Abstract
Objective: To determine induction start time(s) that would maximise daytime deliveries when using prostaglandin vaginal inserts.
Methods: Women enrolled into the Phase III trial, EXPEDITE (clinical trial registration: NCT01127581), had labour induced with either a misoprostol or dinoprostone vaginal insert (MVI or DVI). A secondary analysis was conducted to determine the optimal start times for induction by identifying the 12-h period with the highest proportion of deliveries by parity and treatment.
Results: Optimal start times for achieving daytime deliveries when using MVI appear to be 19:00 in nulliparae and 23:00 in multiparae. Applying these start times, the median time of onset of active labour would be approximately 08:30 for both parities and the median time of delivery would be the following day at approximately 16:30 for nulliparae and 12:00 (midday) for multiparae. Optimal start times when using DVI appear to be 07:00 for nulliparae and 23:00 for multiparae. Using these start times, the median time of onset of active labour would be the following day at approximately 04:00 and 11:50, and the median time of delivery would be approximately 13:40 and 16:10, respectively.
Conclusions: When optimising daytime deliveries, different times to initiate induction of labour may be appropriate depending on parity and the type of retrievable prostaglandin vaginal insert used.
Introduction
Induction of labour (IOL) is a common obstetric procedure; women have labour induced either for medical reasons, such as pregnancy-related maternal or foetal complications, post-date gestation, or for elective reasons. Women with an unfavourable cervix, particularly nulliparous women, provide a greater challenge if labour needs to be induced because the time to delivery is generally longer and more unpredictable compared with IOL in women with a favourable cervix.
Several studies have suggested that in some healthcare settings, daytime deliveries have better safety outcomes compared with deliveries at night [Citation1–10]. Factors postulated to be associated with increases in neonatal morbidity and mortality for night time births include reduced staffing levels during night shifts, fatigue of attending medical staff [Citation11–13], and supervision relying to a higher degree on junior staff [Citation14], particularly during night shifts [Citation15] or simply that more higher-risk neonates are born at night [Citation5,Citation15]. In contrast, Caughey et al. demonstrated that there were no significant differences in neonatal morbidity or mortality in an academic teaching hospital [Citation15].
Few studies have investigated the optimal start time for IOL with prostaglandins with a view to optimising daytime deliveries [Citation16]. Moreover, no studies have looked at the optimal start time for IOL with retrievable prostaglandin vaginal delivery systems (vaginal inserts), which are available for IOL or cervical ripening as misoprostol 200 µg vaginal insert (MVI) or dinoprostone vaginal insert (DVI). Presumably, nurse-staffing decisions are most efficient and cost-effective when the time course of labour induction can be reasonably estimated and the predictability of the time from start of IOL to delivery is better understood. The aim of this investigation is to determine a theoretical induction start time when using prostaglandin vaginal inserts which would result in the highest proportion of deliveries between 07:00 and 19:00 (daytime). The analysis is based on data from nulliparous and parous women at term gestation with an unfavourable cervix who were enrolled in the EXPEDITE (EXogenous Prostaglandin comparing the Efficacy and safety of MVI with the DVI for reducing Time to vaginal delivery in pregnant women at term) trial [Citation17].
Methods
Study design
The EXPEDITE trial was a Phase III, randomised, controlled, double-blind study conducted at 35 sites in the United States (clinical trial registration at www.clinicaltrials.gov; identifier NCT01127581). Women requiring cervical ripening and eligible for IOL using prostaglandins were randomised to receive MVI (Misodel®, Ferring Pharmaceuticals Pty Ltd., Pymble, Australia; Mysodelle®, Ferring Pharmaceuticals Ltd., West Drayton, United Kingdom; or Myspess®, Ferring Pharmaceuticals Méxio, Mexico City, Mexico) or DVI (Cervidil®, Forest Pharmaceuticals Inc., St Louis, MO; Propess®, Ferring Pharmaceuticals Ltd., West Drayton, United Kingdom). Women were treated with a single vaginal insert for up to 24 h. Intravenous oxytocin was permitted if needed, with a minimum waiting period of 30 min following removal of the vaginal insert, assuming no contraindications. Randomisation was stratified by site and parity to include approximately 60% nulliparous and 40% parous women. The primary publication for the EXPEDITE trial contains a detailed description of the study protocol and study population [Citation17].
Eligible participants
Pregnant women, aged ≥18 years were eligible to be included in the trial if they: were ≥36 weeks gestation with a single-live fetus, required cervical ripening and labour induction (baseline modified Bishop score ≤4); parity ≤3; and had no uterine scar.
