Abstract
Objectives: To determine whether a new antibiotic regimen could reduce the frequency of intra-amniotic inflammation/infection in patients with preterm PROM.
Study design: This retrospective cohort study was conducted to evaluate the effect of antibiotics on the frequency of intra-amniotic inflammation/infection based on the results of follow-up transabdominal amniocenteses from 89 patients diagnosed with preterm PROM who underwent serial amniocenteses. From 1993–2003, ampicillin and/or cephalosporins or a combination was used (“regimen 1”). A new regimen (ceftriaxone, clarithromycin and metronidazole) was used from 2003–2012 (“regimen 2”). Amniotic fluid was cultured and matrix metalloproteinase-8 (MMP-8) concentrations were measured.
Results: (1) The rates of intra-amniotic inflammation and intra-amniotic inflammation/infection in patients who received regimen 2 decreased during treatment from 68.8% to 52.1% and from 75% to 54.2%, respectively. In contrast, in patients who received regimen 1, the frequency of intra-amniotic inflammation and infection/inflammation increased during treatment (31.7% to 55% and 34.1% to 58.5%, respectively); and (2) intra-amniotic inflammation/infection was eradicated in 33.3% of patients who received regimen 2, but in none who received regimen 1.
Conclusion: The administration of ceftriaxone, clarithromycin and metronidazole was associated with a more successful eradication of intra-amniotic inflammation/infection and prevented secondary intra-amniotic inflammation/infection more frequently than an antibiotic regimen which included ampicillin and/or cephalosporins in patients with preterm PROM.
Introduction
Premature rupture of the membranes (PROM) occurs in 10% of pregnancies [Citation1–5] and is a risk factor for adverse pregnancy and neonatal outcomes [Citation6–24]. Preterm PROM is a major complication of pregnancy [Citation25–39] and accounts for 30% of spontaneous preterm births [Citation40–42]. Microorganisms in the amniotic fluid are present in approximately 30% of patients, based on the results of culture techniques [Citation43–66], and in 50% of patients using the combination of culture and molecular microbiologic techniques [Citation67–72]. The frequency of infection increases over time (latency period), so that when a patient with preterm PROM eventually goes into labor, microorganisms are detected in 75% of cases [Citation48]. Intra-amniotic inflammation is frequently present in patients with microorganisms in the amniotic fluid even though, in some cases, sterile inflammation is present [Citation69,Citation73–77].
Antibiotic administration has become the standard of care for patients with preterm PROM [Citation1,Citation35,Citation36,Citation78–88]. Antimicrobial agents are prescribed to eradicate existing subclinical intra-amniotic infection or to prevent secondary ascending infection into the amniotic cavity [Citation1,Citation35,Citation36,Citation78–89]. Randomized clinical trials [Citation90–104] and systematic reviews [Citation2,Citation105–108] indicate that antibiotic administration has short-term benefits for pregnant women and their neonates, including prolonged pregnancy [Citation2,Citation91–97,Citation99–102,Citation105,Citation109], reduction of neonatal respiratory distress syndrome [Citation2,Citation92,Citation100,Citation102], infection-related morbidity [Citation2,Citation90–92,Citation94,Citation98–100,Citation102,Citation105,Citation106,Citation109], and necrotizing enterocolitis [Citation2,Citation100,Citation102]. Whether the short-term benefits translate into long-term health outcomes is not clear. Indeed, a follow-up study of infants exposed to antibiotics due to preterm PROM indicated that there was not a demonstrable benefit at 7 years of age [Citation110].
Several antibiotic regimens have been used in patients with preterm PROM, including ampicillin [Citation90,Citation93,Citation97,Citation99,Citation100,Citation108,Citation111–113], amoxicillin–clavulanate [Citation93,Citation102,Citation108], penicillin [Citation94], erythromycin [Citation92,Citation95,Citation97,Citation100,Citation102,Citation108,Citation114], mezlocillin [Citation97], pipericillin [Citation96], cefexin [Citation115], cefizox [Citation116], gentamicin [Citation93], clindamycin [Citation93], azithromycin [Citation113,Citation117], and combinations of different agents [Citation89,Citation109]. The currently recommended practice varies in the United States and Europe. These choices appear to be influenced by randomized clinical trials conducted in each country. For example, the current recommendation in the United States includes intravenous ampicillin and erythromycin, followed by oral amoxicillin and erythromycin [Citation100]. In the United Kingdom, the recommendation is to administer oral erythromycin [Citation102].
