Abstract
Objective: To determine (1) whether maternal plasma concentrations of angiogenic and anti-angiogenic factors can predict which mothers diagnosed with “suspected small for gestational age fetuses (sSGA)” will develop pre-eclampsia (PE) or require an indicated early preterm delivery (≤ 34 weeks of gestation); and (2) whether risk assessment performance is improved using these proteins in addition to clinical factors and Doppler parameters.
Methods: This prospective cohort study included women with singleton pregnancies diagnosed with sSGA (estimated fetal weight <10th percentile) between 24 and 34 weeks of gestation (n = 314). Plasma concentrations of soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), soluble endoglin (sEng) and placental growth factor (PlGF) were determined in maternal blood obtained at the time of diagnosis. Doppler velocimetry of the umbilical (Umb) and uterine (UT) arteries was performed. The outcomes were (1) subsequent development of PE; and (2) indicated preterm delivery at ≤34 weeks of gestation (excluding deliveries as a result of spontaneous preterm labor, preterm pre-labor rupture of membranes or chorioamnionitis).
Results: (1) The prevalence of PE and indicated preterm delivery was 9.2% (n = 29/314) and 7.3% (n = 23/314), respectively; (2) the area under the receiver operating characteristic curve (AUC) for the identification of patients who developed PE and/or required indicated preterm delivery was greater than 80% for the UT artery pulsatility index (PI) z-score and each biochemical marker (including their ratios) except sVEGFR-1 MoM; (3) using cutoffs at a false positive rate of 15%, women with abnormal plasma concentrations of angiogenic/anti-angiogenic factors were 7–13 times more likely to develop PE, and 12–22 times more likely to require preterm delivery than those with normal plasma MoM concentrations of these factors; (4) sEng, PlGF, PIGF/sEng and PIGF/sVEGFR-1 ratios MoM, each contributed significant information about the risk of PE beyond that provided by clinical factors and/or Doppler parameters: women who had low MoM values for these biomarkers were at 5–9 times greater risk of developing PE than women who had normal values, adjusting for clinical factors and Doppler parameters (adjusted odds ratio for PlGF: 9.1, PlGF/sEng: 5.6); (5) the concentrations of sVEGFR-1 and PlGF/sVEGFR-1 ratio MoM, each contributed significant information about the risk of indicated preterm delivery beyond that provided by clinical factors and/or Doppler parameters: women who had abnormal values were at 8–9 times greater risk for indicated preterm delivery, adjusting for clinical factors and Doppler parameters; and (6) for a two-stage risk assessment (Umb artery Doppler followed by Ut artery Doppler plus biochemical markers), among women who had normal Umb artery Doppler velocimetry (n = 279), 21 (7.5%) developed PE and 11 (52%) of these women were identified by an abnormal UT artery Doppler mean PI z-score (>2SD): a combination of PlGF/sEng ratio MoM concentration and abnormal UT artery Doppler velocimetry increased the sensitivity of abnormal UT artery Doppler velocimetry to 76% (16/21) at a fixed false-positive rate of 10% (p = 0.06).
Conclusion: Angiogenic and anti-angiogenic factors measured in maternal blood between 24 and 34 weeks of gestation can identify the majority of mothers diagnosed with “suspected SGA” who subsequently developed PE or those who later required preterm delivery ≤34 weeks of gestation. Moreover, incorporation of these biochemical markers significantly improves risk assessment performance for these outcomes beyond that of clinical factors and uterine and umbilical artery Doppler velocimetry.
Introduction
The identification of fetal growth disorders, and, in particular, the small for gestational age (SGA) fetus, has been a major concern in clinical obstetrics for decades [Citation1–15]. Most efforts have focused on the use of sonographic fetal biometric standards or reference ranges when there is an index of suspicion (i.e. when the fundal height is shorter or larger than expected) [Citation16–33]. However, this approach is not satisfactory, because the precise diagnosis of fetal growth disorders (acceleration or deceleration) require measurement of the fetus rather than assessment of the entire uterine contents (which includes amniotic fluid and the uterine wall) through physical examination of the maternal abdomen [Citation34–49].
Growth is defined as a time-dependent change in bodily dimensions, and therefore, its assessment needs serial examinations [Citation1]. Therefore, many investigators have used longitudinal studies to generate standards or reference ranges to monitor fetal growth. These studies have included individual biometric parameters such as fetal head circumference [Citation50–60], and abdominal circumference [Citation50–54,Citation56,Citation58,Citation60–63], as well as computed-estimated fetal weight [Citation50,Citation51] or volumetric parameters such as limb volume [Citation64,Citation65] or fractional limb volume [Citation66–68]. The conceptual basis consists of comparing the trajectory of growth of a particular fetus with that of a standard. Recently, the possibility of using early biometric measurements to perform individualized growth assessment has been subject of several reports [Citation66–71].
Once an SGA fetus has been diagnosed (using cross-sectional standards, longitudinal standards or individualized fetal growth assessment), a key question is the optimal management of such case [Citation13,Citation72–75]. The differential diagnosis between fetuses which are small because of growth restriction from those who are small because of “constitutional factors” has been considered a major area of interest in clinical obstetrics [Citation13,Citation72–75]. A recent randomized clinical trial of SGA fetuses diagnosed at term has compared induction of labor and expectant management, and there is no evidence that one approach is clearly superior to the other [Citation76]. In the preterm fetus, a Bayesian randomized clinical trial has also shown no clear benefit of induction versus expectant management [Citation77–83].
Mothers of SGA fetuses are at increased risk for developing pre-eclampsia (PE) [Citation84–87], and some SGA fetuses may deteriorate and require early delivery because of suspected fetal compromise [Citation75,Citation88–101]. We and others previously reported that SGA [Citation102–118], PE [Citation102,Citation109,Citation110,Citation113,Citation119–164] and stillbirth [Citation153,Citation165–169] are associated with an abnormal angiogenic to anti-angiogenic ratio by measuring placental growth factor (PlGF), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and soluble endoglin (sEng) in the maternal circulation. Moreover, we have reported that an imbalance in angiogenic and anti-angiogenic factors measured in maternal blood are detectable before the clinical diagnosis, and that such measurements have prognostic value [Citation102,Citation110,Citation119–121,Citation123,Citation127,Citation129,Citation130,Citation132,Citation134–138,Citation140,Citation143–146]. For example, maternal plasma concentrations of such biomarkers can identify women presenting to the obstetrical triage area with symptoms or signs of PE who are at subsequent risk for preterm delivery and the development of adverse outcomes [Citation113,Citation141,Citation142,Citation147,Citation150–152].
Once the diagnosis of an SGA fetus has been made, monitoring consists of umbilical artery Doppler velocimetry and serial biometry [Citation31,Citation170–182]. Systematic reviews have shown that the introduction of umbilical artery Doppler velocimetry reduces perinatal death in high-risk patients [Citation183–185]. The performance of uterine artery Doppler velocimetry can also identify patients at increased risk of obstetrical complications [Citation186–188]; however, the likelihood ratio of a positive test to identify women with SGA fetuses who will develop PE or gestational hypertension is only 4–5 – therefore, this test has not been incorporated into clinical practice [Citation86].
We propose that the measurement of maternal plasma angiogenic and anti-angiogenic factors can identify mothers with SGA fetuses who will develop PE or require an indicated delivery before 34 weeks of gestation. The current study was designed to test this hypothesis and determine if the information gained from the measurement of these biomarkers would have additional value to that derived from evaluation of Doppler velocimetry of the umbilical or uterine artery.