Assessments
As a secondary analysis, times to delivery were summarised by 4-h intervals from the start of the induction (placement of the vaginal insert) with either MVI or DVI for both nulliparous and parous women. The 12-h period (i.e. three consecutive 4-h periods) with the highest proportion of deliveries was identified and used to determine the optimal start times for induction that would then result in the highest chance of having a daytime delivery. Median times to active labour were summarised for both treatment groups by parity. Active labour was defined as progressive cervical dilatation to 4 cm with any frequency of contractions or rhythmic, firm, adequate, quality uterine contractions causing progressive cervical change occurring at a frequency of three or more in 10 min and lasting 45 s or more. This definition of active labour was in line with the American Congress of Obstetricians and Gynecologists (ACOG) guidelines at the time the trial was conducted. The proportion of women who required pre-delivery oxytocin was also described for each treatment group by parity.
Statistical analysis
A Cox regression analysis was conducted using time of day when IOL began and treatment (either MVI or DVI) as predictive variables for interval to delivery to test whether the use of hypothetical start times to model expected delivery times were justified. Assuming theoretical IOL start times, the proportions and 95% confidence intervals (CIs) were calculated for all caesarean or vaginal deliveries for a theoretical 12-h daytime period after start of induction for both treatment groups by parity. Unmodified median times to active labour and delivery (any, vaginal or caesarean) were calculated to determine approximate median time of day of these events given the theoretical IOL start times and summarised for both treatment groups by parity.
Results
Study population
The primary results of EXPEDITE have been published previously [Citation17]. A total of 678 women received MVI (441 nulliparae; 237 multiparae) and 680 women received DVI (451 nulliparae; 229 multiparae). The median baseline modified Bishop score was 2 for nulliparous and parous women in both treatment groups. The majority of women received epidural anaesthesia (MVI group: n = 607 [89.5%]; DVI group: n = 628 [92.4%]).
Relationship of actual start of induction of labour and time to delivery
No relationship was identified between the actual IOL start time and interval to delivery for either induction agent justifying the use of hypothetical start times to model expected time to delivery.
Optimal start times for MVI
A total of 673/678 women (99.3%; 437 nulliparae and 236 multiparae) delivered during the initial induction attempt; vaginal delivery rates were 65.2% (285/437) in nulliparae and 89.8% (212/236) in multiparae. For nulliparae who were induced with MVI, the 12-h interval with the largest proportion of any delivery mode occurred 12–24 h post-insertion; 45.8% (202/441) of all deliveries occurred during this time interval (95% CI: 41.1−50.6; ). For multiparae who were induced with MVI, although the 12-h period with the most deliveries was 4–16 h post-insertion with 63.3% of all deliveries, this would have resulted in a recommended start time of 03:00. Given that 03:00 is an impractical start time, we repeated our model with an 8–20 h post-insertion interval for multiparae. This interval resulted in a similar proportion of deliveries (60.8% [144/237]; 95% CI: 54.2–67.0) and would lead to in a more reasonable start time (). These data suggest that 19:00 in nulliparae and 23:00 in multiparae are the optimal start times for achieving daytime deliveries when using MVI.
Figure 1. Intervals from MVI insertion to delivery using optimised start times. MVI, misoprostol vaginal insert.
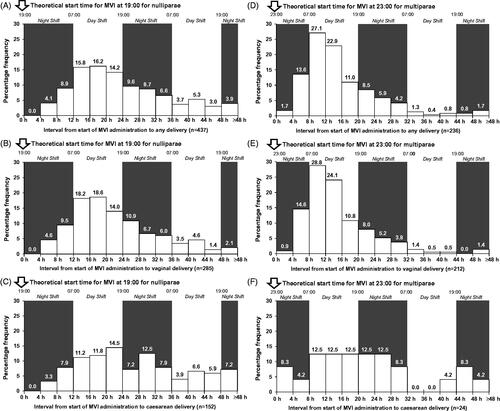
Applying these start times, the median time of onset of active labour would be the following day at approximately 08:30 for both parities and the median time of delivery would be approximately 16:30 for nulliparae and 12:00 (midday) for multiparae (). The time-interval distributions for women in the MVI group for vaginal and caesarean deliveries show a similar pattern to the distribution for any delivery, confirming optimal induction start times as 19:00 for nulliparae and 23:00 for multiparae (, , and ).
Table 1. Median time to events.
