Abstract
Objective: Prostaglandins (PGs) are considered the universal mediators of parturition. Amniotic fluid PGE2 and PGF2α concentrations increase before the onset of spontaneous labor at term, as well as during labor. This study was conducted to determine if the concentrations of umbilical cord PGE2 and PGF2α change with advancing gestational age, spontaneous labor at term, and preterm labor (with and without funisitis).
Methods: Umbilical cord (UC) tissue samples were obtained from women (N = 158) with singleton pregnancies in the following groups: (1) term deliveries without labor (TNL; n = 20); (2) term deliveries with labor (TIL; n = 20); (3) spontaneous preterm deliveries (sPTD) with (n = 20) and without acute funisitis (n = 20); and (4) preeclampsia without labor (n = 78). The concentrations of PGs were determined in different locations of the UC. PGE2 and PGF2α were measured by specific immunoassays. Non-parametric statistics were used for analysis.
Results: (1) In spontaneous preterm deliveries, the median UC PGE2 concentration was higher in cases with funisitis than in those without funisitis (233.7 pg/µg versus 87.4 pg/µg of total protein, p = 0.001); (2) the median UC PGE2 concentration in sPTD with funisitis was also higher than that obtained from samples who had undergone labor at term (233.7 pg/µg versus 116.1 pg/µg of total protein, p = 0.03); (3) the UC PGE2 and PGF2α concentration increased as a function of advancing gestational age before 36 weeks (PGE2: ρ = 0.59, p < 0.001; PGF2α: ρ = 0.39, p = 0.01), but not after 36 weeks (PGE2: ρ = −0.1, p = 0.5; PGF2α: ρ = −0.2, p = 0.2); (4) the median UC concentrations of PGE2 and PGF2α at term was similar in samples obtained from women with and without labor (PGE2: TNL 133.7 pg/µg versus TIL 116.1 pg/µg of total protein, p = 0.9; PGF2α: TNL 8.4 pg/µg versus TIL 8.1 pg/µg of total protein, p = 0.7); and (5) there was no correlation between UC PG concentration and gestational age at term pregnancy (PGE2: ρ = 0.01, p = 0.9; PGF2α: ρ = 0.07, p = 0.7).
Conclusions: (1) PGE2 concentrations in the UC are higher in the presence of acute funisitis than in the absence of this lesion; (2) spontaneous labor at term was not associated with a change in the UC concentration of PGE2 and PGF2α; and (3) the UC concentrations of PGE2 and PGF2α increased as a function of gestational age. We propose that UC PGs act as inflammatory mediators generated in the context of fetal systemic inflammation.
Introduction
Prostaglandins (PGs) are considered key mediators of human parturition [Citation1–19], and can induce myometrial contractility [Citation20–34], cervical remodeling [Citation35–58], and participate in extracellular matrix degradation leading to ruptured membranes [Citation7,Citation59–70]. The main PGs found in amniotic fluid are prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) [Citation16,Citation71–73], and concentrations of these eicosanoids increase prior to the onset of term labor [Citation11,Citation16,Citation17], during labor [Citation8,Citation72] or rupture of membranes at term [Citation7], and in spontaneous preterm labor [particularly in the setting of microbial invasion of the amniotic cavity (MIAC)] [Citation4–6,Citation12,Citation65,Citation73–82].
The increased concentrations of PGs in amniotic fluid have been considered important in both normal labor at term [Citation7,Citation8,Citation11,Citation12,Citation16,Citation71,Citation72,Citation82–84], and preterm labor [Citation4–6,Citation16,Citation65,Citation73–78,Citation81]. The amnion is an important source of PGs in the amniotic fluid [Citation85–94]. This membrane can be anatomically divided into three distinct regions: the reflected, placental, and umbilical amnion [Citation95–97]. Gene expression profile studies indicate that there are differences between placental and reflected amnion [Citation97–100]. Indeed, we previously reported that the placental amnion is responsible for the tonic production of PGs with advancing gestational age, while the reflected amnion increases the production of PGs during labor [Citation66]. Such difference in PG production suggests that the placental amnion and the reflected amnion play different roles in the regulation of eicosanoids during pregnancy and labor [Citation66].
The umbilical cord (UC) is a source of PGs [Citation95,Citation101–103]. PGE2 and PGF2α have been localized in the amniotic cells covering the UC and in the endothelium of the umbilical veins [Citation103]. Furthermore, McCoshen et al. claimed that the UC is the major source of PGE2 found in the amniotic fluid of women in labor at term [Citation95]. However, incubation of a segment of the UC, as well as the placental and reflected amnion in a perfusion chamber, allowed demonstration of the PGE2 output from the UC which did not change before or after labor, and the placental and reflected amnion PGE2 output were two-fold greater in tissues collected after labor [Citation95]. This observation suggests that the pattern of PG production by the UC is different from that of the reflected and placental amnion [Citation95]. The current study is conducted to assess the concentrations of PGE2 and PGF2α in UC segments from women who had undergone labor at term, women delivered by cesarean section without labor, and a group who had preterm labor and delivery (with and without funisitis).
