31)].
Table 1. Clinical characteristics of the study population.
lists the microorganisms identified by AF culture, gestational age at delivery, concentrations of IL-6 (by ELISA and POC test), AF inflammatory response and the type or absence of placental lesions consistent with acute inflammation in women with MIAC. The most frequent microorganism identified was Ureaplasma urealyticum, which was identified in 18% (4/22) of these women.
Table 2. Clinical characteristics, amniotic fluid inflammatory response and acute inflammatory placental lesions in patients with microbial invasion of the amniotic cavity using cultivation techniques.
The diagnostic performance of an AF IL-6 POC test for the identification of intra-amniotic inflammation
Upon inspecting a receiver operating characteristic curve for the identification of intra-amniotic inflammation [area under curve = 0.94 (0.90–0.99)], a threshold of ≥745 pg/ml was selected for the POC test (). lists, at this cut-off, the POC test had a sensitivity of 93% and a specificity of 91%.
Figure 1. Receiver operating characteristic curve that describes the performance of point of care test amniotic fluid interleukin-6 in the identification of intra-amniotic inflammation (determined by ELISA IL-6 ≥ 2600 pg/mL) (area under the curve for amniotic fluid IL-6 point of care test = 0.94; 95% confidence interval: 0.90–0.99, p < 0.001).
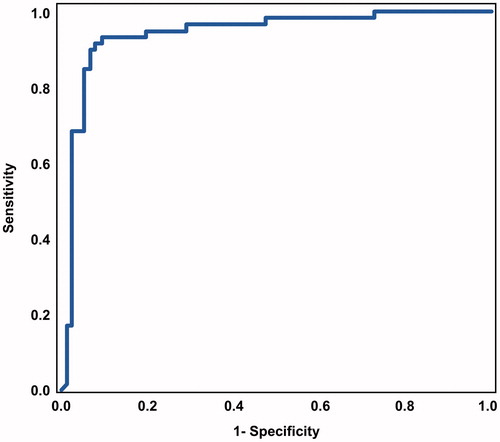
Table 3. Diagnostic performance of point of care AF IL-6 concentrations and ELISA AF IL-6 for identification of intra-amniotic infection and/or inflammation and placental lesions consistent with acute inflammation.
Of seven women with negative ELISA AF IL-6 tests who had positive POC test results, six (85%) delivered within two days of amniocentesis at <34 weeks of gestation. Two of these women also had positive AF cultures: one had Streptococcus spp./Gemella morbillorum in addition to placental lesions consistent with acute inflammation; the other had Gram-negative bacilli, but placental histopathology was not available. On the other hand, four patients had positive ELISA AF IL-6 test results, yet the results of the POC test were negative. Two of these four patients delivered at term, none had a positive AF culture, and one had an AF IL-6 concentration above the cutoff used to define intra-amniotic inflammation (2609 pg/ml; determined by ELISA). This patient did not have placental lesions consistent with acute inflammation.
The diagnostic performance of an AF IL-6 POC test for the identification of MIAC and acute inflammatory lesions of placenta
lists the performance of the POC test in identifying patients with MIAC and those with placental lesions consistent with acute inflammation was equivalent to that of conventional ELISA. Among patients with MIAC, 18.2% (4/22) had negative ELISA IL-6 results (). However, POC AF IL-6 was elevated in two of these four patients. One of these patients had acute histologic chorioamnionitis and funisitis; therefore, this implied true intra-amniotic infection. The placental pathology report was not available for the other patient.
Of note, one of the two patients with MIAC who had negative AF IL-6 test results for both assays (POC and ELISA) delivered at term and did not have placental lesions consistent with acute inflammation, suggesting the possibility of contamination [i.e. false-positive AF culture (U. urealyticum)].
