Abstract
Objective: To estimate the cost-effectiveness of fetal aneuploidy screening in the general pregnancy population using non-invasive prenatal testing (NIPT) as compared to first trimester combined screening (FTS) with serum markers and NT ultrasound.
Methods: Using a decision-analytic model, we estimated the number of fetal T21, T18, and T13 cases identified prenatally, the number of invasive procedures performed, corresponding normal fetus losses, and costs of screening using FTS or NIPT with cell-free DNA (cfDNA). Modeling was based on a 4 million pregnant women cohort, which represents annual births in the U.S.
Results: For the general pregnancy population, NIPT identified 15% more trisomy cases, reduced invasive procedures by 88%, and reduced iatrogenic fetal loss by 94% as compared to FTS. The cost per trisomy case identified with FTS was $497 909. At a NIPT unit, cost of $453 and below, there were cost savings as compared to FTS. Accounting for additional trisomy cases identified by NIPT, a NIPT unit cost of $665 provided the same per trisomy cost as that of FTS.
Conclusions: NIPT in the general pregnancy population leads to more prenatal identification of fetal trisomy cases as compared to FTS and is more economical at a NIPT unit cost of $453.
Introduction
Down syndrome, which is caused by trisomy 21 (T21), is the most common aneuploidy found at birth and is associated with developmental and neurocognitive delay and other medical issues. Prenatal screening for Down syndrome is a standard clinical offering in many countries and has been employed over many years [Citation1,Citation2]. Screening for less common aneuploidies such as trisomy 18 (T18) and trisomy 13 (T13) is often included as well [Citation3].
Prenatal screening for T21 has evolved over the past several decades from initially using only maternal age as the criteria to the addition of serum protein markers as well as specialized ultrasound that allows for measurement of nuchal translucency (NT). First trimester combined screening (FTS) utilizes two serum proteins, beta unit of human chorionic gonadotropin (β-hCG) and pregnancy-associated plasma protein A (PAPP-A), in conjunction with NT measurement to provide women with a risk assessment for fetal T21. While FTS provides for early screening within the first trimester of pregnancy, it has two notable shortcomings. First, it requires ultrasound to be performed by specially trained ultrasonographers to accurately measure the NT [Citation4]. Second, FTS identifies only about 85% of fetal T21 cases with a 5% false-positive rate [Citation2].
Non-invasive prenatal testing (NIPT) with cell-free DNA (cfDNA) has been shown in numerous clinical studies to be highly accurate for screening of fetal trisomies with false-positive rates at 0.1% or less for each trisomy tested [Citation5,Citation6]. The accuracy of NIPT has been consistent in all pregnant women populations, regardless of age or risk status [Citation7,Citation8]. As NIPT only requires a standard blood draw without any special ultrasound assessments, it enables general Ob/Gyns as well as other primary care providers such as midwives to implement prenatal screening for fetal trisomy with high accuracy.
The objective of this study was to compare the cost-effectiveness of prenatal screening for common fetal trisomies with FTS or NIPT within a representative general pregnancy population in the U.S.
Methods
Using DATA Pro (TreeAge Software Inc., Williamston, MA), we modified a previously published decision-analytic model to compare different prenatal screening strategies for fetal T21, T18, and T13 in a general pregnancy screening population [Citation9]. The screening strategies compared consisted of: (1) FTS which included measurement of serum proteins β-hCG and PAPP-A as well as ultrasound assessment for NT measurement and (2) NIPT with cfDNA. For both FTS and NIPT, we assumed both received the same standard obstetrical ultrasounds during pregnancy. However, as only FTS requires NT, which is a specialized ultrasound measurement, we assumed a proportion of patients would need to be referred from their primary care provider to complete screening with FTS.
We searched MEDLINE from 1997 to 2014 for English-language literature using the terms Down syndrome, trisomy 21, trisomy 18, trisomy 13, prenatal screening, non-invasive prenatal diagnosis, NIPT, non-invasive prenatal screening and cell-free DNA analysis. In addition, we reviewed abstracts from national meetings, data from Medicare, and relevant data from companies offering NIPT tests.
For the analysis, we used a cohort of 4 000 000 pregnant women which represents the current estimated annual number of births in the U.S. The first trimester prevalence of each trisomy, the performance of each screening modality in terms of sensitivity and specificity, and the risk of fetal loss from invasive testing are shown in . In the base case, we assumed a 70% screening uptake for both FTS and NIPT. For those that proceed with screening, tests can result in true positives, false positives, true negatives, and false negatives. Any screen positives, whether true or false positives, were assumed to have sufficient follow-up so that any fetal trisomies from a screen positive result were detected. Fetal losses from invasive testing complications were captured.