Materials and methods
Tissue samples were retrieved from the Bank of Biological Specimens at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), U.S. Department of Health and Human Services (DHHS), Detroit, Michigan. Samples were taken from the following clinical groups, all of whom had singleton gestations: (1) women at term not in labor (TNL; n = 20); (2) women at term in labor (TIL; n = 20); (3) women with spontaneous preterm labor and delivery (sPTD; n = 20) without acute funisitis; (4) women with sPTD with acute funisitis (n = 20); and (5) women with preeclampsia who underwent cesarean delivery without labor over a range of gestational ages 25–39 weeks (n = 78). To compare the PG concentrations at different locations in a given cord, three samples from each UC (1 cm apart from UC insertion, 1 cm apart from the cord clamping, and mid-segment) were obtained from additional term delivery (n = 10) cases. Samples of UC tissue were flash-frozen using liquid nitrogen, and stored at −80 °C until use. All women provided written informed consent prior to the collection of samples, and the Institutional Review Boards of the NICHD and Wayne State University approved the collection and use of materials and clinical data for research purposes.
Clinical definition
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination, or by ultrasound examination alone if the sonographic determination of gestational age was not consistent with menstrual dating. Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 min in association with cervical changes in patients with a gestational age between 20 and 36 6/7 weeks which led to preterm delivery (defined as birth prior to the 37th week of gestation). Spontaneous term labor was defined as the presence of regular uterine contractions with a frequency of at least one every 10 min associated with cervical changes after 37 weeks of gestation. Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly, according to criteria previously published [Citation104–109]. Preeclampsia was defined as new onset hypertension during pregnancy (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 h to 1 week apart) and proteinuria (≥300 mg in a 24-h urine collection or one dipstick measurement ≥2+) [Citation110].
Prostaglandins assays
Tissue samples were pulverized in liquid nitrogen using a mortar and pestle for protein isolation. Total protein lysates were obtained using T-PER® Tissue Protein Extraction Reagent with a protease inhibitor cocktail (Thermo Scientific, Rockford, IL). To conduct the immunoassays for PGE2 and PGF2α content, 6.25 µg of total protein in 5 µl were added to 95 µl of assay buffer, and meclofenamic acid (10 µg/ml; Sigma-Aldrich Corporation, St. Louis, MO) was added to all samples. The assays were performed using the Prostaglandin E2 and Prostaglandin F2α enzyme immunoassay kits (Assay Designs, Inc., Ann Arbor, MI). The inter-assay coefficient of variation was 8.1%, and the intra-assay coefficient of variation was 6.7%.
Statistical analyses
Pearson’s chi-square test and Fisher’s exact test were used to compare proportions for categorical variables, and the Mann–Whitney U test was used to compare medians for continuous variables. Spearman’s correlation was used to examine the relationship between continuous variables such as gestational age at delivery and PG concentration. A repeated-measures analysis of variance test was performed to compare PG concentrations in different locations within each UC. A piecewise linear regression was performed to detect a change point in PG concentrations in preeclampsia using the SiZer package in the R program (www.r-project.org). Other statistical analyses were conducted using SPSS version 18.0 software (SPSS, Inc., Chicago, IL).
Results
Prostaglandin concentration in the umbilical cord in term and preterm labor/delivery
The clinical characteristics of the study groups as well as umbilical concentrations of PGE2 and PGF2α are displayed in . The median UC concentrations of PGE2 and PGF2α were not different among women at term, according to whether the samples had been obtained in women who had undergone labor (PGE2: TNL 133.7 pg/μg versus TIL 116.1 pg/µg of total protein, p = 0.9; PGF2α: TNL 8.4 pg/μg versus TIL 8.1 pg/µg of total protein, p = 0.7). The UC concentrations of PGE2 and PGF2α showed no correlation with gestational age at delivery in term pregnancies (PGE2: ρ = 0.01, p = 0.9; PGF2α: ρ = 0.07, p = 0.7).
Table 1. Clinical characteristics and concentrations of prostaglandins (PGE2 and PGF2α) in the umbilical cord for each study group.
The median PGE2 concentration in the UC of patients with spontaneous preterm delivery was significantly higher among patients with acute funisitis than in those without funisitis (233.7 pg/μg versus 87.4 pg/µg of total protein, p = 0.001). The median PGF2α UC concentration was also higher in patients with funisitis than in those without funisitis (9.6 pg/μg versus 7.1 pg/µg of total protein, p = 0.051).
The UC concentration of PGE2 (but not that of PGF2α) in cases of spontaneous preterm labor/delivery with funisitis was higher than that measured in cases who had undergone labor at term (PGE2: 233.7 pg/µg versus 116.1 pg/µg of total protein, p = 0.03; PGF2α: 9.6 pg/µg versus 8.1 pg/µg of total protein, p = 0.3).