Optimal start times for DVI
A total of 671/680 (98.7%; 443 nulliparae and 228 multiparae) delivered during the initial induction attempt; vaginal delivery rates were 62.1% (275/443) in nulliparae and 93.0% (212/228) in multiparae. For nulliparae who received DVI, the 12-h interval with the largest proportion of any delivery mode occurred 24–36 h post-insertion; 32.2% (145/451) of all deliveries occurred during this time interval (95% CI: 27.9–36.7; ). For multiparae who received DVI, the 12-h interval with the largest proportion of any delivery mode occurred 8–20 h post-insertion; 43.7% (100/229) of all deliveries occurred during this time interval (95% CI: 37.1–50.4; ). As such, optimal induction start times for achieving daytime deliveries appear to be 07:00 for nulliparae and 23:00 for multiparae when using DVI. A theoretical start time of 19:00 in multiparae would also result in a similar proportion (40.6%) of deliveries 12–24 h post-insertion, suggesting that a start time between 19:00 and 23:00 would result in a similar number of daytime deliveries in parous women being induced with DVI.
Figure 2. Intervals from DVI insertion to delivery using optimised start times. DVI, dinoprostone vaginal insert.
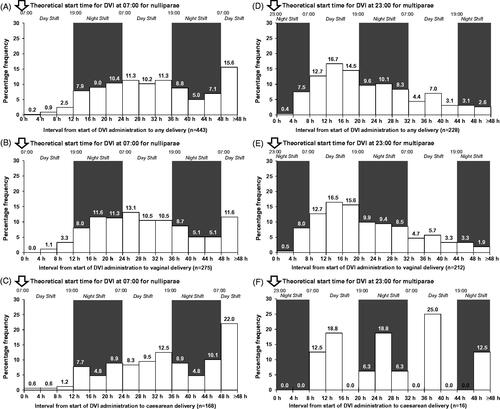
Using these start times for DVI, the median time of onset of active labour would be the following day at approximately 04:00 and 10:50, and the median time of delivery would be approximately 13:40 for nulliparae and 16:10 for multiparae (). The time-interval distributions for women in the DVI group for vaginal and caesarean deliveries show a similar pattern to the distribution for any delivery, confirming 07:00 and 23:00 as optimal induction start times for nulliparae and multiparae, respectively (, , and ).
Pre-delivery oxytocin use
Pre-delivery oxytocin for labour augmentation and its required monitoring are also important factors when determining staffing needs. Table S1 shows the proportion of women requiring oxytocin for labour augmentation following insert removal for each treatment group by parity.
Discussion
Our investigation indicates that pre-determined start times for IOL with prostaglandin vaginal inserts may maximise daytime deliveries. Factors for determining the optimal start time include parity and which agent (MVI or DVI) is being used. Start times of 19:00 and 23:00 optimise daytime deliveries for nulliparae and multiparae who receive MVI, whereas start times of 07:00 and 23:00 optimise daytime deliveries for nulliparae and multiparae who receive DVI; however, a modelled start time of 19:00 also results in a similar number of daytime deliveries as the start time of 23:00 in parous women being induced with DVI, suggesting that any evening induction for parous women would optimise daytime deliveries the following day. As such, to maximise daytime deliveries, it may be appropriate for nulliparous women to receive MVI when admitted during the evening shift whereas it may be more appropriate to induce with DVI when nulliparous women are admitted during the morning. For parous women, it appears that both DVI and MVI should be administered during the evening to maximise daytime deliveries. It should be noted, however, that even when using these optimal IOL start times, at best only around 50% of women would deliver during the first 7:00 am to 7:00 pm (daytime) period after IOL, with the remaining deliveries spread over a period of up to several days. As such, these theoretical start times for either MVI or DVI by parity would likely lead to only a slightly larger proportion of daytime deliveries than those at night, although the proportion of parous women likely to achieve a daytime delivery would be greater than nulliparous women.
Interestingly, the optimal IOL with prostaglandin inserts start times determined in our investigation reflect the circadian rhythm of spontaneous labour, which is more likely to start at night [Citation18–20], with parous women delivering late morning and nulliparous women delivering in early-mid-afternoon [Citation20]. The histograms in and show that MVI-use results in a peak period of deliveries for both nulliparae and multiparae, whereas DVI deliveries are more evenly distributed over time. We postulate that if the optimal times are used for induction with MVI, a higher proportion of women will deliver during the 12-h daytime period compared with women who are induced with DVI. The start times for each type of prostaglandin vaginal insert can be tailored to suit the staffing shift patterns of each individual maternity unit. Some hospitals utilise 8-h shifts, making the 12-h period chosen inappropriate. Nevertheless, understanding the time intervals from start of induction to delivery may help clinicians to determine when best to start induction according to their specific staffing schedules.