Genital mycoplasmas are the most frequent organisms invading the amniotic cavity in preterm PROM [Citation57,Citation69,Citation118–122,Citation123]. The antibiotics used in randomized clinical trials of preterm PROM have limited effectiveness against these organisms. Penicillin and cephalosporins are not effective, and more than 80% of Ureaplasma spp. are resistant to erythromycin [Citation124–129]. Therefore, we designed a combination of antibiotic therapies for patients with preterm PROM, which was implemented in clinical practice in 2003 with the goal of providing antimicrobial activities to most organisms found in the amniotic cavity in preterm PROM. This combination included ceftriaxone, clarithromycin, and metronidazole. We recently reported that the use of this regimen was associated with a prolonged latency period and the reduced frequency of funisitis [Citation109].
We performed follow-up amniocenteses as a part of our standard practice to monitor the response to therapy. The information derived from follow-up amniocenteses and microbiologic studies provides a unique opportunity to gain insight into the efficacy of antibiotic administration in preterm PROM. The purpose of this study was to examine whether the antibiotic regimen with expanded coverage (regimen 2) would decrease the frequency of intra-amniotic inflammation/infection.
Materials and methods
Study design
This is a retrospective cohort study. The effect of antibiotics on the microbial state of the amniotic cavity and intra-amniotic inflammation was analyzed in the samples of amniotic fluid obtained at the time of the initial and follow-up amniocenteses. The study population comprised patients admitted to Seoul National University Hospital, Seoul, Republic of Korea, between January 1993 and June 2012, with the diagnosis of preterm PROM, who met the following criteria: (1) singleton pregnancy, (2) gestational age <34 weeks, and (3) follow-up amniocentesis performed within 15 days of the initial amniocentesis. The last criterion was used to evaluate changes in the microbial state of the amniotic cavity and intra-amniotic inflammation after the initial amniocentesis.
At Seoul National University Hospital, a transabdominal amniocentesis is routinely offered to all patients admitted with the diagnosis of preterm PROM to assess the microbiologic status of the amniotic cavity and fetal lung maturation. A follow-up amniocentesis could then be performed at the discretion of the managing clinician to further assess the microbiologic state of the amniotic cavity and fetal lung maturity after the completion of corticosteroid administration. This could have been performed with either a transabdominal amniocentesis or the aspiration of fluid at the time of cesarean delivery. Although all patients admitted with the diagnosis of preterm PROM were offered an initial amniocentesis, there was no uniform agreement on the performance and timing of a follow-up amniocentesis in our unit. Some physicians elected not to offer a second amniocentesis; therefore, many patients did not undergo the procedure. The diagnosis of rupture of the membranes was based on the patient having a previous history of watery vaginal discharge and a combination of the following tests: confirming leakage of amniotic fluid from the cervical os, vaginal pooling of amniotic fluid, and a positive nitrazine test through a sterile speculum examination.
Antibiotic administration was initiated when rupture of the membranes was diagnosed and continued until delivery. Corticosteroids were administered to patients at 24 weeks of gestation or more for the induction of fetal lung maturity. Between January 1993 and August 2003, patients received ampicillin and/or cephalosporin. Fifteen patients received intravenous ampicillin alone, and seven patients received intravenous cephalosporins. Three patients were treated with intravenous ampicillin combined with cephalosporins. The remaining 16 patients received erythromycin, azithromycin, gentamicin, or metronidazole combined with ampicillin or cephalosporins [regimen 1 (N = 41)]. The precise selection of antimicrobial agents was also at the discretion of the managing clinicians.