The concentration of prostaglandins in the umbilical cord during gestation
The evaluation of UC PG concentration is not possible in women with uncomplicated pregnancies. Therefore, we assembled a study group comprised of women with preeclampsia who underwent indicated cesarean delivery. shows the clinical characteristics of patients with preeclampsia. All women with preeclampsia were delivered by cesarean in the absence of labor (n = 78). None of the patients had acute histologic chorioamnionitis or funisitis after pathologic evaluation of the placenta. We found a positive correlation between PG concentrations in the UC and gestational age at delivery (PGE2: ρ = 0.60, p < 0.001; PGF2α: ρ = 0.35, p = 0.002).
Table 2. Clinical characteristics and the umbilical cord concentrations of PGE2 and PGF2α for term and preterm preeclampsia cases.
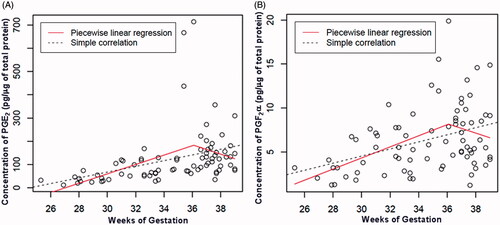
Since our findings showed that PG concentrations in the UC did not change with gestational age at term, we attempted to establish if there was a point of change in UC PG concentration in preeclampsia. A piecewise linear regression revealed that the slopes before 36 weeks provided an estimate of 20.1 pg/µg of total protein/week and 0.63 pg/µg of total protein/week – this difference was statistically significant for both PGE2 and PGF2α. In both cases, the slopes after 36 weeks were estimated as −20.0 pg/µg of total protein/week and −0.53 pg/µg of total protein/week, but they were not significantly different from zero at a 5% significance level (). These results indicate that the correlation between PG concentrations and gestational age changed significantly at about 36 weeks of gestation. Among patients with preeclampsia who were not in labor, there was an increase of PGE2 and PGF2α concentrations in the UC as a function of gestational age before 36 weeks (PGE2: ρ = 0.59, p < 0.001; PGF2α: ρ = 0.39, p = 0.01) but not after 36 weeks (PGE2: ρ = −0.1, p = 0.5; PGF2α: ρ = −0.2, p = 0.2).
Figure 1. PGE2 and PGF2α concentrations in the umbilical cord as a function of gestational age including preterm and term gestations with preeclampsia. Umbilical cord PGE2 and PGF2α concentrations increased with advancing gestational age until 36 weeks (PGE2: ρ = 0.59, p < 0.001; PGF2α: ρ = 0.39, p = 0.01), but not after 36 weeks (PGE2: ρ = −0.1, p = 0.5; PGF2α: ρ = −0.2, p = 0.2).
A comparison of the concentrations of prostaglandins at different locations in the same umbilical cord
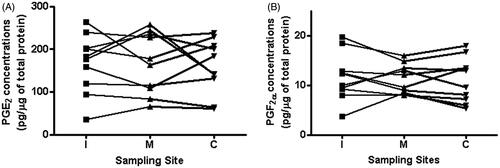
The concentrations of PGs at three different locations in the same UC were determined. illustrates concentrations of PGE2 and PGF2α at each UC sampling site. There were no significant differences in the concentrations of PGE2 and PGF2α among the three sampling sites (PGE2 and PGF2α: p = 0.7 and p = 0.7, respectively).
Figure 2. PGE2 and PGF2α umbilical cord concentrations in different sites of the umbilical cord. There was no significant difference in the umbilical cord concentrations of PGE2 and PGF2α among the three different sampling sites (PGE2; p = 0.7; and PGF2α; p = 0.7). (I: 1 cm apart from umbilical cord insertion. C: 1 cm apart from the cord clamping. M: mid-segment). Connected lines referred to prostaglandin concentration in a particular segment of the umbilical and in another segment in each patient.
Discussion
Principal findings of the study
(1) The UC concentrations of PGF2α and PGE2 after spontaneous preterm labor with funisitis were significantly greater than in preterm labor without this lesion; (2) spontaneous labor at term was not associated with a change in the UC concentrations of PGF2α and PGE2; and (3) among women who underwent a cesarean delivery without labor (because of preeclampsia), the UC concentrations of PGF2α and PGE2 increased as a function of advancing gestational age in the second and third trimesters.
Prostaglandins, different regions of the amnion, and the umbilical cord
Amniotic fluid PGs increase prior to the onset of labor [Citation11,Citation16,Citation17] and further increase during labor [Citation8,Citation72]. The amnion is considered to be the main source of PGs (in particular, PGE2) in the amniotic fluid [Citation85,Citation87–94]. The human amnion can be divided into three areas: (1) placental amnion; (2) reflected amnion; and (3) amnion covering the UC. The term “placental amnion” refers to that covering the chorionic plate of the placenta, while the reflected amnion is part of the extraplacental membranes. A small portion of amnion covers the surface of the UC [Citation95–97]. McCoshen et al. proposed that each component of amnion contributes to the production of PGE2, and that the UC is the major source of prostaglandin in the amniotic fluid in patients in labor at term [Citation95]. This work was based on in vitro experiments in which the production of PGs by different types of amnion was studied, and calculations about the relative contribution of each type were made. The investigators reported that before labor, the UC accounts for the greatest tissue mass (76%), compared to the placental amnion (14%) and reflected amnion (10%) [Citation95]. They further claimed that the UC accounts for 66% of the total PGE2 output before labor, and 44% after labor, proposing that the UC was the primary source of PGE2 in the amniotic fluid [Citation95].