Table 1. Probability and cost variables.
All costs are represented in 2014 USD. Cost items, which are listed in , included those associated with screening tests, invasive testing, office visits and counseling, termination procedures, and cost of each trisomy birth. A range of unit costs for NIPT were used for the analysis. When possible, the Medicare 2014 Fee Schedule was used to estimate cost inputs. Any cost inputs relying on data prior to 2014 were adjusted taking into account inflation based on the Bureau of Labor Statistics. A range of cost values based on published literature were used for sensitivity analysis. The cost for screening and invasive testing was based on the total cost which included any expected payments by insurance as well as patient co-pays. For the base case, we assumed 35% of FTS would require referral from a primary care provider to a specialist to perform the NT, which would incur the additional cost of an office visit. We did not assume any other downstream additional costs from the specialist referral. All screen positive tests were assumed to have follow-up counseling, which generated an office visit cost. For cost analysis, the cost of screening was inclusive of the screening test(s) and any associated office visits. Invasive testing costs included the cost of the invasive procedure as well as any terminations. The baseline cost for a given trisomy birth was estimated based on direct medical costs as well as indirect costs.
The primary outcomes of the analyses were separated into clinical and economic outcomes. For the clinical outcomes, the number of fetal trisomies detected based on confirmatory testing and number of normal fetus losses due to invasive procedures for each screening strategy was determined. For the economic outcomes, the NIPT unit cost at which it was cost savings and cost equivalent on a per trisomy case as compared to FTS was determined. Sensitivity analyses were performed on all cost and effectiveness variables over the ranges specified in .
Results
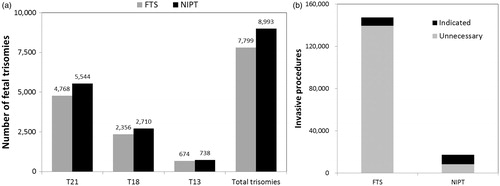
Based on a theoretical general pregnancy population of 4 million women, which represents the annual number of U.S. births, we assumed a 70% screening uptake representing 2.8 million women undergoing screening with either FTS or NIPT. NIPT led to the identification of 8993 trisomy cases, of which 5544 were T21 whereas FTS led to the identification of 7799 trisomy cases of which 4768 were T21 (). The total number of invasive procedures with NIPT was 17 303 of which 8342 were unnecessary due to false-positive NIPT screening results. This led to 42 normal fetal losses. With FTS, the total number of invasive procedures was 147 311 of which 139 540 were unnecessary due to false-positive FTS screening results (). This led to 698 normal fetal losses. As compared to FTS, NIPT identified 15% more trisomy cases, reduced invasive procedures by 88%, and reduced iatrogenic normal fetal loss by 94%.
Figure 1. (a) Number of trisomies cases identified with FTS or NIPT by trisomy type and total. (b) Number of invasive procedures that were indicated or unnecessary due to false-positive results with FTS or NIPT.
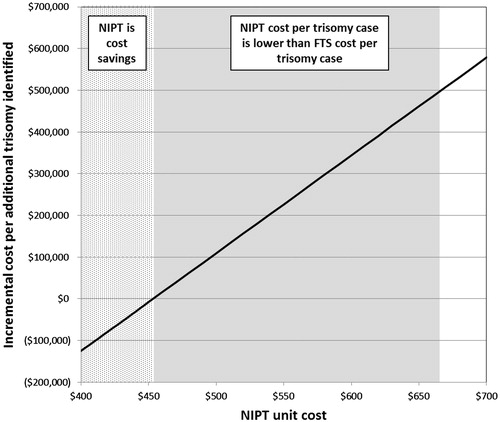
The total costs of screening the cohort with FTS was $3.88B with each trisomy case identified costing $497 909. Taking into account only costs, at a NIPT unit cost of $453 and less, NIPT demonstrated cost savings over FTS. When accounting for the additional trisomy cases identified with NIPT, at a NIPT unit cost of $665, the cost per trisomy case identified was equivalent to that of FTS (). No economic value was assigned in the model for any normal fetus losses averted.
Figure 2. NIPT unit cost analysis. The dotted area shows NIPT unit costs at which total costs are less compared to FTS. The shaded area shows NIPT unit costs at which the cost per trisomy case identified with NIPT is equivalent or lower than that of FTS, but the total overall costs are higher.