Between September 2003 and June 2012, the antibiotic regimen administered was 1 g of intravenous ceftriaxone every 24 h, 500 mg of oral clarithromycin every 12 h, and 500 mg of intravenous metronidazole every 8 h until delivery [regimen 2 (N = 48)] with the exception of metronidazole, which was administered for a maximum of 4 weeks. The study population was divided into two groups according to the antibiotic regimen 1 or 2.
Written informed consent was obtained from all participants. The Institutional Review Board of the Seoul National University Hospital approved the collection and use of samples and clinical information for research purposes. The Seoul National University Hospital has a Federalwide Assurance with the Office for Human Research Protection of the U.S. Department of Health and Human Services.
Amniotic fluid
Amniotic fluid was retrieved by transabdominal amniocentesis and cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas using methods previously described [Citation52,Citation57,Citation130–133]. Amniotic fluid not used for diagnostic studies was centrifuged and stored at −70 °C until assay. Matrix metalloproteinase-8 (MMP-8) concentrations in the amniotic fluid were used to diagnose intra-amniotic inflammation based on the criteria of previous studies [Citation55,Citation131,Citation134–141]. Stored amniotic fluid as well as a commercially available enzyme-linked immunosorbent assay (ELISA) (Amersham Pharmacia Biotech, Inc, Bucks, UK), according to the manufacturer’s instructions, were used. The sensitivity of the test was 0.3 ng/mL, and the intra- and interassay coefficients of variation were less than 10%.
Criteria for the diagnosis of intra-amniotic inflammation, intra-amniotic infection/inflammation, acute histologic chorioamnionitis and funisitis
Intra-amniotic inflammation was defined as an elevated amniotic fluid MMP-8 concentration (>23 ng/mL), as reported previously [Citation55,Citation131,Citation134–141]. Intra-amniotic inflammation/infection was diagnosed when either a positive amniotic fluid culture or the presence of intra-amniotic inflammation was detected. Using criteria previously described, the diagnosis of acute histologic chorioamnionitis was made upon examination of the extraplacental chorioamniotic membrane roll and/or the chorionic plate of the placenta when a patient presented with acute inflammatory changes [Citation142–145]; funisitis was diagnosed when neutrophil infiltration was detected in the umbilical vessel walls or Wharton’s jelly [Citation135,Citation143–148].
Statistical analysis
To determine continuous variables, the Mann-Whitney U test was used. Paired nonparametric analyses were used to compare the changes in the amniotic fluid MMP-8 concentration between the initial and follow-up amniocenteses. For categorical variables, the χ2 test or Fisher’s exact test was used. McNemar’s test was used to compare the frequency of positive amniotic fluid culture, intra-amniotic inflammation, and intra-amniotic infection/inflammation between the initial and follow-up amniocenteses. Medians and ranges were reported for continuous variables, whereas frequencies and percentages were calculated for categorical variables. Statistical analyses were conducted using SPSS Version 19.0 (SPSS Inc., Chicago, IL). All p values were two-sided and a p value of <0.05 was considered statistically significant.
Results
Eighty-nine patients with preterm PROM and a singleton gestation (<34 weeks) met the inclusion criteria of this study; 41 patients received regimen 1 and 48 patients received regimen 2. After the initial amniocentesis, the antibiotic regimen was changed in 12 patients and discontinued in one patient among those who received regimen 1, and also changed in 4 patients and discontinued in one patient among those who received regimen 2. After the follow-up amniocentesis, the antibiotic treatment regimen was changed in 6 patients and discontinued in another 6 patients among those who received regimen 1, and also changed in 6 patients and discontinued in 4 patients who received regimen 2. shows the clinical and demographical characteristics of the study population. Patients who received regimen 2 (ceftriaxone, clarithromycin, and metronidazole) had a significantly lower median gestational age at the initial amniocentesis, follow-up amniocentesis, and delivery as well as a significantly lower rate of funisitis, a marker of fetal systemic inflammatory response syndrome, than those who received regimen 1 (p < 0.05 for each).