Previously, we compared the expression of prostaglandin-endoperoxide synthase 2 (PTGS2), a key enzyme involved in prostaglandin production by amnion [Citation111–114], in placenta versus reflected amnion [Citation66]. We reported that: 1) before the onset of labor, PTGS2 expression was greater in placental amnion, rather than reflected amnion; 2) after labor, there was a significant increase in PTGS2 expression in reflected, but not in placental amnion; and 3) there was little expression of PTGS2 in preterm labor in the absence of acute inflammatory lesions [Citation66].
Changes in umbilical cord prostaglandins with advancing gestational age, labor, and acute inflammatory lesions of the placenta
The results of the current study indicate that the UC concentrations of PGE2 and PGF2α increase with advancing gestational age. Such findings were based on studies of UC derived from patients with the diagnosis of preeclampsia who underwent cesarean delivery for obstetrical indications. It is noteworthy that the prostaglandin concentration increased until approximately 36 weeks of gestation, and there was no detectable increase thereafter. Labor at term was not associated with an increase in the prostaglandin concentration in the UC. However, in the presence of funisitis (acute inflammation of the UC) the prostaglandin concentration increased significantly in cases with spontaneous preterm labor.
Amniotic fluid prostaglandins with microbial invasion of the amniotic cavity
Amniotic fluid concentrations of eicosanoids and arachidonate lipoxygenase products are increased in the amniotic fluid of women with preterm labor and MIAC [Citation4–6,Citation59,Citation74,Citation75,Citation82]. MIAC is frequently associated with acute histologic chorioamnionitis and funisitis [Citation82,Citation107,Citation108,Citation115–148]. Amniotic fluid PGs in cases of MIAC and/or intra-amniotic inflammation are thought to derive from amnion cells and local cells in the amniotic cavity, as well as from the human fetus. Microorganisms and their products (e.g. lipopolysaccharide endotoxin) could stimulate resident macrophages in the amniotic fluid, as well as fetal cells suspended in amniotic fluid or contained within fetal mucosa or skin to produce PGs which could be found in amniotic fluid. In a similar way, bacterial products could stimulate the amniotic epithelial cells surrounding the UC to produce PGs, which could find their way into the amniotic fluid. In the context of a fetal systemic inflammatory response syndrome (FIRS) [Citation104,Citation131,Citation149–156], prostaglandin production could be increased, as is the case for the adult systemic inflammatory response syndrome [Citation157–162]. PGs contained within the UC may exert a local effect, such as modifying vascular reactivity of the umbilical arteries and vein [Citation102,Citation163–168], altering vessel permeability [Citation169] or changing extracellular matrix metabolism in Wharton’s jelly. McCoshen et al. proposed that PGs generated in the UC could diffuse into the amniotic cavity [Citation95]. Experimental evidence is required to prove this concept.
Conclusion
Prostaglandin concentrations in the UC are increased in the context of funisitis. We propose that PGE2 and PGF2α act as inflammatory mediators in the fetal inflammatory response syndrome, whose pathologic hallmark is funisitis.
Declaration of interest
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD, and Detroit, MI, USA. The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to the patients who agreed to provide the materials of our studies; to the nurses, laboratory staff, and clinicians who made this work possible; and to Maureen McGerty (Wayne State University) for her critical reading of the manuscript. J.-S. H., R. R., N. G. T., L. Y., P. C., C. J. K., Y. M. K. worked on the design of the study, data analysis, manuscript preparation; D. C. L., S. A. performed experiments, data analysis; and J. S. K. contributed in histopathological analysis and manuscript preparation.