Methods
Study design
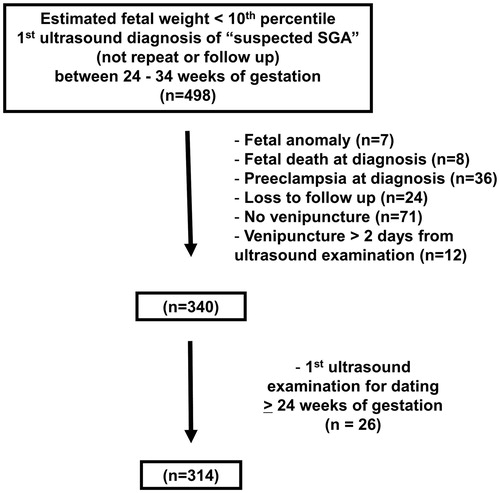
This study was a subcohort of women who were enrolled in a prospective longitudinal study conducted to identify biological markers for the prediction of PE and SGA. This subcohort included women who had sSGA fetuses based on ultrasonographic estimated fetal weight between 24 and 33 + 6/7 weeks of gestation from November 2007 to August 2011. Patients were enrolled in the Center for Advanced Obstetrical Care at Hutzel Hospital, Detroit, MI, and followed until delivery. Inclusion criteria were: (1) singleton pregnancy; (2) ultrasound biometry below 10th percentile for gestational age; (3) first ultrasound for dating performed prior to 24 weeks of gestation; (4) no evidence of PE (hypertension and proteinuria) at the ultrasonographic examination; and (5) venipuncture was performed within 2 days of the ultrasonographic examination. Exclusion criteria were: (1) preterm labor, preterm pre-labor rupture of membranes, PE or impaired fetal growth at the time of recruitment; (2) known major fetal anomaly or fetal demise; (3) active vaginal bleeding; and (4) serious medical illness (renal insufficiency, congestive heart disease, chronic respiratory insufficiency or active hepatitis).
Maternal blood was collected by venipuncture, and Doppler examination of the UT and Umb arteries were performed, at enrollment and during examinations scheduled every 4 weeks until 24 weeks, and every 2 weeks thereafter until delivery. Ultrasonographic biometry was also performed every 3–4 weeks to assess fetal growth.
The primary and secondary outcomes of the study were development of PE and indicated preterm delivery before 34 weeks of gestation, respectively. All participants provided written informed consent and the research protocol was approved by the Institutional Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS) and Wayne State University, Detroit, Michigan.
Definitions
The diagnosis of “suspected SGA” was based on sonographic estimated fetal weight below the 10th percentile for gestational age [Citation189] determined using fetal head circumference, biparietal diameter, abdominal circumference and femur length measurements. SGA was defined as a birth weight below the 10th percentile for gestational age [Citation190]. Gestational age was determined by last menstrual period and confirmed by the earliest ultrasound examination. PE was defined as new-onset hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on 2 measurements at least 4 h apart) and proteinuria after 20 weeks gestation in a previously normotensive woman [Citation191]. Proteinuria was defined as ≥300 mg protein in a 24 h urine collection specimen [Citation191,Citation192] or, if a 24 h urine was not completed, at least 1 + protein (30 mg/dL) on 2 urine dipsticks, measured at least 4 h apart. An “indicated preterm delivery” was defined as delivery for maternal indications or concerns regarding fetal well-being. For the objectives of this study, this definition did not include deliveries occurring as a result of spontaneous preterm labor, preterm pre-labor rupture of membranes or clinical chorioamnionitis.
Sonographic examination
Fetal biometry was obtained every 3–4 weeks and the pulse wave and color Doppler ultrasound examination was performed on the Umb and both UT arteries using a 3.5- or 5-MHz curvilinear probe at every visit as previously described [Citation108,Citation193]. The PI of the right and left UT arteries was measured and the mean PI of the two vessels was calculated. UT artery Doppler velocimetry was defined as abnormal if the mean PI was above the second standard deviation (SD) for gestational age using the reference range from our institution. The Doppler signal of the Umb artery was obtained from a free floating loop of the umbilical cord during the absence of fetal breathing and body movement. When three similar consecutive waveforms were obtained, the PI was measured. Umb artery Doppler velocimetry was defined as abnormal if the PI was above the second SD for gestational age using the reference range from our institution or if abnormal waveforms (absent or reversed end-diastolic velocities) were present as described by Trudinger et al. [Citation194]. The inter- and intra-observer CV for UT Doppler measurement were 11.6 and 5.4%, and for Umb, 9.5 and 7%, respectively.
Sample collection and immunoassay
Maternal plasma was collected into tubes containing EDTA. Samples were centrifuged and stored at −70 °C. Sensitive and specific immunoassays (R&D systems, Minneapolis, MN) utilizing a quantitative sandwich enzyme technique were used to determine maternal plasma concentrations of PlGF, sEng and sVEGFR-1. The inter- and intra-assay coefficients of variation (CV) were: PlGF, 6.02 and 4.8%; sEng, 2.3 and 4.6%; sVEGFR-1, 1.4 and 3.9%, respectively. The lower limits of the detection were: PlGF, 9.52 pg/mL; sEng, 0.08 ng/mL; and sVEGFR-1, 16.97 pg/mL.
Statistical analysis
Descriptive analysis of demographic and obstetrical characteristics of the study cohort was performed using SPSS Version 15.0 (SPSS, Inc., Chicago, IL). Umb artery Doppler pulsatility index (PI) and UT artery Doppler (UT) mean PI were converted to z-scores, whereas biochemical markers were converted to multiples of the expected median (MoM) for gestational age, each based on reference values from uncomplicated pregnancies with appropriately grown neonates. The unpaired t-test or Mann–Whitney U statistic was used to test for differences in the distributions of arithmetic variables, as appropriate. Logistic regression models were fitted to determine the crude and adjusted magnitude of association between biochemical markers, Doppler parameter z-scores and PE, as well as indicated preterm delivery before 34 weeks of gestation.
Multivariable models were fitted to determine whether incorporation of biochemical markers improved performance of risk assessment compared to use of clinical factors and Umb and UT artery Doppler PI z-scores, both individually and in combination [Citation195,Citation196]. Biochemical markers that had significant independent associations with the outcome of interest were interpreted as providing information that significantly improved risk assessment performance compared to use of the other predictors included in each model [Citation195,Citation197].
To describe performance in discriminating cases from non-cases, receiver-operating characteristic (ROC) curves were constructed for biochemical markers that significantly improved risk assessment performance. Statistical significance was defined either by non-overlapping 95%CIs or p values < 0.05. These analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Demographic and clinical characteristics
A total of 314 women were included in this study (). Demographic and obstetric characteristics of the study population are summarized in . The prevalence of PE was 9.2% (29/314) and 7.3% (23/314) of the women required indicated preterm delivery because of maternal and/or fetal indications at ≤34 weeks of gestation.
Table 1. Demographic and clinical characteristics of the study population.
Among patients with PE, 11 (38%) were diagnosed with early onset (≤34 weeks of gestation) disease. The median interval from venipuncture to the diagnosis of PE was 43 days (interquartile range 24–63). displays the indications for delivery of patients who required a preterm delivery at ≤34 weeks of gestation. The two most common indications were PE [47.8% (11/23)] and abruptio placentae [30.4% (7/23)].
Table 2. Indications for preterm delivery ≤ 34 weeks of gestation (n = 23).
Differences in biomarker distributions
Patients who subsequently developed PE () or required an indicated delivery at ≤34 weeks () had significantly higher median plasma sVEGFR-1 and sEng MOM concentrations, and higher mean Umb and UT artery Doppler PI z-scores, but significantly lower median plasma PlGF, PlGF/sEng ratio and PlGF/sVEGFR-1 ratio MOM concentrations, than those who did not develop these complications (each p < 0.005).