Table 1. Demographics and clinical characteristics of the study population.
compares the rates of positive amniotic fluid culture, intra-amniotic inflammation, intra-amniotic inflammation/infection, and the median MMP-8 concentration according to the antibiotic regimen and timing of amniocentesis. Microorganisms isolated at the initial amniocentesis in patients who received regimen 1 included Ureaplasma urealyticum (n = 7), coagulase (−) Staphylococcus (n = 2), Streptococcus anginosus (n = 1), and peptostreptococcus (n = 1), and in those who received regimen 2 were Ureaplasma urealyticum (n = 7), Mycoplasma hominis (n = 4), Staphylococcus anginosus (n = 1), Gram (+) cocci (n = 2), Lactobacillus (n = 1), and Enterococcus faecium (n = 1). Microorganisms identified at the follow-up amniocentesis in patients who received regimen 1 included Ureaplasma urealyticum (N = 8), Mycoplasma hominis (N = 1), Klebsiella pneumoniae (N = 2), Escherichia coli (N = 1), coagulase (−) Staphylococcus (N = 1), Staphylococcus epidermidis (N = 2), Staphylococcus aureus (N = 1), and Streptococcus agalactiae (N = 1), and in those who received regimen 2, we found Ureaplasma urealyticum (N = 5), Mycoplasma hominis (N = 7), and Candida albicans (N = 3).
Table 2. Amniotic fluid culture, intra-amniotic inflammation and intra-amniotic infection/inflammation according to the antibiotics regimen and timing of amniocentesis.
The frequency of intra-amniotic inflammation in patients who received regimen 1 increased over time [initial amniocentesis: 31.7% (13/41) versus follow-up amniocentesis: 55% (22/40); p = 0.006, McNemar’s test]. Similarly, the frequency of intra-amniotic inflammation/infection also increased over time in patients who received regimen 1 [initial amniocentesis: 34.1% (14/41) versus follow-up amniocentesis 58.5%: (24/41), p = 0.002, McNemar’s test] ().
In contrast, the rates of intra-amniotic inflammation and intra-amniotic inflammation/infection in patients who received regimen 2 decreased over time from 68.8% (33/48) to 52.1% (25/48) and from 75% (36/48) to 54.2% (26/48), respectively (p < 0.05, for each, McNemar’s test) (). In other words, the frequency of intra-amniotic inflammation/infection in patients who received regimen 2 decreased while it increased over time in those who received regimen 1.
There was no difference in the frequency of positive amniotic fluid cultures between the initial and follow-up amniocenteses in patients who received either regimen 1 or regimen 2 (p > 0.2, for each, McNemar’s test). However, the magnitude of the intra-amniotic inflammatory response significantly increased over time in patients who received regimen 1 (median amniotic fluid MMP-8 concentration 1.8 ng/mL versus 29.0 ng/mL, p = 0.003, Wilcoxon signed rank test), while there was no difference in the magnitude of the intra-amniotic inflammatory response between the initial and follow-up amniocenteses in patients who received regimen 2 ().
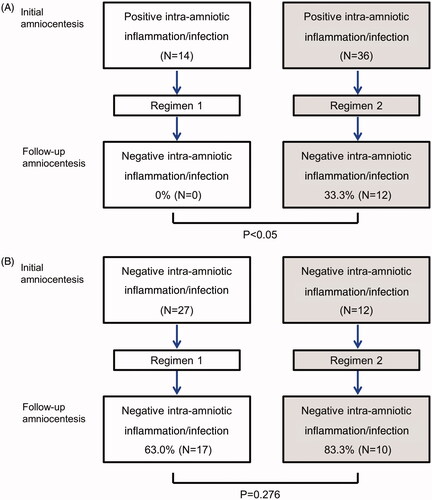
In terms of treatment of an existing intra-amniotic inflammation/infection, regimen 2 was superior to regimen 1 (). Specifically, 33.3% (12/36) of patients with intra-amniotic inflammation/infection who received regimen 2 at the initial amniocentesis had a negative amniotic fluid culture and no evidence of intra-amniotic inflammation at the follow-up amniocentesis. In contrast, none (0/14) of the patients who had intra-amniotic inflammation/infection at the time of the initial amniocentesis who received antibiotic regimen 1 had a negative amniotic fluid culture and intra-amniotic inflammation at the follow-up repeated amniocentesis [33.3% (12/36) versus 0% (0/14); p < 0.05] (). Among the patients without intra-amniotic inflammation/infection at the time of the initial amniocentesis, 83.3% (10/12) of patients who received regimen 2 and 63% (17/27) of those who received regimen 1 remained without evidence of intra-amniotic inflammation/infection ().