References
- Maddipati KR, Romero R, Chaiworapongsa T, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J 2014;28:4835–46
- Karim SM. The role of prostaglandins in human parturition. Proc Royal Soc Med 1971;64:10–12
- Mitchell MD. Prostaglandins during pregnancy and the perinatal period. J Reprod Fertil 1981;62:305–15
- Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–7
- Romero R, Wu YK, Mazor M, et al. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1988;34:141–5
- Romero R, Wu YK, Sintori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–61
- Romero R, Baumann P, Gomez R, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol 1993;168:1654–64; discussion 1664–8
- Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol 1994;171:1613–20
- Romero R, Gonzalez R, Baumann P, et al. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids 1994;50:97–104
- Mitchell MD, Romero RJ, Edwin SS, et al. Prostaglandins and parturition. Reprod Fertil Dev 1995;7:623–32
- Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 1996;54:187–91
- Gibb W. The role of prostaglandins in human parturition. Ann Med 1998;30:235–41
- Challis JR, Sloboda DM, Alfaidy N, et al. Prostaglandins and mechanisms of preterm birth. Reproduction 2002;124:1–17
- Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol 2003;17:717–30
- Challis JR, Bloomfield FH, Bocking AD, et al. Fetal signals and parturition. J Obstet Gynaecol Res 2005;31:492–9
- Lee SE, Romero R, Park IS, et al. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med 2008;21:89–94
- Mitchell MD, Chang MC, Chaiworapongsa T, et al. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab 2005;90:4244–8
- Vidaeff AC, Ramin SM. Potential biochemical events associated with initiation of labor. Curr Med Chem 2008;15:614–19
- Sykes L, MacIntyre DA, Teoh TG, et al. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction 2014;148:R29–40
- Kirton KT, Wyngarden LJ, Bergstrom KK. Prostaglandins and myometrial contractility. Adv Biosci 1973;9:651–5
- Novy MJ, Thomas CL, Lees MH. Uterine contractility and regional blood flow responses to oxytocin and prostaglandin E2 in pregnant rhesus monkeys. Am J Obstet Gynecol 1975;122:419–33
- Wikland M, Lindblom B, Wilhelmsson L, et al. Oxytocin, prostaglandins, and contractility of the human uterus at term pregnancy. Acta Obstet Gynecol Scand 1982;61:467–72
- Rydnert J, Joelsson I. Effect of the naturally occurring prostaglandins E2 and F2 alpha on the human myometrium in vivo during pregnancy. Acta Obstet Gynecol Scand 1985;64:577–82
- Wiqvist N, Bryman I, Lindblom B, et al. The role of prostaglandins for the coordination of myometrial forces during labour. Acta Physiol Hung 1985;65:313–22
- Asboth G, Phaneuf S, Lopez Bernal AL. Prostaglandin E receptors in myometrial cells. Acta Physiol Hung 1997;85:39–50
- Astle S, Thornton S, Slater DM. Identification and localization of prostaglandin E2 receptors in upper and lower segment human myometrium during pregnancy. Mol Hum Reprod 2005;11:279–87
- Woodcock NA, Taylor CW, Thornton S. Prostaglandin F2alpha increases the sensitivity of the contractile proteins to Ca2+ in human myometrium. Am J Obstet Gynecol 2006;195:1404–6
- Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front Biosci 2007;12:1329–43
- Hurd WW, Gibbs SG, Rudinsky KA. Differential regulation of myometrial prostaglandin production by changes in length. Am J Obstet Gynecol 2008;198:225 e221–4
- Durn JH, Marshall KM, Farrar D, et al. Lipidomic analysis reveals prostanoid profiles in human term pregnant myometrium. Prostaglandins Leukot Essent Fatty Acids 2010;82:21–6
- Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol 2011;204:177 e115–33
- Chiossi G, Costantine MM, Bytautiene E, et al. The effects of prostaglandin E1 and prostaglandin E2 on in vitro myometrial contractility and uterine structure. Am J Perinatol 2012;29:615–22
- Arulkumaran S, Kandola MK, Hoffman B, et al. The roles of prostaglandin EP 1 and 3 receptors in the control of human myometrial contractility. J Clin Endocrinol Metab 2012;97:489–98
- Conde-Agudelo A, Nieto A, Rosas-Bermudez A, et al. Misoprostol to reduce intraoperative and postoperative hemorrhage during cesarean delivery: a systematic review and metaanalysis. Am J Obstet Gynecol 2013;209:40. e1–40. e17
- Keirse MJ, Thiery M, Parewijck W, et al. Chronic stimulation of uterine prostaglandin synthesis during cervical ripening before the onset of labor. Prostaglandins 1983;25:671–82
- Norman M, Ekman G, Malmstrom A. Prostaglandin E2-induced ripening of the human cervix involves changes in proteoglycan metabolism. Obstet Gynecol 1993;82:1013–20
- Voss DH, Cumminsky KC, Cook VD, et al. Effect of three concentrations of intracervical prostaglandin E2 gel for cervical ripening. J Matern Fetal Med 1996;5:186–93
- Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol 1999;180:614–20
- Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol with vaginally administered misoprostol for cervical ripening and labor induction. Am J Obstet Gynecol 1999;180:1155–60
- Fujimoto T, Savani RC, Watari M, et al. Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2). Am J Pathol 2002;160:1495–502
- Ben-Aroya Z, Hallak M, Segal D, et al. Ripening of the uterine cervix in a post-cesarean parturient: prostaglandin E2 versus Foley catheter. J Matern Fetal Neonatal Med 2002;12:42–5