Figure 2. Median plasma concentration of angiogenic/anti-angiogenic factors and pulsatility index (PI) of the umbilical (Umb) and uterine (UT) artery Doppler velocimetry in women who did or did not subsequently develop PE. (A) Patients with “suspected SGA” who subsequently developed PE had higher median plasma multiple of the expected median (MoM) concentrations of sVEGFR-1 and sEng than those who did not. [sVEGFR-1 MoM: median 2.4, interquartile range (IQR) 1.2–4.0 versus median 1.1, IQR 0.7–1.7; p < 0.001; and sEng MoM: median 0.96, IQR 0.84–1.2 versus median 1.7, IQR 1.3–4.2; p < 0.001]. (B) Median plasma MoM concentration of placental growth factor (PlGF), the ratio of PlGF/sVEGFR-1 and the ratio of PlGF/sEng in women who did or did not subsequently develop PE. Patients with “suspected SGA” who subsequently developed PE had lower median plasma MoM concentrations of PlGF, PlGF/sVEGFR-1 and PlGF/sEng than those who did not. [PlGF MoM: median 0.34, IQR 0.16–0.71 versus median 1.3, IQR 0.71–2.1; p < 0.001; PlGF/sVEGFR-1 MoM: median 0.12, IQR 0.06–0.72 versus median 1.1, IQR 0.59–2.0; p < 0.001 and PlGF/sEng MoM: median 0.20, IQR 0.05–0.64 versus median 1.3, IQR 0.74–2.2; p < 0.001]. (C) Median umbilical and uterine artery PI z-scores in women who did and did not subsequently develop PE. Patients with “suspected SGA” who subsequently developed PE had higher median umbilical and uterine artery PI z-scores than those who did not. [Umbilical artery PI z-score: median 0.83, IQR 0.17–1.7 versus median 0.35, IQR −0.35 to 1.0; p < 0.001; Uterine artery mean PI z-score: median 2.8, IQR 0.55–3.4 versus median 0.065, IQR −0.44 to 0.68; p < 0.001.]
![Figure 2. Median plasma concentration of angiogenic/anti-angiogenic factors and pulsatility index (PI) of the umbilical (Umb) and uterine (UT) artery Doppler velocimetry in women who did or did not subsequently develop PE. (A) Patients with “suspected SGA” who subsequently developed PE had higher median plasma multiple of the expected median (MoM) concentrations of sVEGFR-1 and sEng than those who did not. [sVEGFR-1 MoM: median 2.4, interquartile range (IQR) 1.2–4.0 versus median 1.1, IQR 0.7–1.7; p < 0.001; and sEng MoM: median 0.96, IQR 0.84–1.2 versus median 1.7, IQR 1.3–4.2; p < 0.001]. (B) Median plasma MoM concentration of placental growth factor (PlGF), the ratio of PlGF/sVEGFR-1 and the ratio of PlGF/sEng in women who did or did not subsequently develop PE. Patients with “suspected SGA” who subsequently developed PE had lower median plasma MoM concentrations of PlGF, PlGF/sVEGFR-1 and PlGF/sEng than those who did not. [PlGF MoM: median 0.34, IQR 0.16–0.71 versus median 1.3, IQR 0.71–2.1; p < 0.001; PlGF/sVEGFR-1 MoM: median 0.12, IQR 0.06–0.72 versus median 1.1, IQR 0.59–2.0; p < 0.001 and PlGF/sEng MoM: median 0.20, IQR 0.05–0.64 versus median 1.3, IQR 0.74–2.2; p < 0.001]. (C) Median umbilical and uterine artery PI z-scores in women who did and did not subsequently develop PE. Patients with “suspected SGA” who subsequently developed PE had higher median umbilical and uterine artery PI z-scores than those who did not. [Umbilical artery PI z-score: median 0.83, IQR 0.17–1.7 versus median 0.35, IQR −0.35 to 1.0; p < 0.001; Uterine artery mean PI z-score: median 2.8, IQR 0.55–3.4 versus median 0.065, IQR −0.44 to 0.68; p < 0.001.]](/cms/asset/959ed3e4-46b8-4b59-b5ff-0de19484aa11/ijmf_a_1048431_f0002_c.jpg)
Figure 3. Median plasma concentration of angiogenic/anti-angiogenic factors as well as pulsatility index (PI) of the umbilical (Umb) and uterine (UT) artery Doppler velocimetry in women who subsequently required indicated preterm delivery at or before 34 weeks of gestation and those who did not. (A) Patients suspected to have SGA fetuses who subsequently required indicated preterm delivery had higher median plasma multiple of the medians (MoM) concentrations of sVEGFR-1 and sEng than those who did not. [sVEGFR-1 MoM: median 3.0, interquartile range (IQR) 1.4–6.4 versus median 1.1, IQR 0.72–1.7; p < 0.001; and sEng MoM: median 2.4, IQR 1.1–6.6 versus median 0.98, IQR 0.85–1.2; p < 0.001]. (B) Median plasma MoM concentration of placental growth factor (PlGF), the ratio of PlGF/sVEGFR-1 and the ratio of PlGF/sEng in women who subsequently required indicated preterm delivery and those who did not. Patients with “suspected SGA” who subsequently developed PE had lower median plasma MoM concentrations of PlGF, PlGF/sVEGFR-1 and PlGF/sEng than those who did not. [PlGF MoM: median 0.18, IQR 0.072–1.3 versus median 1.2, IQR 0.70–2.1; p < 0.001; PlGF/sVEGFR-1 MoM: median 0.066, IQR 0.013–0.41 versus median 1.0, IQR 0.59–1.9; p < 0.001 and PlGF/sEng MoM: median 0.074, IQR 0.012–1.7 versus median 1.2, IQR 0.70–2.1; p < 0.001]. (C) Median umbilical and uterine artery PI z-scores in women who subsequently required indicated preterm delivery and those who did not. Patients with “suspected SGA” who required indicated preterm delivery had higher median umbilical and uterine artery PI z-scores than those who did not. [Umbilical artery PI z-Score: median 1.6, interquartile range (IQR) −0.21 to 2.8 versus median 0.36, IQR −0.32 to 0.89; p < 0.001; and Uterine artery mean PI z-Score: median 3.3, IQR 2.4–3.9 versus median 0.071, IQR −0.44 to 0.66; p < 0.001.]
![Figure 3. Median plasma concentration of angiogenic/anti-angiogenic factors as well as pulsatility index (PI) of the umbilical (Umb) and uterine (UT) artery Doppler velocimetry in women who subsequently required indicated preterm delivery at or before 34 weeks of gestation and those who did not. (A) Patients suspected to have SGA fetuses who subsequently required indicated preterm delivery had higher median plasma multiple of the medians (MoM) concentrations of sVEGFR-1 and sEng than those who did not. [sVEGFR-1 MoM: median 3.0, interquartile range (IQR) 1.4–6.4 versus median 1.1, IQR 0.72–1.7; p < 0.001; and sEng MoM: median 2.4, IQR 1.1–6.6 versus median 0.98, IQR 0.85–1.2; p < 0.001]. (B) Median plasma MoM concentration of placental growth factor (PlGF), the ratio of PlGF/sVEGFR-1 and the ratio of PlGF/sEng in women who subsequently required indicated preterm delivery and those who did not. Patients with “suspected SGA” who subsequently developed PE had lower median plasma MoM concentrations of PlGF, PlGF/sVEGFR-1 and PlGF/sEng than those who did not. [PlGF MoM: median 0.18, IQR 0.072–1.3 versus median 1.2, IQR 0.70–2.1; p < 0.001; PlGF/sVEGFR-1 MoM: median 0.066, IQR 0.013–0.41 versus median 1.0, IQR 0.59–1.9; p < 0.001 and PlGF/sEng MoM: median 0.074, IQR 0.012–1.7 versus median 1.2, IQR 0.70–2.1; p < 0.001]. (C) Median umbilical and uterine artery PI z-scores in women who subsequently required indicated preterm delivery and those who did not. Patients with “suspected SGA” who required indicated preterm delivery had higher median umbilical and uterine artery PI z-scores than those who did not. [Umbilical artery PI z-Score: median 1.6, interquartile range (IQR) −0.21 to 2.8 versus median 0.36, IQR −0.32 to 0.89; p < 0.001; and Uterine artery mean PI z-Score: median 3.3, IQR 2.4–3.9 versus median 0.071, IQR −0.44 to 0.66; p < 0.001.]](/cms/asset/69c72b27-99a8-4a0c-895b-bfcae20bd26a/ijmf_a_1048431_f0003_c.jpg)
Single-marker prognostic performance
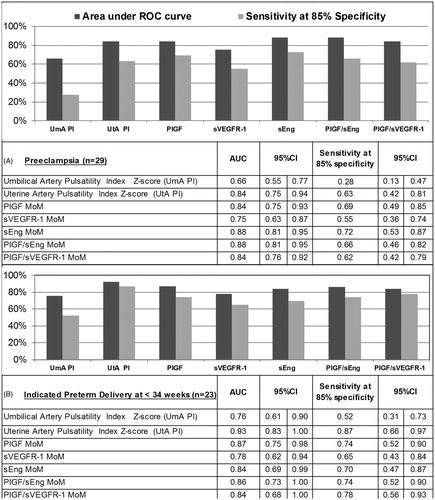
shows the AUC and sensitivity at 85% specificity for each biochemical and Doppler parameter in identifying patients at risk of PE or indicated preterm delivery at ≤34 weeks of gestation. The AUC in identifying either outcome was greater than 80% for UT artery PI z-score (AUC 84% for PE and 93% for indicated delivery ≤ 34 weeks) and each biochemical marker (AUC 84–88% for PE and 84–87% for indicated delivery ≤ 34 weeks) except the sVEGFR-1 MoM (AUC 75% for PE and 78% for indicated delivery ≤ 34 weeks). UT artery PI z-score performed comparably to the biochemical markers in identifying patients at risk of each outcome. In addition, at a specificity of 85%, the sensitivity of biochemical markers for identifying women at risk for PE (sensitivity 55–72%) or indicated delivery (sensitivity 65–78%) was more than double that of Umb artery PI z-score alone (sensitivity 28% for PE and 52% for indicated delivery).