The diagnostic performance of an AF IL-6 POC test for the identification of impending preterm delivery
lists the performance of the POC and ELISA AF IL-6 tests in identifying women who had spontaneous preterm deliveries. Both tests had equivalent positive likelihood ratios in identifying patients who had a spontaneous preterm delivery within one day of amniocentesis, or those who would subsequently deliver spontaneously at <28 weeks of gestation. Sensitivity and specificity were each marginally higher for the POC test than for the ELISA in identifying women who would deliver spontaneously within either two or seven days of amniocentesis. In contrast, sensitivity was slightly higher, whereas specificity was slightly lower, when comparing the performance of the POC test to that of the ELISA for the identification of spontaneous preterm delivery at <34 weeks of gestation. Yet, confidence intervals for estimates characterizing the diagnostic performance of the POC test overlapped with those of the ELISA test, indicating statistically equivalent performance in assessing the risk of spontaneous preterm delivery.
Table 4. Diagnostic performance of point of care AF IL-6 concentrations and ELISA AF IL-6 for identification of patients with spontaneous preterm delivery.
Discussion
Principal findings of the study: (1) AF IL-6 concentrations determined by a POC test have high sensitivity (93%) and specificity (91%) for the identification of intra-amniotic inflammation, by using a threshold of 745 pg/ml and (2) the POC test and ELISA for IL-6 determination perform similarly in identifying patients with MIAC, acute inflammatory lesions of placenta and risk of impending spontaneous preterm delivery in patients with preterm labor with intact membranes.
AF IL-6 POC test for the identification of intra-amniotic inflammation and impending preterm delivery
Compelling evidence indicates that patients with intra-amniotic inflammation are at greater risk for impending preterm delivery and other adverse outcomes, even without identifiable microorganisms [Citation46,Citation72,Citation133–139,Citation158]. We have previously demonstrated that sterile intra-amniotic inflammation (inflammatory process in which microorganisms are neither detected by cultivation nor molecular methods) is more common than microbial-associated intra-amniotic inflammation in patients with preterm labor and intact membranes [Citation72,Citation134], asymptomatic sonographic short cervix [Citation139] and preterm PROM [Citation138]. Moreover, we have shown that sterile intra-amniotic inflammation is associated with adverse pregnancy outcomes; hence the importance of identifying patients with intra-amniotic inflammation [Citation72,Citation134,Citation139].
In this study, we have demonstrated that a POC AF IL-6 test has high sensitivity and specificity in the identification of intra-amniotic inflammation and spontaneous preterm delivery. While its performance in identifying infection/inflammation-related obstetrical outcomes was comparable to that of AF IL-6 concentrations determined by ELISA, the POC assay can yield results within 20 min. Hence, unlike conventional ELISA, POC AF IL-6 results can be available in time to inform clinical decisions, similar to a rapid matrix metalloproteinase-8 (MMP-8) test, which has been shown to identify intra-amniotic infection/inflammation in patients with preterm labor and intact membranes with >80% sensitivity and >90% specificity [Citation103]. Furthermore, the MMP-8 test was found to be useful in the identification of intra-amniotic inflammation in patients with preterm PROM [Citation104], MIAC in patients at risk for preterm delivery [Citation159] and funisitis in patients with preterm delivery [Citation160].
It is interesting that six of seven patients with positive POC test and negative ELISA results had early spontaneous preterm deliveries (<34 weeks) within two days of amniocentesis. This suggests that the POC test contributes additional risk information beyond that provided by conventional ELISA. It is further noteworthy that two of the four patients with negative POC and positive ELISA test results delivered at term, one of them did not have placental lesions consistent with acute inflammation, and this patient had a positive ELISA result (2609 pg/ml).