- Larmon JE, Magann EF, Dickerson GA, et al. Outpatient cervical ripening with prostaglandin E2 and estradiol. J Matern Fetal Neonatal Med 2002;11:113–17
- D'Aniello G, Bocchi C, Florio P, et al. Cervical ripening and induction of labor by prostaglandin E2: a comparison between intracervical gel and vaginal pessary. J Matern Fetal Neonatal Med 2003;14:158–62
- Chien EK, Macgregor C. Expression and regulation of the rat prostaglandin E2 receptor type 4 (EP4) in pregnant cervical tissue. Am J Obstet Gynecol 2003;189:1501–10
- Yogev Y, Ben-Haroush A, Gilboa Y, et al. Induction of labor with vaginal prostaglandin E2. J Matern Fetal Neonatal Med 2003;14:30–4
- Garry D, Figueroa R, Kalish RB, et al. Randomized controlled trial of vaginal misoprostol versus dinoprostone vaginal insert for labor induction. J Matern Fetal Neonatal Med 2003;13:254–9
- Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids 2004;70:207–22
- Crane JM, Delaney T, Butt KD, et al. Predictors of successful labor induction with oral or vaginal misoprostol. J Matern Fetal Neonatal Med 2004;15:319–23
- Tornblom SA, Patel FA, Bystrom B, et al. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab 2004;89:2909–15
- Word RA, Li XH, Hnat M, et al. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007;25:69–79
- Ji H, Dailey TL, Long V, et al. Prostaglandin E2-regulated cervical ripening: analysis of proteoglycan expression in the rat cervix. Am J Obstet Gynecol 2008;198:536 e531–7
- Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 2010;21:353–61
- Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction 2012;143:429–38
- Favilli A, Acanfora MM, Bini V, et al. Single indication of labor induction with prostaglandins: is advanced maternal age a risk factor for cesarean section? A matched retrospective cohort study. J Matern Fetal Neonatal Med 2013;26:665–8
- Haas J, Barzilay E, Chayen B, et al. Safety of labor induction with prostaglandin E2 in grandmultiparous women. J Matern Fetal Neonatal Med 2013;26:49–51
- Melamed N, Yariv O, Hiersch L, et al. Labor induction with prostaglandin E2: characteristics of response and prediction of failure. J Matern Fetal Neonatal Med 2013;26:132–6
- Timmons BC, Reese J, Socrate S, et al. Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening. Endocrinology 2014;155:287–98
- Kishore AH, Owens D, Word RA. Prostaglandin E2 regulates its own inactivating enzyme, 15-PGDH, by EP2 receptor-mediated cervical cell-specific mechanisms. J Clin Endocrinol Metab 2014;99:1006–18
- McLaren J, Taylor DJ, Bell SC. Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod 2000;6:1033–40
- Ulug U, Goldman S, Ben-Shlomo I, et al. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod 2001;7:1187–93
- Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig 2004;11:427–37
- Moore RM, Mansour JM, Redline RW, et al. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta 2006;27:1037–51
- Oger S, Mehats C, Dallot E, et al. Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin E2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J Immunol 2005;174:8082–9
- Makino S, Zaragoza DB, Mitchell BF, et al. Prostaglandin F2alpha and its receptor as activators of human decidua. Semin Reprod Med 2007;25:60–8
- Lee SE, Park IS, Romero R, et al. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern-Fetal Neonat Med 2009;22:880–6
- Lee DC, Romero R, Kim JS, et al. Evidence for a spatial and temporal regulation of prostaglandin-endoperoxide synthase 2 expression in human amnion in term and preterm parturition. J Clin Endocrinol Metab 2010;95:E86–91
- Rossi D, Pianta S, Magatti M, et al. Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One 2012;7:e46956
- Alzamil HA, Pawade J, Fortier MA, et al. Expression of the prostaglandin F synthase AKR1B1 and the prostaglandin transporter SLCO2A1 in human fetal membranes in relation to spontaneous term and preterm labor. Front Physiol 2014;5:272
- Phillips RJ, Fortier MA, Lopez Bernal A. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregn Childbirth 2014;14:241
- Menon R, Fortunato SJ, Yu J, et al. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta 2011;32:317–22
- Dray F, Frydman R. Primary prostaglandins in amniotic fluid in pregnancy and spontaneous labor. Am J Obstet Gynecol 1976;126:13–19
- Nieder J, Augustin W. Increase of prostaglandin E and F equivalents in amniotic fluid during late pregnancy and rapid PG F elevation after cervical dilatation. Prostagland Leukotr Med 1983;12:289–97
- Mazor M, Wiznitzer A, Maymon E, et al. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990;26:425–8
- Romero R, Emamian M, Quintero R, et al. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet 1986;1:1380
- Lopez Bernal A, Hansell DJ, Canete Soler R, et al. Prostaglandins, chorioamnionitis and preterm labour. Br J Obstet Gynaecol 1987;94:1156–8
- Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol 1989;9:60–5
- Romero R, Wu YK, Mazor M, et al. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1989;36:69–75
- Lamont RF, Anthony F, Myatt L, et al. Production of prostaglandin E2 by human amnion in vitro in response to addition of media conditioned by microorganisms associated with chorioamnionitis and preterm labor. Am J Obstet Gynecol 1990;162:819–25
- Mitchell MD, Romero RJ, Avila C, et al. Prostaglandin production by amnion and decidual cells in response to bacterial products. Prostaglandins Leukot Essent Fatty Acids 1991;42:167–9