Women enrolled in the EXPEDITE study could have the prostaglandin vaginal insert in situ for up to 24 h. This is an important consideration when predicting the optimal start time for induction in some regions, including the United States, where DVI is indicated to be removed 12 h after insertion. Furthermore, time from start of induction to the onset of active labour may vary depending on how active labour is defined. During the trial period (between September 2010 and March 2012) [Citation17] the then current ACOG definition was used to define active labour: cervical dilation to 4 cm or when three or more rhythmic and regular uterine contractions occurred within 10 min and lasting 45 s or more. Recently, however, ACOG guidance has changed, with the suggestion that cervical dilation of 6 cm should be considered as the start of active phase labour [Citation21].
For women who undergo IOL, daytime deliveries may be advantageous in terms of reducing the risk of neonatal mortality and morbidity [Citation4,Citation5]. Furthermore, other studies indicate better safety outcomes for daytime deliveries in certain clinical settings. For example, neonates admitted to intensive care units when born during the night shift had a higher rate of mortality, morbidity and brain asphyxia compared with those born during dayshifts in four hospitals in Lebanon [Citation6]. Another study with data from 3.36 million births in California, United States identified that early night births (19:00–00:00) had a 12% increase and late night births (01:00–06:00) had a 16% increase in the odds of neonatal deaths in hospitals which provided intermediate, community and regional neonatal intensive care. This increased risk was not shown for hospitals which provide primary care [Citation5]. Another report of 694 888 singleton Swedish births between 1991 and 1997 showed that, after adjusting for potential differences in gestational age, birth weight, breech presentation, malformations, IOL, and year of birth, singletons born at night had a 28% increase in the risk of death during the first week of life [Citation4]. As such, timing the start of IOL to maximise the number of daytime deliveries may merit consideration. Moreover, by understanding the distribution of induction−delivery intervals, the durations of labour for women enrolled in the EXPEDITE trial and the use of pre-delivery oxytocin for labour augmentation, the duration of labour can more accurately be predicted for women who are induced with either MVI or DVI in clinical practice, thereby enhancing staff-resourcing decisions and planning more efficient use of labour suites.
Current National Institute for Health and Care Excellence (NICE) IOL guidelines [CG70] recommends induction with vaginally administered dinoprostone should be initiated in the morning because of higher maternal satisfaction [Citation22]; however, as stated in the Cochrane review by Bakker et al., only two trials have compared the administration of prostaglandins in the morning versus evening in women with an unfavourable cervix, with few differences between study groups for maternal or neonatal outcomes and with one trial stating that women had a preference for morning inductions [Citation16]. Indeed, published studies investigating morning versus evening start times for pharmacological IOL show a variety of findings, partly because of the range of protocols used for labour induction as well as different criteria for participation [Citation16,Citation23–26]. These variables make it difficult to make comparisons between the studies or compare them with our current investigation. In addition, our investigation is limited in that the time of vaginal insert administration used to determine the optimal start time was modelled and thus represents theoretical results rather than the actual time used. Further prospective studies with assigned start times for MVI and DVI administration would be required to confirm whether our suggested start times for nulliparae and multiparae would result in a higher incidence of daytime deliveries as well as improved outcomes.
In summary, when outlining the strategy to achieve a higher proportion of daytime deliveries, our results suggest that different times to initiate IOL may be appropriate depending on parity and on the type of retrievable prostaglandin vaginal insert used. Time from administration start to delivery is shorter when using MVI compared with DVI and also shorter for parous women compared with nulliparous women. Pre-delivery oxytocin requirements for labour augmentation are also important when determining staffing needs. This information can be used to optimise resource utilisation around expected active labour and delivery times.
Supplementary material available online
Supplementary Table S1.
Table_SI_revised_clean.pdf
Download PDF (19.6 KB)Declaration of interest
The study was fully funded by Ferring Pharmaceuticals. Medical writing and editorial assistance for manuscript development was provided by C. J. Parkyn, PhD, and was funded by Ferring Pharmaceuticals. H. M. has received research and travel support from Ferring Pharmaceuticals, Inc., and has attended an advisory board as a panel member and consultant; L. G. has received research support from Ferring Pharmaceuticals, Inc., and has attended an advisory board as a panel member and consultant. D. W. has been a consultant for Ferring Pharmaceuticals, Inc.; prior to that she received research support for the conduct of this trial. She has attended an advisory board as a panel member and received support for travel to a FDA meeting on behalf of the sponsor; B. P. is a former employee of and current consultant to Ferring International; O. R. is an employee of Ferring Pharmaceuticals.