Figure 1. Conversion of intra-amniotic infection/inflammation according to the antibiotic regimen. (A) Intra-amniotic infection/inflammation was eradicated in 33.3% of patients who had intra-amniotic infection/inflammation at the time of initial amniocentesis and received regimen 2, while none of patients who received regimen 1 showed negative conversion of intra-amniotic infection/inflammation (p < 0.05). (B) Among the patients without intra-amniotic inflammation/infection at the time of the first amniocentesis, 83.3% of patients who received regimen 2% and 63% of those who received regimen 1 remained without evidence of intra-amniotic infection/inflammation, which did not reach statistical significance (p = 0.276).
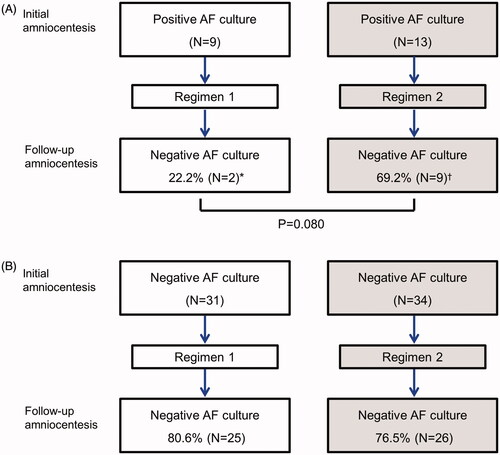
Twenty-two percent (2/9) of patients with a positive amniotic fluid culture at the time of the initial amniocentesis who received regimen 1 had a negative amniotic fluid culture at the follow-up amniocentesis. In contrast, in 69.2% (9/13) of patients who received regimen 2, the amniotic cavity became sterile; however, this trend did not reach statistical significance (p = 0.08) (). There was no difference in the rate of development of de novo intra-amniotic infection according to the antibiotic regimen ().
Figure 2. Conversion of intra-amniotic infection according to the antibiotic regimen. (A) 22.2% of patients with a positive amniotic fluid culture at the time of initial amniocentesis and who received regimen 1 had a conversion to a negative amniotic fluid culture at follow-up amniocentesis, while 69.2% of patients who received regimen 2, the amniotic cavity became sterile (p = 0.080) (B) There was no difference in the rate of development of de novo intra-amniotic infection according to the antibiotic regimen. *, All cases (2/2) with negative conversion of intra-amniotic infection had an intra-amniotic inflammation. †, Four (44.4%) of 9 cases with negative conversion of intra-amniotic infection had an intra-amniotic inflammation.
Discussion
Principal findings of the study
The administration of ceftriaxone, clarithromycin, and metronidazole was associated with a more successful eradication of intra-amniotic inflammation/infection and also prevented secondary intra-amniotic inflammation/infection more frequently than that observed with a conventional antimicrobial regimen used in preterm PROM. Moreover, this new combination was associated with a lower rate of funisitis, the histologic hallmark of the fetal inflammatory response syndrome.