Multi-marker prognostic performance
and show the unadjusted and adjusted magnitudes of the association between each biochemical and Doppler parameter marker (expressed either as a continuous or categorical variable) with PE and indicated preterm delivery at ≤ 34 weeks, respectively. Four columns of odds ratios are shown in each table. The first column describes unadjusted magnitudes of association between each biomarker and outcome. The remaining three columns of odds ratios are shown to describe whether inclusion of each biochemical marker improved risk assessment beyond: a combination of all clinical factors (model II); a combination of both Doppler parameters (model III); or a reduced combination of factors included in either of these two models that were statistically associated with PE or indicated preterm delivery at ≤ 34 weeks (model IV), respectively. Factors with statistically significant odds ratios are interpreted as contributing significant risk information beyond other factors included in each model.
Table 3. Magnitudes of association between subsequent diagnosis of pre-eclampsia and maternal plasma concentrations of angiogenic and anti-angiogenic factors, unadjusted and adjusted for clinical factors and uterine and umbilical artery Doppler velocimetry.
Table 4. Magnitudes of association between subsequent indicated preterm delivery ≤34 weeks of gestation and maternal plasma concentrations of angiogenic and anti-angiogenic factors, unadjusted and adjusted for clinical factors and uterine and umbilical artery Doppler velocimetry.
Identifying patients at risk for pre-eclampsia
Each biochemical and Doppler parameter was significantly associated with subsequent development of PE (). Patients with “abnormal” biochemical marker values (threshold defined using a fixed specificity of 85%) were 7–13 times (without multivariable adjustment) more likely to develop PE than those without such values. The odds of subsequently developing PE were 5–9 times greater among women who had “abnormal” compared to normal sEng, PlGF, PlGF/sEng or PlGF/sVEGFR-1 MoM concentrations (thresholds defined at 85% specificity), after adjusting for parity and prior history of PE, BMI, MAP and abnormal UT artery PI z-score (>2SD). Therefore, all of the biochemical markers, except sVEGFR-1, contributed significant risk information above that provided by a combination of clinical factors, a combination of Doppler parameters or a combination of significantly associated clinical factors and Doppler parameters. For example, women who had low PlGF/sVEGFR-1 MoM concentrations were at five-fold greater risk of PE [odds ratio (OR), 5.0; 95% confidence interval (CI), 1.5–17.2], whereas those with low PlGF MoM concentrations were at 9-fold greater risk [OR 9.1; 95% CI 2.5–33.3], when each was compared to women who had normal values, adjusting for both clinical factors and Doppler parameters ().
Identifying patients at risk for indicated delivery at ≤34 weeks of gestation
Similarly, each biochemical marker and Doppler parameter was significantly associated with indicated preterm delivery (); without multivariable adjustment, patients with abnormal biochemical marker concentrations (cutoff defined at 85% specificity) were at 12–22 times greater risk of early preterm delivery. Women who had low PIGF/sVEGFR-1 MoM concentration ratio or high sVEGFR-1 MoM concentrations, were at 8–9 times [OR 8.4; 95% CI 1.4–48.8 and OR 9.1; 95% CI 1.5–57.3, respectively] greater risk of indicated preterm delivery at <34 weeks than women who did not, adjusting for MAP and Doppler velocimetry findings.
Two-stage risk assessment
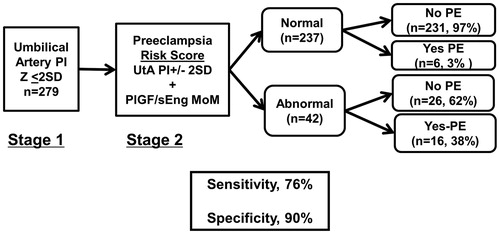
Since the current management of patients diagnosed with “suspected SGA” is to triage those who require intensive surveillance with Umb artery Doppler velocimetry assessment, this study evaluated potential benefits of the incorporation of angiogenic biomarkers in this clinical scenario using a two-stage selection procedure. In the first stage, Umb artery Doppler PI z-scores were used, and women with normal values were triaged to further testing. In the second stage, a combination of UT artery Doppler velocimetry abnormality (>2SD) and plasma concentrations of angiogenic/anti-angiogenic factors were used to identify patients who subsequently developed adverse outcomes.
Stage 1
Umb artery Doppler velocimetry was abnormal (>2SD) in 10% (n = 30) of the study population, and 32% (9/28) (2 patients did not have information on UT artery Doppler) of these women had abnormal UT artery Doppler PI z scores (>2SD). Each of the nine patients with abnormal Umb and UT artery Doppler velocimetry also had an imbalance in angiogenic/anti-angiogenic factors, whereas such imbalance was found for only 16% (n = 3/19) of those who had abnormal Umb artery and normal UT artery Doppler velocimetry. Among these three patients with abnormal angiogenic profile, only one delivered before 34 weeks and none developed PE. There was accordingly no benefit to use biochemical markers in addition to UT artery Doppler velocimetry to identify women with abnormal Umb artery Doppler velocimetry who were at risk of PE or indicated preterm delivery at ≤34 weeks.
Stage 2
Among women with normal Umb artery Doppler velocimetry (≤2SD, n = 279), 10% (n = 28) had abnormal UT artery Doppler PI z scores. UT artery Doppler velocimetry was abnormal in 85% (11/13) of the women who subsequently required indicated preterm delivery at ≤34 weeks of gestation, and therefore, this biophysical marker had a high sensitivity and a reasonable positive predictive value (39%, 11/28) for this outcome. Addition of angiogenic/anti-angiogenic markers to UT artery Doppler velocimetry did not significantly improve diagnostic performance.
In contrast, the sensitivity of UT artery Doppler velocimetry in identifying women who subsequently developed PE, when Umb artery Doppler velocimetry was normal, was only 52% (11/21). The sensitivity of abnormal UT artery Doppler velocimetry increased to 76% (16/21) at a specificity of 90% (52% versus 76%, p = 0.06; ), when the PIGF/sEng concentration ratio was added.
Figure 5. Risk of PE and/or indicated preterm delivery at or before 34 weeks of gestation conditional on a two-step screening approach. In the first step, Umb artery Doppler PI z-score was used to triage patients. In the second step, UT artery Doppler mean PI z-score abnormality and MoM plasma angiogenic/anti-angiogenic factor concentrations are used to identify patients that subsequently had the targeted outcome. Among patients who had normal Umb artery Doppler PI z-scores, 52% of those who subsequently developed PE had UT artery Doppler mean PI z-scores > 2SD; adding PlGF/sEng MoM to abnormal UT artery Doppler mean PI z-score increased the sensitivity to 76% at a fixed false positive rate of 10% (p = 0.06).