Sensitivity analysis was performed on key variables using the ranges shown in . In one-way sensitivity analysis, NIPT remained the dominant strategy over FTS in all analyses, except when the cost of NIPT exceeded $453. A two-way sensitivity analysis was performed looking at improved adherence to screening with NIPT over FTS and NIPT cost. We evaluated NIPT screening adherence at 70% (baseline, same as FTS) and at 5% increased increments at 75%, 80%, and 85% while keeping FTS screening adherence at 70%. At increased screening adherence with NIPT of 75%, 80%, and 85%, NIPT remained cost savings over FTS at a NIPT unit cost up to $490, $522, and $550, respectively.
Discussion
For the general pregnancy population, NIPT at the appropriate cost is the preferred and dominant primary screening strategy for fetal trisomies. We decided to compare NIPT to FTS as both can be performed in the first trimester of pregnancy and therefore provide earlier information to best manage the pregnancy. In our study, NIPT was able to identify 15% more trisomy cases than FTS as well as significantly reducing the amount of invasive procedures and as a consequence leading to 656 normal fetuses being saved annually, the benefits of which were not quantified economically in the model. The clinical superiority of NIPT is expected, given its higher accuracy as compared to FTS [Citation2,Citation6].
The initial implementation of NIPT has primarily been in pregnant women classified as “high risk” based on maternal age or other risk factors. ACOG issued a statement in 2012 that supported NIPT as an option only in “high risk” women [Citation10]. Since then, numerous clinical studies have validated the performance of NIPT in “average risk” or “low risk” women and professional groups have supported NIPT as an option in any pregnant woman, regardless of age or risk [Citation7,Citation8,Citation11,Citation12]. The primary barrier to adoption of NIPT in the general pregnancy population appears to be one of cost. Our analysis shows that at a NIPT unit cost of $453 or less, it is cost savings over FTS. At this NIPT unit cost, NIPT is clearly the dominant screening strategy since the overall costs are lower with additional clinical benefits. The cost analysis could also be evaluated based on the cost per trisomy case identified. As NIPT identifies more fetal trisomies than FTS, a NIPT unit cost of $665 allows a cost per trisomy case identified to be equivalent to that of FTS. However, in this latter case, the total overall costs with NIPT are higher than that of FTS.
Conventional screening methods, such as FTS, not only have less accuracy than NIPT, but can be more cumbersome to implement. FTS requires the assessment of both blood serum protein markers as well as NT. As NT is a specialized ultrasound procedure, primary care providers including Ob/Gyns may need to refer their pregnant patients to a specialist to carry out screening. Specialist referral leads to additional costs to the healthcare system as well as placing an inconvenience to patients, especially those who live in less populated areas and therefore may need to travel considerable distances to obtain the NT. NIPT allows for all pregnant women to have equal access to a highly accurate screening test for fetal trisomies and also provides a means for screening to be performed by primary care providers.
Given the higher accuracy and ease of implementation, we performed a sensitivity analysis in which we assumed higher uptake of screening with NIPT as compared to FTS. Studies suggest that NIPT may lead to higher uptake of prenatal screening [Citation13,Citation14]. Our analysis showed that NIPT could remain cost savings up to a unit price of $550, if NIPT allowed for improved screening adherence to 85%, a 15% improvement over the base case assumption for FTS.
Several recent cost-effectiveness analyses on NIPT in the general pregnancy population have been published highlighting the broader use of NIPT in all pregnant women as a timely topic [Citation15–17]. These studies looked at screening for Down syndrome only and compared various conventional screening methods, but all found NIPT to be clinically superior. None of these studies directly compared NIPT to FTS nor took into account the additional costs of specialist referral for FTS to perform NT. The cost of NIPT appeared to be the primary open issue in these other published studies. One study found NIPT to be more costly, but also assumed a NIPT unit cost of $1000 [Citation15]. At a lower unit cost of $453, it is probable that NIPT would have been found to be cost savings.
As with any cost-effectiveness analysis, there are limitations. The analysis is based on a theoretical cohort of women as well as assumptions on screening performance, uptake, and cost. The analysis was also performed based on a U.S. population. Screening practices and costs can be quite different in other countries and so the findings here may not be generalizable outside the U.S. Our analysis also focused only on fetal trisomies 21, 18, and 13. We decided to focus on these conditions as they are the ones commonly being screened for today and supported by clinical standards. While both FTS and NIPT have the possibility to pick up other rare medical conditions, we are not aware of any analysis that demonstrates clinical utility or supports assessment of these other medical conditions for screening the general pregnancy population.