In a prior study, we showed that AF IL-6 concentrations determined by a POC test were on an average 30% lower than that determined by conventional ELISA. Thus, it is not surprising that a lower AF IL-6 cut-off was selected for the POC to identify patients with intra-amniotic inflammation in this study (≥745 pg/ml). Kacerovsky et al. proposed a higher cut-off (1000 pg/ml) in a study using the same assay to determine AF IL-6 concentrations among women with preterm PROM for the identification of MIAC (or the combination of MIAC with acute histological chorioamnionitis) [Citation146]. Other investigators who used a POC test to determine IL-6 concentrations in vaginal fluid from women with preterm PROM have reported high negative predictive value for the detection of intra-amniotic inflammation, comparable to that observed in our study (97.4% versus 94.5%) [Citation147]. Yet, the positive predictive value in our study was higher than that of vaginal fluid IL-6 concentration POC test (88.9% versus 50%). Vousden et al. reported the use of POC IL-6 in vaginal fluid to determine pregnancy outcomes in asymptomatic high-risk patients of preterm birth [Citation145]. Using a cut-off of 56 pg/ml, vaginal fluid IL-6 concentrations had 81% sensitivity and 65% specificity to identify patients who delivered <28 weeks of gestation [Citation145]. This diagnostic performance is slightly lower than that of POC AF IL-6 in this study. The optimal cutoff value is to be determined in accordance with regard to risk/benefit ratios for specific interventions.
Strengths and limitations
The strengths of this study include: (1) we included a homogenous group of patients with preterm labor with intact membranes rather than including patients with preterm pre-labor rupture of membranes who have a higher prevalence of intra-amniotic infection/inflammation and (2) the POC test was not used to inform treatment. A limitation is that we used cultivation technique to identify microorganisms in the amniotic cavity. Thus, non-culturable bacteria, which could have been identified by molecular microbiologic techniques, may be not able to be detected.
Conclusion
A POC AF IL-6 test can identify intra-amniotic inflammation as determined by ELISA in women with preterm labor and intact membranes, and it also performs equivalently in identifying those who subsequently deliver spontaneously before term. Further studies are warranted to determine whether POC AF IL-6 results can inform treatment decisions sufficient to improve pregnancy outcomes in such patients.
Declaration of interest
Authors declare no conflict of interest.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract no. HHSN275201300006C.
References
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113:17–42
- www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf. 2014
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529–35
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72
- Lawn JE, Kinney M. Preterm birth: now the leading cause of child death worldwide. Sci Transl Med 2014;6:263ed221
- Landmann E, Misselwitz B, Steiss JO, Gortner L. Mortality and morbidity of neonates born at <26 weeks of gestation (1998-2003). A population-based study. J Perinat Med 2008;36:168–74
- Lipkind HS, Slopen ME, Pfeiffer MR, McVeigh KH. School-age outcomes of late preterm infants in New York City. Am J Obstet Gynecol 2012;206:222.e221–6
- Taylor HG. Outcomes of late preterm birth: who is at risk and for what? Am J Obstet Gynecol 2012;206:181–2
- Zanardo V, Straface G, Trevisanuto D. Future learning abilities of late preterm infants. Am J Obstet Gynecol 2012;207:e17; author reply e17–18
- Hofer N, Kothari R, Morris N, et al. The fetal inflammatory response syndrome is a risk factor for morbidity in preterm neonates. Am J Obstet Gynecol 2013;209:542.e541–2 e511
- Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol 2014;210:426.e421–9
- Bastek JA, Weber AL, McShea MA, et al. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol 2014;210:450.e1–10
- Strunk T, Inder T, Wang X, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62
- Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med 1977;19:8–12
- Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol 1978;51:56–62
- Miller JM, Jr., Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol 1980;136:796–804
- Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol 1981;57:483–6
- Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52
- Wahbeh CJ, Hill GB, Eden RD, et al. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43
- Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol 1984;149:726–30
- Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37
- Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol 1986;67:500–6
- Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–19
- Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 1988;5:64–9
- Romero R, Scharf K, Mazor M, et al. The clinical value of gas-liquid chromatography in the detection of intra-amniotic microbial invasion. Obstet Gynecol 1988;72:44–50
- Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79
- Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24
- Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol 1989;161:813–16
- Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–30
- Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 1991;165:1105–10
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–7
- Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1992;167:1231–42
- Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82
- Hussey MJ, Levy ES, Pombar X, et al. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol 1998;179:650–6
- Oyarzun E, Yamamoto M, Kato S, et al. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol 1998;179:1115–19
- Elimian A, Figueroa R, Canterino J, et al. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol 1998;92:72–6
- Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999;8:155–8
- Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–7
- Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6
- Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003;82:120–8
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39
- Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23
- Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990;35:235–8
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Romero R, Gomez R, Galasso M, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol 1994;171:912–21
- Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–30
- Arntzen KJ, Kjollesdal AM, Halgunset J, et al. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998;26:17–26
- Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–16
- Cobo T, Tsiartas P, Kacerovsky M, et al. Maternal inflammatory response to microbial invasion of the amniotic cavity: analyses of multiple proteins in the maternal serum. Acta Obstet Gynecol Scand 2013;92:61–8
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 220–3
- Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Allbert JR, Naef RW 3rd, Perry KG Jr., et al. Amniotic fluid interleukin-6 and interleukin-8 levels predict the success of tocolysis in patients with preterm labor. J Soc Gynecol Investig 1994;1:264–8
- Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol 1998;179:1267–70
- Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–9
- Rogers BB, Alexander JM, Head J, et al. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol 2002;33:335–40
- Jacobsson B, Mattsby-Baltzer I, Hagberg H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG 2005;112:719–24
- Holst RM, Mattsby-Baltzer I, Wennerholm UB, et al. Interleukin-6 and interleu kin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand 2005;84:551–7
- Holst RM, Laurini R, Jacobsson B, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med 2007;20:885–93
- Menon R, Camargo MC, Thorsen P, et al. Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black Americans. Am J Obstet Gynecol 2008;198:77.e1–7
- Marconi C, de Andrade Ramos BR, Peracoli JC, et al. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol 2011;65:549–56
- Cobo T, Kacerovsky M, Holst RM, et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012;91:930–5
- Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014;27:757–69
- Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325.e1–5.e10
- Armstrong-Wells J, Donnelly M, Post MD, et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol 2014. [Epub ahead of print]. doi: 10.1016/j.ajog.2014.09.016
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–47
- Kacerovsky M, Celec P, Vlkova B, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One 2013;8:e60399
- Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41
- Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–87
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999;181:1142–8
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 1993;169:1299–303
- Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994;32:108–13
- Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol 1996;87:94–8
- Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10
- Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol 1998;178:428–32
- Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81
- Chaiworapongsa T, Romero R, Tolosa JE, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med 2002;12:159–64
- Keelan JA, Wang K, Chaiworapongsa T, et al. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod 2003;9:535–40
- Jacobsson B, Holst RM, Wennerholm UB, et al. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol 2003;189:1161–7
- Keelan JA, Yang J, Romero RJ, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod 2004;70:253–9
- Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73
- Jacobsson B, Holst RM, Andersson B, Hagberg H. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand 2005;84:566–71
- Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 2008;60:246–57
- Nhan-Chang CL, Romero R, Kusanovic JP, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2008;21:763–75
- Athayde N, Romero R, Gomez R, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med 1999;8:213–19
- Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–53
- Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–9
- Maymon E, Romero R, Pacora P, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000;183:887–94
- Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol 2001;185:1156–61
- Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–16
- Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1149–55
- Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol 2006;195:1025–30
- Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292.e1–5
- Park CW, Yoon BH, Kim SM, et al. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet Gynecol Sci 2013;56:167–75