Antibiotic administration to patients with preterm PROM based on randomized clinical trials
The practice of administering antibiotics to patients with preterm PROM is grounded in the results of multiple randomized clinical trials [Citation90–104] and a large systematic review and meta-analysis [Citation2,Citation105,Citation106]. Antimicrobial agents have been shown to prolong the latency period [Citation2,Citation91–97,Citation99–102,Citation105], decrease neonatal infection [Citation2,Citation90–92,Citation94,Citation98–100,Citation102,Citation105,Citation106], and reduce respiratory morbidity, including the need for oxygen and surfactant [Citation2,Citation92,Citation100,Citation102]. Moreover, antibiotic administration also reduces the frequency of clinical chorioamnionitis [Citation2,Citation91,Citation94,Citation97,Citation98,Citation100,Citation102,Citation105]. Long-term follow-up of infants to the age of 7 exposed to antimicrobial agents in utero because of preterm PROM has not shown convincing evidence that the short-term gain translates into long-term benefits [Citation110].
Are antibiotics effective in treating and eradicating intra-amniotic infection in preterm PROM?
A challenge in assessing the efficacy of antibiotic administration is the clinical context in which they are prescribed. Pregnant women with subclinical intra-amniotic infection are difficult to monitor noninvasively. Serial amniocenteses were performed in the current study, and this provided a unique opportunity to assess which antibiotic regimen eradicated intra-amniotic inflammation/infection present at the time of admission or prevented secondary intra-amniotic inflammation/infection which frequently occurs over time prior to the onset of spontaneous labor or induction of labor.
Although eradication of intra-amniotic infection has been demonstrated in patients with preterm PROM by using serial amniocenteses, one study has raised important questions about efficacy [Citation149]. Gomez et al. followed 46 patients with preterm PROM for whom amniocenteses were performed between 18–32 weeks (median 27 weeks). The prevalence of intra-amniotic inflammation for the initial amniocentesis was 39% (18/46); seven patients had a positive amniotic fluid culture for bacteria [Citation149]. At the time of the follow-up amniocentesis, six of these seven patients still had a positive amniotic fluid culture despite having received antibiotics (ampicillin and erythromycin) for 7 days; the intra-amniotic inflammatory process had been successfully treated in only three of the 18 patients. Importantly, among patients with no evidence of intra-amniotic inflammation, 32% (9/28) developed intra-amniotic inflammation despite antibiotic therapy, and five of the nine had a positive amniotic fluid culture [Citation149]. These observations suggest that antimicrobial agents are not effective in eradicating or preventing intra-amniotic inflammation/infection in preterm PROM.
Potential risks associated with inadequate therapy or prophylaxis of intra-amniotic infection
Experimental studies designed to generate an animal model of brain injury after intrauterine infection have shown that inoculation of bacteria is followed by the onset of labor [Citation150]. If antibiotics are administered around the time of bacterial inoculation, this prevents the onset of labor, but intrauterine infection can be observed at the time of euthanasia [Citation151]. Moreover, the fetuses whose gestation was prolonged by the administration of antibiotics were subsequently found to have lesions of periventricular leukomalacia, a major risk factor for cerebral palsy [Citation151]. Studies in humans have suggested an association between acute chorioamnionitis and neurodevelopmental disorders [Citation136,Citation151–159]. The observation that the administration of ampicillin and erythromycin in the randomized clinical trial conducted by Mercer et al. [Citation100] did not result in a lower rate of funisitis (compared to the placebo group) [Citation160] indicates that short-term antimicrobial therapy did not reduce the likelihood of a fetal inflammatory response [Citation79]. This observation is consistent with a subsequent report indicating that there was no change in the concentrations of inflammatory cytokines in umbilical cord blood after the administration of antibiotics [Citation161]. Therefore, in women with preterm PROM, antibiotic administration prolongs pregnancy, but does not eradicate infection or inflammation.
Efficacy of erythromycin and azithromycin in eradicating intra-amniotic infection in animal models
Recent studies of sheep and nonhuman primates have reported that the administration of erythromycin does not eradicate intrauterine infection [Citation162,Citation163]. This has been attributed to the fact that maternal administration of macrolide antibiotics achieved only subtherapeutic doses in the amniotic fluid and fetal plasma. In contrast, studies of rhesus monkeys [Citation164,Citation165] and sheep [Citation166] demonstrated that the administration of azithromycin can eradicate Ureaplasma parvum from the amniotic fluid and fetal organs. Yet, residual acute histologic chorioamniotis has been observed, indicating that such therapy is not completely successful [Citation164,Citation166].