NIPT represents a technological advance in prenatal screening that has high accuracy for prenatal assessment of fetal trisomies. Based on our cost-effectiveness model looking at the U.S. general pregnancy population, NIPT can identify more fetal trisomy cases and at the same time reduce unnecessary invasive procedures and in turn fewer related normal fetus losses. These clinical benefits are realized in the setting of also achieving cost savings at the appropriate unit cost of NIPT.
Declaration of interest
K. S. is a paid employee of Ariosa Diagnostics, Inc.
References
- Driscoll DA, Gross S. Clinical practice. Prenatal screening for aneuploidy. N Engl J Med 2009;360:2556–62
- ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obs Gynecol 2007;109:217–27
- Kagan KO, Wright D, Valencia C, et al. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta-hCG and pregnancy-associated plasma protein-A. Hum Reprod 2008;23:1968–75
- Evans MI, Krantz DA, Hallahan TW, Sherwin J. Impact of nuchal translucency credentialing by the FMF, the NTQR or both on screening distributions and performance. Ultrasound Obs Gynecol 2012;39:181–4
- Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obs Gynecol 2012;207:137.e1–8
- Gil MM, Akolekar R, Quezada MS, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: meta-analysis. Fetal Diagn Ther 2014;35:156–73
- Nicolaides KH, Syngelaki A, Ashoor G, et al. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obs Gynecol 2012;207:374.e1–6
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015;372:1589--97
- Song K, Musci TJ, Caughey AB. Clinical utility and cost of non-invasive prenatal testing with cfDNA analysis in high-risk women based on a US population. J Matern Fetal Neonatal Med 2013;7058:1–6
- Committee opinion no. 545: noninvasive prenatal testing for fetal aneuploidy. Obs Gynecol 2012;120:1532–4
- Gregg AR, Gross SJ, Best RG, et al. ACMG statement on noninvasive prenatal screening for fetal aneuploidy. Genet Med 2013;15:395–8
- Dondorp W, de Wert G, Bombard Y, et al. Non-invasive prenatal testing for aneuploidy and beyond: challenges of responsible innovation in prenatal screening. Eur J Hum Genet 2015. [Epub ahead of print]. doi: 10.1038/ejhg.2015.57
- Larion S, Warsof SL, Romary L, et al. Uptake of noninvasive prenatal testing at a large academic referral center. Am J Obs Gynecol 2014;211:651.e1–7
- Hill M, Fisher J, Chitty LS, Morris S. Women’s and health professionals’ preferences for prenatal tests for Down syndrome: a discrete choice experiment to contrast noninvasive prenatal diagnosis with current invasive tests. Genet Med 2012;14:905–13
- Evans MI, Sonek JD, Hallahan TW, Krantz DA. Cell-free fetal DNA screening in the USA: a cost analysis of screening strategies. Ultrasound Obs Gynecol 2015;45:74–83
- Walker BS, Jackson BR, LaGrave D, et al. A cost-effectiveness analysis of cell free DNA as a replacement for serum screening for Down syndrome. Prenat Diagn 2014 . [Epub ahead of print]. doi: 10.1002/pd.4511
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome – a cost sensitivity analysis. Prenat Diagn 2013;33:636–42
- Cuckle HS, Wald NJ, Thompson SG. Estimating a woman’s risk of having a pregnancy associated with Down’s syndrome using her age and serum alpha-fetoprotein level. Br J Obs Gynaecol 1987;94:387–402
- Snijders RJ, Sebire NJ, Souka A, et al. Fetal exomphalos and chromosomal defects: relationship to maternal age and gestation. Ultrasound Obs Gynecol 1995;6:250–5
- Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995–2011). Prenat Diagn 2012;32:142–53
- Shaffer BL, Caughey AB, Norton ME. Variation in the decision to terminate pregnancy in the setting of fetal aneuploidy. Prenat Diagn 2006;26:667–71
- Caughey AB, Hopkins LM, Norton ME. Chorionic villus sampling compared with amniocentesis and the difference in the rate of pregnancy loss. Obs Gynecol 2006;108:612–16
- Centers for Disease Control and Prevention (CDC). Economic costs of birth defects and cerebral palsy–United States, 1992. MMWR Morb Mortal Wkly Rep 1995;44:694–9