Discussion
Principal findings of the study
Among patients diagnosed with “suspected SGA” by ultrasonographic estimated fetal weight <10th percentile for gestational age between 24 and 34 weeks of gestation: (1) women with abnormal plasma concentrations of angiogenic/anti-angiogenic factors were 7–13 times more likely to develop PE, and 12–22 times more likely to require preterm delivery ≤34 weeks, than women with normal plasma concentrations; (2) sEng, PlGF, PlGF/sEng or PlGF/sVEGFR-1, each contributed significant information about the risk of PE beyond that provided by clinical factors and/or Doppler parameters; (3) sVEGFR-1 and PlGF/sVEGFR-1, each contributed significant information about the risk of indicated preterm delivery at ≤ 34 weeks, beyond that provided by clinical factors and/or Doppler parameters; and (4) For the two-step risk assessment, angiogenic biomarkers did not improve risk assessment performance among patients with abnormal Umb artery Doppler velocimetry. In contrast, among patients with normal Umb artery Doppler velocimetry, the combination of PlGF/sEng with abnormal UT artery Doppler velocimetry had a sensitivity of 76% at a specificity of 90% for identifying women destined to develop PE (compared to a sensitivity of 52% using abnormal UT artery Doppler alone; p = 0.06). Together, these findings indicate that angiogenic and anti-angiogenic factors measured in maternal plasma at the time of “suspected SGA” diagnosis between 24 and 34 weeks of gestation had prognostic value for subsequent development of PE or indicated early preterm delivery.
An anti-angiogenic state is present and has prognostic value in a subset of pregnancies with sSGA fetuses
An imbalance of angiogenic and anti-angiogenic factor concentrations in maternal blood was originally reported in patients with PE, both prior to [Citation102,Citation110,Citation119–121,Citation123,Citation127,Citation129,Citation130,Citation132,Citation134–138,Citation140,Citation143–146] and at the time of diagnosis [Citation122,Citation124–126,Citation128,Citation131,Citation133]. Yet, we and others have demonstrated that an anti-angiogenic state is not specific to PE, but can be observed in other obstetrical syndromes, including SGA [Citation102–110,Citation112,Citation113,Citation124], especially in patients with abnormal UT and Umb artery Doppler velocimetry findings [Citation108]. Doppler examination of the Umb artery has been the mainstay of fetal surveillance in pregnancies with “suspected SGA” [Citation172–174,Citation181,Citation198–200]. While UT artery Doppler velocimetry can identify an additional proportion of these pregnancies that are at risk of adverse perinatal outcomes [Citation186–188], the predictive value has thus far been insufficient to warrant acceptance into clinical practice [Citation86].
In this study, we demonstrated that plasma angiogenic/anti-angiogenic factor concentrations have prognostic value at the time of “suspected SGA” diagnosis (24–34 weeks of gestation) for the identification of patients destined to develop PE and/or require indicated preterm delivery at ≤34 weeks. The performance of these biomarkers alone was comparable to that of UT artery Doppler velocimetry. Thus, this approach may be useful in areas where accessible to expertise in Doppler examination is limited. Indeed, plasma concentration of angiogenic/anti-angiogenic factors contributed significant information about the risk of PE or indicated preterm delivery at ≤ 34 weeks beyond that provided by abnormal Doppler velocimetry and/or clinical factors alone. Adjusting for these factors, patients with abnormal angiogenic/anti-angiogenic factor plasma concentrations were 5–9 and 8–9 times more likely than women with normal concentrations to subsequently develop PE or require delivery at ≤34 weeks, respectively.
Our findings are consistent with a prior study of 38 women with suspected SGA fetuses that showed an association between low plasma PlGF concentrations and early preterm delivery (<34 weeks of gestation) [Citation113], and with another report that revealed an association between an anti-angiogenic state and subsequent development of PE in pregnancies with suspected sSGA. The authors concluded that the plasma angiogenic/anti-angiogenic factors concentration at diagnosis and Doppler ultrasound, both predict adverse outcome with a similar performance and that adding these biomarkers to the uterine artery Doppler did not improve predictive performance, defined as a significantly larger areas under the ROC curve [Citation115]. In cardiovascular fields, it is recognized that the AUC test [Citation201] can be too conservative [Citation202], and have lower power than other statistical tests in examining incremental improvement in prognostic performance with the addition of a new marker [Citation203]. The significant independent magnitudes of association for the studied biochemical markers did not always translate into significant improvements in AUC. In addition, the median gestational age at diagnosis of sSGA in that study was 34 (IQR 32.9–36.3) weeks as opposed to 27.7 weeks (IQR 26.0–29.6) in the current study and the median time between the diagnosis of sSGA and venipuncture was 14 (range 4–26) days as opposed to within 2 days in the current study.
Two-stage risk assessment for indicated preterm delivery
Among patients with “suspected SGA” who had abnormal Umb artery Doppler velocimetry, incorporation of these biochemical markers with UT artery Doppler PI z-score did not improve prognostic performance in identifying patients who would require indicated delivery ≤34 weeks of gestation, since all patients with abnormal Umb and UT arteries Doppler PI z-scores also had abnormal plasma concentrations of PlGF/sEng. Similarly, among patients who had normal Umb artery Doppler velocimetry, the incorporation of angiogenic/anti-angiogenic factors and UT artery Doppler velocimetry failed to improve prognostic performance in identifying patients who would require indicated delivery at or before 34 weeks of gestation, since 90% of these women were identified by abnormal UT artery Doppler velocimetry alone.
Two-stage risk assessment for subsequent development of PE
Among patients with normal Umb artery Doppler velocimetry, a combination of maternal plasma angiogenic/anti-angiogenic factor concentrations and UT artery Doppler velocimetry improved the identification of patients who subsequently developed PE. This combination of tests correctly identified 76% of the women with “suspected SGA” who subsequently developed PE at a false positive rate of 10%, whereas abnormal UT artery Doppler velocimetry alone identified only 52% of these women. These findings are important since potential interventions to reverse an anti-angiogenic state are currently under extensive investigations and may be available in the near future. The use of these biomarkers may help identify patients at risk to include in the studies investigating the efficacy of these interventions.
Strengths and limitations
The strengths of this study are its prospective design and that examination of plasma angiogenic/anti-angiogenic factor concentrations were measured in combination with Doppler velocimetry in women suspected of having SGA fetuses. Limitations of this study are that: (1) women diagnosed as having severe PE or SGA and absent or reversed end-diastolic velocity of the Umb artery Doppler, were electively induced at 32–34 weeks of gestation, and, therefore, the outcomes associated with intervention and no intervention could not be compared; and (2) early preterm delivery (≤34 weeks of gestation) was used as a surrogate for several neonatal morbidities. Larger studies are needed to examine the association between abnormal plasma angiogenic/anti-angiogenic factor concentrations and neonatal complications among offspring of patients with SGA.
Conclusion
Among patients suspected of having SGA fetuses at less than 34 weeks of gestation, the determination of angiogenic and anti-angiogenic factors in maternal plasma can identify a majority of mothers who subsequently developed PE or those who later required early preterm delivery. Determination of these biomarkers can be obtained from a simple blood test, and thus, this approach may be useful in areas where accessible to expertise in Doppler examination is limited. Moreover, incorporation of these biochemical markers significantly improves risk assessment performance for these outcomes, beyond that of clinical factors and uterine and umbilical artery Doppler velocimetry in patients diagnosed with “suspected SGA”.