A new antibiotic regimen for patients with preterm PROM
Our study has examined the changes in microbiology and intra-amniotic inflammation in patients with preterm PROM treated with two different antimicrobial regimens. The first regimen used antibiotics used by clinicians concerned with polymicrobial infections. The second regimen was developed and implemented based upon studies of the microbiology of amniotic fluid in patients with preterm PROM, which showed that Ureaplasmas were a predominant species not treated successfully with erythromycin [Citation124–129]. The rationale for using a combination of clarithromycin [Citation167,Citation168], ceftriaxone [Citation169–171], and metronidazole [Citation172–175] was based on previous studies about the pharmacokinetics of antibiotics during pregnancy and the need for expanded coverage and duration of treatment [Citation129,Citation167,Citation168,Citation176,Citation177]. The key finding of the current study is that antimicrobial therapy is more effective in eradicating intra-amniotic inflammation/infection than the antimicrobial therapy used in the past. The improved efficacy can be attributed to the improved bioavailability of antibiotics and expanded coverage. It is unlikely that the improved result can be attributed to the duration of therapy, as patients in both groups were treated from admission to delivery. The clinical benefit of this therapy is the subject of a separate communication. However, there is evidence that this new antimicrobial therapy is associated with prolonged pregnancy and a lower frequency of neonatal complications, acute histologic chorioamnionitis, and funisitis [Citation109]. When broadspectrum antibiotics are used, the emergence of antibiotic-resistant bacteria is a possibility.
Strengths and limitations
The major strength of this study is that it is the first to examine the effect of the new antibiotic regimen for the treatment of intra-amniotic inflammation/infection. In our practice, we used serial amniocenteses to evaluate the microbial state and inflammatory response in the amniotic fluid. Moreover, we have also well-established assays to detect inflammation in the amniotic cavity.
A limitation of this study is that it was not a randomized clinical trial, but rather a retrospective cohort study conducted over a period of 20 years. However, none of the parameters serving as an endpoint (amniotic fluid culture, MMP-8 determination, and histologic examination of the placenta) changed fundamentally during this time, so it is unlikely that the historical nature of the control group could have affected the result reported herein. To conduct this study required the performance of serial amniocenteses, a procedure demanding considerable skill; this could introduce bias because not all patients are able to undergo a serial amniocentesis. We have recently developed a transcervical amniotic fluid collector to obtain this material for the assessment of intra-amniotic inflammation [Citation178]. Preliminary evidence indicates that fluid retrieved with this device can be used to reliably identify intra-amniotic inflammation, and this could enable serial evaluation of a large number of patients and reduce potential biases introduced by the requirement of amniocentesis [Citation178].
Conclusion
The combination of ceftriaxone, clarithromycin, and metronidazole can treat and prevent intra-amniotic inflammation/infection in patients with preterm PROM.
Declaration of interest
The authors report no conflicts of interest. This research was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C0768). This research was also supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS).