References
- Paccaud F, Martin-Beran B, Gutzwiller F. Hour of birth as a prognostic factor for perinatal death. Lancet 1988;1:340–3
- Stewart JH, Andrews J, Cartlidge PH. Numbers of deaths related to intrapartum asphyxia and timing of birth in all Wales perinatal survey, 1993–5. BMJ 1998;316:657–60
- Chalmers JW, Shanks E, Paterson S, et al. Scottish data on intrapartum related deaths are in same direction as Welsh data. BMJ 1998;317:539–40
- Stephansson O, Dickman PW, Johansson AL, et al. Time of birth and risk of intrapartum and early neonatal death. Epidemiology 2003;14:218–22
- Gould JB, Qin C, Chavez G. Time of birth and the risk of neonatal death. Obstet Gynecol 2005;106:352–8
- Badr LK, Abdallah B, Balian S, et al. The chasm in neonatal outcomes in relation to time of birth in Lebanon. Neonatal Netw 2007;26:97–102
- Gijsen R, Hukkelhoven CW, Schipper CM, et al. Effects of hospital delivery during off-hours on perinatal outcome in several subgroups: a retrospective cohort study. BMC Pregn Childbirth 2012;12:92
- Kalogiannidis I, Margioula-Siarkou C, Petousis S, et al. Infant births during the internal night are at increased risk for operative delivery and NICU admission. Arch Gynecol Obstet 2011;284:65–71
- Heller G, Misselwitz B, Schmidt S. Early neonatal mortality, asphyxia related deaths, and timing of low risk births in Hesse, Germany, 1990–8: observational study. BMJ 2000;321:274–5
- Luo ZC, Karlberg J. Timing of birth and infant and early neonatal mortality in Sweden 1973–95: longitudinal birth register study. BMJ 2001;323:1327–30
- de Cordova PB, Phibbs CS, Bartel AP, Stone PW. Twenty-four/seven: a mixed-method systematic review of the off-shift literature. J Adv Nurs 2012;68:1454–68
- de Cordova PB, Phibbs CS, Stone PW. Perceptions and observations of off-shift nursing. J Nurs Manag 2013;21:283–92
- Gaba DM, Howard SK. Patient safety: fatigue among clinicians and the safety of patients. N Engl J Med 2002;347:1249–55
- Jagsi R, Kitch BT, Weinstein DF, et al. Residents report on adverse events and their causes. Arch Intern Med 2005;165:2607–13
- Caughey AB, Urato AC, Lee KA, et al. Time of delivery and neonatal morbidity and mortality. Am J Obstet Gynecol 2008;199:496 e1–5
- Bakker JJ, van der Goes BY, Pel M, et al. Morning versus evening induction of labour for improving outcomes. Cochrane Database Syst Rev 2013;2:CD007707
- Wing DA, Brown R, Plante LA, et al. Misoprostol vaginal insert and time to vaginal delivery: a randomized controlled trial. Obstet Gynecol 2013;122:201–9
- Fraser WD, McLean FH, Usher RH. Diurnal variation in admission to hospital of women in labour. Can J Surg 1989;32:33–5
- Cagnacci A, Soldani R, Melis GB, Volpe A. Diurnal rhythms of labor and delivery in women: modulation by parity and seasons. Am J Obstet Gynecol 1998;178:140–5
- Anderka M, Declercq ER, Smith W. A time to be born. Am J Public Health 2000;90:124–6
- American College of Obstetricians Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol 2014;123:693–711
- National Institute for Health and Care Excellence. NICE guidelines [CG70] Induction of labour. Available from: http://www.nice.org.uk/guidance/CG70 [last accessed 20 Dec 2014]
- Dodd JM, Crowther CA, Robinson JS. Morning compared with evening induction of labor: a nested randomized controlled trial. A nested randomized controlled trial. Obstet Gynecol 2006;108:350–60
- Bakker JJ, De Vos R, Pel M, et al. Start of induction of labour with oxytocin in the morning or in the evening. A randomised controlled trial. BJOG 2009;116:562–8
- Oei SG, Jongmans L, Mol BW. Randomized trial of administration of prostaglandin E2 gel for induction of labor in the morning or the evening. J Perinat Med 2000;28:20–5
- Thorsell M, Lyrenas S, Andolf E, Kaijser M. Starting time for induction of labor and the risk for night-time delivery. Sex Reprod Healthc 2011;2:113–17