Declaration of interest
The authors report no conflicts of interest. This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Notes
* Presented as an oral presentation at the World Congress of Perinatal Medicine, June 19–22, 2013, Moscow, Russia
References
- Romero R, Jeanty P. The detection of fetal growth disorders. Sem Ultrasound CT MR 1984;5:130–43
- Goldenberg RL, Cutter GR, Hoffman HJ, et al. Intrauterine growth retardation: standards for diagnosis. Am J Obstet Gynecol 1989;161:271–7
- Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011;204:288–300
- Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat Diagn 2014;34:655–9
- Gardosi J. Intrauterine growth restriction: new standards for assessing adverse outcome. Best Pract Res Clin Obstet Gynaecol 2009;23:741–9
- Baschat AA. Fetal growth restriction – from observation to intervention. J Perinat Med 2010;38:239–46
- Kinzler WL, Vintzileos AM. Fetal growth restriction: a modern approach. Curr Opin Obstet Gynecol 2008;20:125–31
- Mongelli M, Gardosi J. Fetal growth. Curr Opin Obstet Gynecol 2000;12:111–15
- Miller J, Turan S, Baschat AA. Fetal growth restriction. Semin Perinatol 2008;32:274–280
- Mandruzzato G, Antsaklis A, Botet F, et al. Intrauterine restriction (IUGR). J Perinat Med 2008;36:277–81
- Caughey AB. How best to diagnose and treat the small-for-gestational-age fetus. Am J Obstet Gynecol 2013;209:397–9
- Bamberg C, Kalache KD. Prenatal diagnosis of fetal growth restriction. Semin Fetal Neonatal Med 2004;9:387–94
- Zhang J, Merialdi M, Platt LD, et al. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol 2010;202:522–8
- Bamfo JE, Odibo AO. Diagnosis and management of fetal growth restriction. J Pregnancy 2011;2011:640715
- Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol 2013;41:136–45
- Deter RL, Harrist RB, Hadlock FP, et al. The use of ultrasound in the assessment of normal fetal growth: a review. J Clin Ultrasound 1981;9:481–93
- Deter RL, Harrist RB, Hadlock FP, et al. The use of ultrasound in the detection of intrauterine growth retardation: a review. J Clin Ultrasound 1982;10:9–16
- Geirsson RT, Persson PH. Diagnosis of intrauterine growth retardation using ultrasound. Clin Obstet Gynaecol 1984;11:457–80
- Wladimiroff JW, Bloemsma CA, Wallenburg HC. Ultrasonic assessment of fetal head and body sizes in relation to normal and retarded fetal growth. Am J Obstet Gynecol 1978;131:857–60
- Wittmann BK, Robinson HP, Aitchison T, et al. The value of diagnostic ultrasound as a screening test for intrauterine growth retardation: comparison of nine parameters. Am J Obstet Gynecol 1979;134:30–5
- Martinez DA, Barton JL. Estimation of fetal body and fetal head volumes: description of technique and nomograms for 18 to 41 weeks of gestation. Am J Obstet Gynecol 1980;137:78–84
- Hadlock FP, Deter RL, Harrist RB, et al. A date-independent predictor of intrauterine growth retardation: femur length/abdominal circumference ratio. AJR Am J Roentgenol 1983;141:979–84
- Jordaan HV. Estimation of fetal weight by ultrasound. J Clin Ultrasound 1983;11:59–66
- Chervenak FA, Romero R, Berkowitz RL, et al. Use of sonographic estimated fetal weight in the prediction of intrauterine growth retardation. Am J Perinatol 1984;1:298–301
- Divon MY, Chamberlain PF, Sipos L, et al. Identification of the small for gestational age fetus with the use of gestational age-independent indices of fetal growth. Am J Obstet Gynecol 1986;155:1197–201
- Yagel S, Zacut D, Igelstein S, et al. In utero ponderal index as a prognostic factor in the evaluation of intrauterine growth retardation. Am J Obstet Gynecol 1987;157:415–19
- Reece EA, Goldstein I, Pilu G, et al. Fetal cerebellar growth unaffected by intrauterine growth retardation: a new parameter for prenatal diagnosis. Am J Obstet Gynecol 1987;157:632–8
- Berkowitz GS, Chitkara U, Rosenberg J, et al. Sonographic estimation of fetal weight and Doppler analysis of umbilical artery velocimetry in the prediction of intrauterine growth retardation: a prospective study. Am J Obstet Gynecol 1988;158:1149–53
- Gaziano E, Knox GE, Wager GP, et al. The predictability of the small-for-gestational-age infant by real-time ultrasound-derived measurements combined with pulsed Doppler umbilical artery velocimetry. Am J Obstet Gynecol 1988;158:1431–9
- Lee W, Barton S, Comstock CH, et al. Transverse cerebellar diameter: a useful predictor of gestational age for fetuses with asymmetric growth retardation. Am J Obstet Gynecol 1991;165:1044–50
- Galan HL, Ferrazzi E, Hobbins JC. Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn 2002;22:331–7
- Carberry AE, Gordon A, Bond DM, et al. Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. Cochrane Database Syst Rev 2011;(12):CD008549
- Serena C, Marchetti G, Rambaldi MP, et al. Stillbirth and fetal growth restriction. J Matern Fetal Neonatal Med 2013;26:16–20
- Beazley JM, Underhill RA. Fallacy of the fundal height. Br Med J 1970;4:404–6
- Belizan JM, Villar J, Nardin JC, et al. Diagnosis of intrauterine growth retardation by a simple clinical method: measurement of uterine height. Am J Obstet Gynecol 1978;131:643–6
- Rosenberg K, Grant JM, Tweedie I, et al. Measurement of fundal height as a screening test for fetal growth retardation. Br J Obstet Gynaecol 1982;89:447–50
- Calvert JP, Crean EE, Newcombe RG, et al. Antenatal screening by measurement of symphysis-fundus height. Br Med J (Clin Res Ed) 1982;285:846–9
- Rogers MS, Needham PG. Evaluation of fundal height measurement in antenatal care. Aust N Z J Obstet Gynaecol 1985;25:87–90
- Persson B, Stangenberg M, Lunell NO, et al. Prediction of size of infants at birth by measurement of symphysis fundus height. Br J Obstet Gynaecol 1986;93:206–11
- Hepburn M, Rosenberg K. An audit of the detection and management of small-for-gestational age babies. Br J Obstet Gynaecol 1986;93:212–16
- Lockwood CJ, Weiner S. Assessment of fetal growth. Clin Perinatol 1986;13:3–35
- Lindhard A, Nielsen PV, Mouritsen LA, et al. The implications of introducing the symphyseal-fundal height-measurement. A prospective randomized controlled trial. Br J Obstet Gynaecol 1990;97:675–80
- Backe B, Nakling J. Effectiveness of antenatal care: a population based study. Br J Obstet Gynaecol 1993;100:727–32
- Mongelli M, Gardosi J. Symphysis-fundus height and pregnancy characteristics in ultrasound-dated pregnancies. Obstet Gynecol 1999;94:591–4
- Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. Br J Obstet Gynaecol 1999;106:309–17
- Bais JM, Eskes M, Pel M, et al. Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol 2004;116:164–9
- Griffiths A, Pinto A, Margarit L. A survey of methods used to measure symphysis fundal height. J Obstet Gynaecol 2008;28:692–4
- Morse K, Williams A, Gardosi J. Fetal growth screening by fundal height measurement. Best Pract Res Clin Obstet Gynaecol 2009;23:809–18
- Sparks TN, Cheng YW, McLaughlin B, et al. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med 2011;24:708–12
- Deter RL, Harrist RB, Hadlock FP, et al. Longitudinal studies of fetal growth with the use of dynamic image ultrasonography. Am J Obstet Gynecol 1982;143:545–54
- Jeanty P, Cantraine F, Romero R, et al. A longitudinal study of fetal weight growth. J Ultrasound Med 1984;3:321–8
- Deter RL, Rossavik IK, Harrist RB, et al. Mathematic modeling of fetal growth: development of individual growth curve standards. Obstet Gynecol 1986;68:156–61
- Deter RL, Harrist RB. Growth standards for anatomic measurements and growth rates derived from longitudinal studies of normal fetal growth. J Clin Ultrasound 1992;20:381–8
- Mongelli M, Gardosi J. Longitudinal study of fetal growth in subgroups of a low-risk population. Ultrasound Obstet Gynecol 1995;6:340–4
- Falkner F. Ultrasonography and fetal growth: key perinatal factors. J Perinatol 1995;15:114–18
- Nasrat HA. Use of ultrasound longitudinal data in the diagnosis of abnormal fetal growth. J Matern Fetal Med 1997;6:209–14
- Owen P, Khan KS. Fetal growth velocity in the prediction of intrauterine growth retardation in a low risk population. Br J Obstet Gynaecol 1998;105:536–40
- Villar J, Altman DG, Purwar M, et al. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG 2013;120:9–26, v
- Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384:857–68
- Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014;384:869–79
- Eriksen PS, Secher NJ, Weis-Bentzon M. Normal growth of the fetal biparietal diameter and the abdominal diameter in a longitudinal study. An evaluation of the two parameters in predicting fetal weight. Acta Obstet Gynecol Scand 1985;64:65–70
- Nazarian LN, Halpern EJ, Kurtz AB, et al. Normal interval fetal growth rates based on obstetrical ultrasonographic measurements. J Ultrasound Med 1995;14:829–36