- van Meir CA, Matthews SG, Keirse MJ, et al. 15-hydroxyprostaglandin dehydrogenase: implications in preterm labor with and without ascending infection. J Clin Endocrinol Metab 1997;82:969–76
- Hsu CD, Meaddough E, Aversa K, et al. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol 1998;15:683–7
- Romero R, Espinoza J, Goncalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–26
- Leaver HA, MacPherson HD, Hutchon DJ. Amniotic fluid prostaglandins F2 alpha and E2, measured at artificial rupture of the membranes, predict the subsequent progress of labour. Prostagland Leukotr Med 1987;28:237–42
- Menon R, Fortunato SJ, Milne GL, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol 2011;118:121–34
- Olson DM, Skinner K, Challis JR. Prostaglandin output in relation to parturition by cells dispersed from human intrauterine tissues. J Clin Endocrinol Metab 1983;57:694–9
- Di Renzo GC, Anceschi MM, Bleasdale JE. Beta-adrenergic stimulation of prostaglandin production by human amnion tissue. Prostaglandins 1984;27:37–49
- Teixeira FJ, Zakar T, Hirst JJ, et al. Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term, and during labor. J Clin Endocrinol Metab 1994;78:1396–402
- Hirst JJ, Teixeira FJ, Zakar T, et al. Prostaglandin H synthase-2 expression increases in human gestational tissues with spontaneous labour onset. Reprod Fertil Dev 1995;7:633–7
- Gibb W, Sun M. Localization of prostaglandin H synthase type 2 protein and mRNA in term human fetal membranes and decidua. J Endocrinol 1996;150:497–503
- Fuentes A, Spaziani EP, O'Brien WF. The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins 1996;52:261–7
- Brennand JE, Leask R, Kelly RW, et al. Mechanisms involved in the stimulatory effect of amniotic fluid on prostaglandin production by human fetal membranes. Prostaglandins Leukot Essent Fatty Acids 1998;58:369–75
- Furuta I, Yamada H, Sagawa T, et al. Effects of inflammatory cytokines on prostaglandin E(2) production from human amnion cells cultured in serum-free condition. Gynecol Obstetr Investig 2000;49:93–7
- Challis JRG, Matthews SG, Gibb W, et al. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000;21:514–50
- Harirah H, Thota C, Wentz MJ, et al. Elevated expression of catechol-O-methyltransferase is associated with labor and increased prostaglandin E(2) production by human fetal membranes. Am J Obstet Gynecol 2009;201:496 e491–7
- McCoshen JA, Tulloch HV, Johnson KA. Umbilical cord is the major source of prostaglandin E2 in the gestational sac during term labor. Am J Obstet Gynecol 1989;160:973–8
- Benirschke K, Kaufmann P, Baergen R. Pathology of the human placenta. New York: Springer; 2006
- Han YM, Romero R, Kim JS, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod 2008;79:954–61
- Marvin KW, Keelan JA, Eykholt RL, et al. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol 2002;187:728–34
- Erez O, Romero R, Tarca AL, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2009;22:1103–15
- Kim SY, Romero R, Tarca AL, et al. miR-143 regulation of prostaglandin-endoperoxidase synthase 2 in the amnion: implications for human parturition at term. PLoS One 2011;6:e24131
- Willman EA, Collins WP. The concentrations of prostaglandin E2 and prostaglandin F2alpha in tissues within the fetoplacental unit after spontaneous or induced labour. Br J Obstet Gynaecol 1976;83:786–9
- Willman EA, Collins WP. Distribution of prostaglandins E2 and F2 alpha within the foetoplacental unit throughout human pregnancy. J Endocrinol 1976;69:413–19
- Siegel RJ, Villa LC, Fishbein MC. Immunohistochemical localization of 6-keto-prostaglandin F1 alpha and prostaglandin E2 in the human umbilical cord before and after labor. Lab Invest 1987;56:550–3
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–48
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Mi Lee S, Romero R, Lee KA, et al. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. J Matern Fetal Neonatal Med 2011;24:37–42
- Kim SM, Romero R, Park JW, et al. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Kim YM, Chaemsaithong P, Romero R, et al. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997;177:1003–10
- Slater D, Berger L, Newton R, et al. The relative abundance of type 1 to type 2 cyclo-oxygenase mRNA in human amnion at term. Biochem Biophys Res Commun 1994;198:304–8
- Slater DM, Berger LC, Newton R, et al. Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol 1995;172:77–82
- Hirst JJ, Teixeira FJ, Zakar T, et al. Prostaglandin endoperoxide-H synthase-1 and -2 messenger ribonucleic acid levels in human amnion with spontaneous labor onset. J Clin Endocrinol Metab 1995;80:517–23
- Slater D, Dennes W, Sawdy R, et al. Expression of cyclo-oxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol 1999;22:125–30
- Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–8
- Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol 1990;75:622–6
- Romero R, Salafia CM, Athanassiadis AP, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992;166:1382–8
- Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–8
- Greig PC, Ernest JM, Teot L, et al. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol 1993;169:1035–44
- Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179:1254–60
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–9
- Williams MC, O'Brien WF, Nelson RN, et al. Histologic chorioamnionitis is associated with fetal growth restriction in term and preterm infants. Am J Obstet Gynecol 2000;183:1094–9
- Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6
- Ustun C, Kocak I, Baris S, et al. Subclinical chorioamnionitis as an etiologic factor in preterm deliveries. Int J Gynaecol Obstet 2001;72:109–15
- Romero R, Espinoza J, Chaiworapongsa T, et al. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–74
- Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13
- Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45
- Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55
- De Paepe ME, Friedman RM, Gundogan F, et al. The histologic fetoplacental inflammatory response in fatal perinatal group B-streptococcus infection. J Perinatol 2004;24:441–5
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113:17–42
- Lee SE, Romero R, Kim CJ, et al. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern-Fetal Neonat Med 2006;19:693–7
- Romero R, Gotsch F, Pineles B, et al. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65:S194–202
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39
- Holzman C, Lin X, Senagore P, et al. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol 2007;166:786–94
- Seong HS, Lee SE, Kang JH, et al. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol 2008;199:375 e371–5
- Romero R, Lockwood CJ. Pathogenesis of spontaneous preterm labor. In: Creasy RK, Resnik R, Iams JD, editors. Creasy and Resnik's maternal-fetal medicine: principles and practice. Philadelphia: Elsevierl 2009:521–43
- Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36
- DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57
- Park HS, Romero R, Lee SM, et al. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta 2010;31:792–5
- Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis–a complex pathophysiologic syndrome. Placenta 2010;31:113–20
- Lee SM, Lee KA, Kim SM, et al. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta 2011;32:516–21
- Bersani I, Thomas W, Speer CP. Chorioamnionitis–the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med 2012;25:12–16
- Martinelli P, Sarno L, Maruotti GM, et al. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med 2012;25:29–31
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5
- Vajrychova M, Kacerovsky M, Tambor V, et al. Microbial invasion and histological chorioamnionitis upregulate neutrophil-gelatinase associated lipocalin in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]
- Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e121–25 e115
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325 e321–25 e310
- Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014;211:385 e381–9
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83
- Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92
- Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med 2011;40:19–32
- Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med 2011;39:653–66
- Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:795–801
- Hofer N, Kothari R, Morris N, et al. The fetal inflammatory response syndrome is a risk factor for morbidity in preterm neonates. Am J Obstet Gynecol 2013;209:542 e541–42 e511
- Baracos V, Rodemann HP, Dinarello CA, et al. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 1983;308:553–8
- Huribal M, Cunningham ME, D'Aiuto ML, et al. Endothelin-1 and prostaglandin E2 levels increase in patients with burns. J Am Coll Surg 1995;180:318–22
- Haupt W, Fritzsche H, Hohenberger W, et al. Selective cytokine release induced by serum and separated plasma from septic patients. Eur J Surg 1996;162:769–76
- Hahn EL, Clancy KD, Tai HH, et al. Prostaglandin E2 alterations during sepsis are partially mediated by endotoxin-induced inhibition of prostaglandin 15-hydroxydehydrogenase. J Trauma 1998;44:777–81; discussion 781–2
- Brogliato AR, Antunes CA, Carvalho RS, et al. Ketoprofen impairs immunosuppression induced by severe sepsis and reveals an important role for prostaglandin E2. Shock 2012;38:620–9
- Kwiatkoski M, Soriano RN, Araujo RM, et al. Hydrogen sulfide inhibits preoptic prostaglandin E2 production during endotoxemia. Exp Neurol 2013;240:88–95
- Kawano M, Mori N. Prostacyclin producing activity of human umbilical, placental and uterine vessels. Prostaglandins 1983;26:645–62
- Monuszko E, Halevy S, Freese KJ, et al. Umbilical vessels endothelium and vascular reactivity. Microcirc Endothelium Lymphatics 1990;6:183–208
- Scalera F. Intracellular glutathione and lipid peroxide availability and the secretion of vasoactive substances by human umbilical vein endothelial cells after incubation with TNF-alpha. Eur J Clin Investig 2003;33:176–82
- Daray FM, Minvielle AI, Puppo S, et al. Vasoconstrictor effects of 8-iso-prostaglandin E2 and 8-iso-prostaglandin F(2alpha) on human umbilical vein. Eur J Pharmacol 2004;499:189–95
- Errasti AE, del-Rey G, Cesio CE, et al. Expression and functional evidence of the prostaglandin F2alpha receptor mediating contraction in human umbilical vein. Euro J Pharmacol 2009;610:68–74
- Topal G, Foudi N, Uydes-Dogan BS, et al. Involvement of prostaglandin F2alpha in preeclamptic human umbilical vein vasospasm: a role of prostaglandin F and thromboxane A2 receptors. J Hypertens 2010;28:2438–45
- Kim SR, Bae SK, Park HJ, et al. Thromboxane A(2) increases endothelial permeability through upregulation of interleukin-8. Biochem Biophys Res Commun 2010;397:413–19