- Kim SM, Romero R, Park JW, et al. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med 2014;1--10. [Epub ahead of print]. doi: 10.3109/14767058.2014.961009
- Romero R, Quintero R, Emamian M, et al. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454–60
- Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–7
- Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–61
- Mazor M, Wiznitzer A, Maymon E, et al. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990;26:425–8
- Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–96
- Soto E, Romero R, Richani K, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med 2009;22:983–92
- Vaisbuch E, Romero R, Erez O, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–16
- Cruciani L, Romero R, Vaisbuch E, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med 2010;38:161–71
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:34–47
- Cobo T, Palacio M, Martinez-Terron M, et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol 2011;205:126.e1–8
- Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–55
- Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–19
- Park KH, Lee SY, Kim SN, et al. Prediction of imminent preterm delivery in women with preterm premature rupture of membranes. J Perinat Med 2012;40:151–7
- Kacerovsky M, Drahosova M, Krejsek J, et al. Amniotic fluid CD200 levels in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:1416–24
- Andrys C, Kacerovsky M, Drahosova M, et al. Amniotic fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:520–7
- Park CW, Yoon BH, Park JS, et al. An elevated maternal serum C-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and AF inflammatory response are likely in patients with preterm labor: clinical implications. J Matern Fetal Neonatal Med 2013;26:847–53
- Stampalija T, Chaiworapongsa T, Romero R, et al. Soluble ST2, a modulator of the inflammatory response, in preterm and term labor. J Matern Fetal Neonatal Med 2014;27:111–21
- Waring PM, Romero R, Laham N, et al. Leukemia inhibitory factor: association with intraamniotic infection. Am J Obstet Gynecol 1994;171:1335–41
- Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 2009;144:S2–10
- Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 2009;23:947–54
- Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 2010;21:353–61
- Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11
- Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–8
- El-Bastawissi AY, Williams MA, Riley DE, et al. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol 2000;95:1056–64
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010;116:393–401
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG 2011;118:1042–54
- Lee SE, Romero R, Jung H, et al. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294.e1–6
- Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58
- Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125.e1–125.e15
- Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;211:708
- Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45
- Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014;1--16. [Epub ahead of print]. doi: 10.3109/14767058.2014.958463
- Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014;1--17. [Epub ahead of print]. doi: 10.3109/14767058.2014.954243
- Schefold JC, Hasper D, von Haehling S, et al. Interleukin-6 serum level assessment using a new qualitative point-of-care test in sepsis: a comparison with ELISA measurements. Clin Biochem 2008;41:893–8
- Meem M, Modak JK, Mortuza R, et al. Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. J Glob Health 2011;1:201–9
- Batfalsky A, Lohr A, Heussen N, et al. Diagnostic value of an interleukin-6 bedside test in term and preterm neonates at the time of clinical suspicion of early- and late-onset bacterial infection. Neonatology 2012;102:37–44
- Dengler J, Schefold JC, Graetz D, et al. Point-of-care testing for interleukin-6 in cerebro spinal fluid (CSF) after subarachnoid haemorrhage. Med Sci Monit 2008;14:BR265–8
- Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med 2014;1–10. [Epub ahead of print]. doi: 10.3109/14767058.2014.961417
- Vousden N, Chandiramani M, Seed P, Shennan A. Interleukin-6 bedside testing in women at high risk of preterm birth. J Matern Fetal Neonatal Med 2011;24:1301–4
- Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014;211:385.e1–9
- Berthiaume M, Rousseau E, Rola-Pleszczynski M, Pasquier JC. Rapid evaluation of the absence of inflammation after rupture of membranes. J Matern Fetal Neonatal Med 2014;27:865–9
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26:S114–17
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–9
- Yoon BH, Romero R, Shim JY, et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90
- DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–13
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Yoon BH, Romero R, Moon JB, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–42
- DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056
- Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med 2012;40:329–43
- Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2014;43:19--36
- Lee SJ, Won HS, Kim MN, et al. Diagnostic value of the matrix metalloproteinase-8 rapid test for detecting microbial invasion of the amniotic cavity. Eur J Clin Microbiol Infect Dis 2008;27:1257–60
- Park CW, Lee SM, Park JS, et al. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008;36:497–502