- Papageorghiou AT, Sarris I, Ioannou C, et al. Ultrasound methodology used to construct the fetal growth standards in the INTERGROWTH-21st Project. BJOG 2013;120:27–32, v
- Deter RL, Harrist RB, Hadlock FP, et al. Longitudinal studies of fetal growth using volume parameters determined with ultrasound. J Clin Ultrasound 1984;12:313–24
- Jeanty P, Romero R, Hobbins JC. Fetal limb volume: a new parameter to assess fetal growth and nutrition. J Ultrasound Med 1985;4:273–82
- Lee W, Deter RL, McNie B, et al. Individualized growth assessment of fetal soft tissue using fractional thigh volume. Ultrasound Obstet Gynecol 2004;24:766–74
- Lee W, Deter RL, McNie B, et al. The fetal arm: individualized growth assessment in normal pregnancies. J Ultrasound Med 2005;24:817–28
- Lee W, Deter RL, Sameera S, et al. Individualized growth assessment of fetal thigh circumference using three-dimensional ultrasonography. Ultrasound Obstet Gynecol 2008;31:520–8
- Deter RL, Lee W, Sangi-Haghpeykar H, et al. Personalized third-trimester fetal growth evaluation: comparisons of individualized growth assessment, percentile line and conditional probability methods. J Matern Fetal Neonatal Med 2014. [Epub ahead of print]. doi: 10.3109/14767058.2014.995083
- Deter RL, Lee W, Sangi-Haghpeykar H, et al. A modified prenatal growth assessment score for the evaluation of fetal growth in the third trimester using single and composite biometric parameters. J Matern Fetal Neonatal Med 2015;28:745–54
- Deter RL, Lee W, Sangi-Haghpeykar H, et al. Individualized fetal growth assessment: critical evaluation of key concepts in the specification of third trimester size trajectories. J Matern Fetal Neonatal Med 2014;27:543–51
- Ananth CV, Vintzileos AM. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev 2009;85:653–8
- Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr 2010;99:550–5
- Galan HL. Timing delivery of the growth-restricted fetus. Semin Perinatol 2011;35:262–9
- von Beckerath AK, Kollmann M, Rotky-Fast C, et al. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol 2013;208:130.e131–6
- Boers KE, Vijgen SM, Bijlenga D, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ 2010;341:c7087
- GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG 2003;110:27–32
- Thornton JG, Hornbuckle J, Vail A, et al. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004;364:513–20
- Grimes DA. When to deliver the stunted fetus? Lancet 2004;364:483–4
- Gardosi J. GRIT: concern about external validity. Lancet 2005;365:384; author reply 385
- Walker DM, Marlow N, Upstone L, et al. The Growth Restriction Intervention Trial: long-term outcomes in a randomized trial of timing of delivery in fetal growth restriction. Am J Obstet Gynecol 2011;204:34.e31–9
- Trudell AS, Cahill AG, Tuuli MG, et al. Risk of stillbirth after 37 weeks in pregnancies complicated by small-for-gestational-age fetuses. Am J Obstet Gynecol 2013;208:376.e371–7
- Trudell AS, Tuuli MG, Cahill AG, et al. Balancing the risks of stillbirth and neonatal death in the early preterm small-for-gestational-age fetus. Am J Obstet Gynecol 2014;211:295.e291–7
- Eskenazi B, Fenster L, Sidney S, et al. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol 1993;169:1112–18
- Churchill D, Perry IJ, Beevers DG. Ambulatory blood pressure in pregnancy and fetal growth. Lancet 1997;349:7–10
- McCowan LM, North RA, Harding JE. Abnormal uterine artery Doppler in small-for-gestational-age pregnancies is associated with later hypertension. Aust N Z J Obstet Gynaecol 2001;41:56–60
- Mitani M, Matsuda Y, Makino Y, et al. Clinical features of fetal growth restriction complicated later by preeclampsia. J Obstet Gynaecol Res 2009;35:882–7
- Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol 2003;188:1596–9; discussion 1599–601
- Kamoji VM, Dorling JS, Manktelow BN, et al. Extremely growth-retarded infants: is there a viability centile? Pediatrics 2006;118:758–63
- Slattery MM, Geary M, Morrison JJ. Obstetric antecedents for preterm delivery. J Perinat Med 2008;36:306–9
- Carreno CA, Costantine MM, Holland MG, et al. Approximately one-third of medically indicated late preterm births are complicated by fetal growth restriction. Am J Obstet Gynecol 2011;204:263.e261–4
- Kramer MS, Olivier M, McLean FH, et al. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics 1990;86:707–13
- Simchen MJ, Beiner ME, Strauss-Liviathan N, et al. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol 2000;17:187–92
- Resnik R. Intrauterine growth restriction. Obstet Gynecol 2002;99:490–6
- Ego A, Subtil D, Grange G, et al. Customized versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study. Am J Obstet Gynecol 2006;194:1042–9
- Getahun D, Ananth CV, Kinzler WL. Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol 2007;196:499–507
- Giapros V, Drougia A, Krallis N, et al. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J Matern Fetal Neonatal Med 2012;25:153–7
- Qiu X, Lodha A, Shah PS, et al. Neonatal outcomes of small for gestational age preterm infants in Canada. Am J Perinatol 2012;29:87–94
- Pilliod RA, Cheng YW, Snowden JM, et al. The risk of intrauterine fetal death in the small-for-gestational-age fetus. Am J Obstet Gynecol 2012;207:318.e311–16
- Voskamp BJ, Kazemier BM, Ravelli AC, et al. Recurrence of small-for-gestational-age pregnancy: analysis of first and subsequent singleton pregnancies in The Netherlands. Am J Obstet Gynecol 2013;208:374.e371–6
- Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013;382:417–25
- Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23
- Boutsikou T, Malamitsi-Puchner A, Economou E, et al. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum Dev 2006;82:235–9
- Crispi F, Dominguez C, Llurba E, et al. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol 2006;195:201–7
- Savvidou MD, Yu CK, Harland LC, et al. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol 2006;195:1668–73
- Schlembach D, Wallner W, Sengenberger R, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2007;29:407–13
- Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–7
- Chaiworapongsa T, Espinoza J, Gotsch F, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40
- Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008;21:41–52
- Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–87
- Crispi F, Llurba E, Dominguez C, et al. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303–9
- Asvold BO, Vatten LJ, Romundstad PR, et al. Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am J Epidemiol 2011;173:630–9
- Sibiude J, Guibourdenche J, Dionne MD, et al. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS One 2012;7:e50208
- Benton SJ, Hu Y, Xie F, et al. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction? Am J Obstet Gynecol 2012;206:163.e161–7
- Lobmaier SM, Figueras F, Mercade I, et al. Angiogenic factors versus Doppler follow up in the prediction of adverse outcome among late pregnancy small-for-gestational-age fetuses. Ultrasound Obstet Gynecol 2014;43:533–40
- Alahakoon TI, Zhang W, Trudinger BJ, et al. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J Matern Fetal Neonatal Med 2014;27:1854–9
- Lobmaier SM, Figueras F, Mercade I, et al. Angiogenic factors vs Doppler surveillance in the prediction of adverse outcome among late-pregnancy small-for- gestational-age fetuses. Ultrasound Obstet Gynecol 2014;43:533–40
- Darling AM, McDonald CR, Conroy AL, et al. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. Am J Obstet Gynecol 2014;211:509.e501–8
- Torry DS, Wang HS, Wang TH, et al. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–44
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, et al. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–22
- Tidwell SC, Ho HN, Chiu WH, et al. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001;184:1267–72
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–58
- Taylor RN, Grimwood J, Taylor RS, et al. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–82
- Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–63
- Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 2003;111:600–2
- Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–51
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83
- Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–7; discussion 1547–50
- Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18
- Park CW, Park JS, Shim SS, et al. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–9
- Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med 2005;18:9–16
- Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005
- Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–9
- Unal ER, Robinson CJ, Johnson DD, et al. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol 2007;197:211.e211–14
- Widmer M, Villar J, Benigni A, et al. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol 2007;109:168–80
- Moore Simas TA, Crawford SL, Solitro MJ, et al. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol 2007;197:244.e241–8
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–38
- Chaiworapongsa T, Romero R, Tarca AL, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol 2010;202:550.e1–10
- Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med 2012;25:498–507
- Molvarec A, Szarka A, Walentin S, et al. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res 2010;33:892–8
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med 2011;24:1187–207
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012;60:451–8
- Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension 2012;59:555–7
- Ghosh SK, Raheja S, Tuli A, et al. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet 2012;285:417–22
- Hagmann H, Thadhani R, Benzing T, et al. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem 2012;58:837–45
- Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;122:43–52
- Rana S, Cerdeira AS, Wenger J, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One 2012;7:e48259
- Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand 2012;91:1388–94
- McElrath TF, Lim KH, Pare E, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 2012;207:407.e401–7
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012;125:911–19
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med 2012;25:2651–7
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med 2014;27:132–44
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol 2013;208:287.e1–15
- Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013;128:2121–31
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014;63:346–52
- Goel A, Rana S. Angiogenic factors in preeclampsia: potential for diagnosis and treatment. Curr Opin Nephrol Hypertens 2013;22:643–50
- Moore Simas TA, Crawford SL, Bathgate S, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med 2014;27:1038–48
- Rizos D, Eleftheriades M, Karampas G, et al. Placental growth factor and soluble fms-like tyrosine kinase-1 are useful markers for the prediction of preeclampsia but not for small for gestational age neonates: a longitudinal study. Eur J Obstet Gynecol Reprod Biol 2013;171:225–30
- Schaarschmidt W, Rana S, Stepan H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med 2013;41:511–16
- Maynard SE, Crawford SL, Bathgate S, et al. Gestational angiogenic biomarker patterns in high risk preeclampsia groups. Am J Obstet Gynecol 2013;209:53.e1–9
- Reimer T, Rohrmann H, Stubert J, et al. Angiogenic factors and acute-phase proteins in serum samples of preeclampsia and HELLP patients: a matched-pair analysis. J Matern Fetal Neonatal Med 2013;26:263–9
- Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014;10:466–80
- Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, et al. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol 2014;10:531–40
- Moore Simas TA, Crawford SL, Bathgate S, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med 2014;27:1038–48
- Romero R, Chaiworapongsa T, Erez O, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med 2010;23:1384–99
- Espinoza J, Chaiworapongsa T, Romero R, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507
- Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol 2007;109:1316–24
- Chaiworapongsa T, Kusanovic JP, Savasan ZA, et al. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med 2010;23:960–72
- Daponte A, Pournaras S, Polyzos NP, et al. Soluble FMS-like tyrosine kinase-1 (sFlt-1) and serum placental growth factor (PlGF) as biomarkers for ectopic pregnancy and missed abortion. J Clin Endocrinol Metab 2011;96:E1444–51
- Ferrazzi E, Vegni C, Bellotti M, et al. Role of umbilical Doppler velocimetry in the biophysical assessment of the growth-retarded fetus. Answers from neonatal morbidity and mortality. J Ultrasound Med 1991;10:309–15
- Romero R, Kalache KD, Kadar N. Timing the delivery of the preterm severely growth-restricted fetus: venous Doppler, cardiotocography or the biophysical profile? Ultrasound Obstet Gynecol 2002;19:118–21
- Ferrazzi E, Bozzo M, Rigano S, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol 2002;19:140–6
- Soregaroli M, Bonera R, Danti L, et al. Prognostic role of umbilical artery Doppler velocimetry in growth-restricted fetuses. J Matern Fetal Neonatal Med 2002;11:199–203
- Baschat AA, Gembruch U, Weiner CP, et al. Qualitative venous Doppler waveform analysis improves prediction of critical perinatal outcomes in premature growth-restricted fetuses. Ultrasound Obstet Gynecol 2003;22:240–5
- Berkley E, Chauhan SP, Abuhamad A. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol 2012;206:300–8
- Unterscheider J, Daly S, Geary MP, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol 2013;208:290.e291–6
- Unterscheider J, Daly S, Geary MP, et al. Predictable progressive Doppler deterioration in IUGR: does it really exist? Am J Obstet Gynecol 2013;209:539.e531–7
- Macones GA, Cahill A, Odibo A. Doppler deterioration in intrauterine growth restriction: Unterscheider et al. Am J Obstet Gynecol 2013;209:589
- Ferrazzi E. The PORTO study and the gestational age perspective of Doppler interrogation of IUGR fetuses. Am J Obstet Gynecol 2014;211:314–15
- Flood K, Unterscheider J, Daly S, et al. The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: results of the multicenter PORTO Study. Am J Obstet Gynecol 2014;211:288.e281–5
- O'Dwyer V, Burke G, Unterscheider J, et al. Defining the residual risk of adverse perinatal outcome in growth-restricted fetuses with normal umbilical artery blood flow. Am J Obstet Gynecol 2014;211:420.e421–5
- Savchev S, Figueras F, Gratacos E. Survey on the current trends in managing intrauterine growth restriction. Fetal Diagn Ther 2014;36:129–35
- Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis. Am J Obstet Gynecol 1995;172:1379–87
- Morris RK, Malin G, Robson SC, et al. Fetal umbilical artery Doppler to predict compromise of fetal/neonatal wellbeing in a high-risk population: systematic review and bivariate meta-analysis. Ultrasound Obstet Gynecol 2011;37:135–42
- Imdad A, Yakoob MY, Siddiqui S, et al. Screening and triage of intrauterine growth restriction (IUGR) in general population and high risk pregnancies: a systematic review with a focus on reduction of IUGR related stillbirths. BMC Public Health 2011;11:S1
- Vergani P, Roncaglia N, Andreotti C, et al. Prognostic value of uterine artery Doppler velocimetry in growth-restricted fetuses delivered near term. Am J Obstet Gynecol 2002;187:932–6
- Severi FM, Bocchi C, Visentin A, et al. Uterine and fetal cerebral Doppler predict the outcome of third-trimester small-for-gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2002;19:225–8
- Ghosh GS, Gudmundsson S. Uterine and umbilical artery Doppler are comparable in predicting perinatal outcome of growth-restricted fetuses. BJOG 2009;116:424–30
- Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991;181:129–33
- Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–8
- ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 2002;99:159–67
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–22
- Chaiworapongsa T, Romero R, Kusanovic JP, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol 2010;35:155–62
- Trudinger BJ, Cook CM, Giles WB, et al. Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. Br J Obstet Gynaecol 1991;98:378–84
- Chen WJ, Samuelson FW, Gallas BD, et al. On the assessment of the added value of new predictive biomarkers. BMC Med Res Methodol 2013;13:98
- Demler OV, Pencina MJ, D'Agostino RB. Misuse of DeLong test to compare AUCs for nested models. Stat Med 2012;31:2577–87
- Pepe MS, Kerr KF, Longton G, et al. Testing for improvement in prediction model performance. Stat Med 2013;32:1467–82
- Nicolaides KH, Bilardo CM, Soothill PW, et al. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. BMJ 1988;297:1026–7
- Yoon BH, Oh IH, Lee PR, et al. Is an abnormal Doppler umbilical artery waveform ratio a risk factor for poor perinatal outcome in the non-small for gestational age fetus? Am J Perinatol 1993;10:245–9
- Giles W, Bisits A. Clinical use of Doppler ultrasound in pregnancy: information from six randomised trials. Fetal Diagn Ther 1993;8:247–55
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–35
- Vickers A, Cronin A, Begg C. One statistical test is sufficient for assessing new predictive markers. BMC Med Res Methodol 2011;11:13