References
- Mercer B. Antibiotics in the management of PROM and preterm labor. Obstet Gynecol Clin North Am 2012;39:65–76
- Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2013;12:CD001058
- Parry S, Strauss JF. 3rd. Premature rupture of the fetal membranes. N Engl J Med 1998;338:663–70
- Romero R, Yeo L, Gotsch F, et al. Prelabor rupture of the membranes. In: Winn H, Chervanak F, Romero R, eds. Clinical maternal-fetal medicine online, 2nd ed. London (UK): Informa healthcare;2011:1–24
- Santolaya-Forgas J, Romero R, Espinoza J, et al. Prelabour rupture of the membranes. In: Reece E, Hobbins J, eds. Clinical obstetrics the fetus & mothers, 3rd ed. Malden (MA): Blackwell;2008:1130–88
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9
- March of Dimes, Premature Birth. Available from: http://www.marchofdimes.com/prematurity/21191.asp
- Deutsch A, Deutsch E, Totten C, et al. Maternal and neonatal outcomes based on the gestational age of midtrimester preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2010;23:1429–34
- Teune MJ, van Wassenaer AG, van Dommelen P, et al. Perinatal risk indicators for long-term neurological morbidity among preterm neonates. Am J Obstet Gynecol 2011;204:396. e1–14
- Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med 2012;25:1428–32
- Tsiartas P, Kacerovsky M, Musilova I, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2013;26:1332–6
- Grobman WA, Lai Y, Rouse DJ, et al. The association of cerebral palsy and death with small-for-gestational-age birthweight in preterm neonates by individualized and population-based percentiles. Am J Obstet Gynecol 2013;209:340. e1–5
- Acaia B, Crovetto F, Ossola MW, et al. Predictive factors for neonatal survival in women with periviable preterm rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:1628–34
- van der Heyden JL, van der Ham DP, van Kuijk S, et al. Outcome of pregnancies with preterm prelabor rupture of membranes before 27 weeks' gestation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol 2013;170:125–30
- Al-Mandeel H, Alhindi MY, Sauve R. Effects of intentional delivery on maternal and neonatal outcomes in pregnancies with preterm prelabour rupture of membranes between 28 and 34 weeks of gestation: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2013;26:83–9
- Strunk T, Inder T, Wang X, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62
- Manuck TA, Varner MW. Neonatal and early childhood outcomes following early vs later preterm premature rupture of membranes. Am J Obstet Gynecol 2014;211:308. e1–6
- Herber-Jonat S, Streiftau S, Knauss E, et al. Long-term outcome at age 7-10 years after extreme prematurity - a prospective, two centre cohort study of children born before 25 completed weeks of gestation (1999-2003). J Matern Fetal Neonatal Med 2014;27:1620–6
- Bastek JA, Weber AL, McShea MA, et al. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol 2014;210:450. e1–10
- Manuck TA, Sheng X, Yoder BA, et al. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol 2014;210:426. e1–9
- Armstrong-Wells J, Donnelly M, Post MD, et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol 2015;212:212. e1–9
- Ekin A, Gezer C, Taner CE, et al. Perinatal outcomes in pregnancies with oligohydramnios after preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1918–22
- Jeong H, Han SJ, Yoo HN, et al. Comparison of changes in etiologic microorganisms causing early-onset neonatal sepsis between preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1923–8
- Wong LF, Holmgren CM, Silver RM, et al. Outcomes of expectantly managed pregnancies with multiple gestations and preterm premature rupture of membranes prior to 26 weeks. Am J Obstet Gynecol 2015;212:215. e1–9
- Lebherz TB, Hellman LP, Madding R, et al. Double-blind study of premature rupture of the membranes. A report of 1,896 cases. Am J Obstet Gynecol 1963;87:218–25
- Sacks M, Baker TH. Spontaneous premature rupture of the membranes. A prospective study. Am J Obstet Gynecol 1967;97:888–93
- Gunn GC, Mishell DR Jr. Morton DG. Premature rupture of the fetal membranes. A review. Am J Obstet Gynecol 1970;106:469–83
- Premature rupture of the membranes. Br Med J 1979;1:1165–6
- Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol 1982;60:671–9
- Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clin Perinatol 2001;28:721–34
- Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol 2011;54:307–12
- Henderson JJ, McWilliam OA, Newnham JP, et al. Preterm birth aetiology 2004-2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J Matern Fetal Neonatal Med 2012;25:642–7
- Hunter TJ, Byrnes MJ, Nathan E, et al. Factors influencing survival in pre-viable preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2012;25:1755–61
- American Colloege of Obstetricians and Gynecologists. Practice bulletins No. 139: premature rupture of membranes. Obstet Gynecol 2013;122:918–30
- Ismail AQ, Lahiri S. Management of prelabour rupture of membranes (PROM) at term. J Perinat Med 2013;41:647–9
- Grunebaum A. Reply to “Management of prelabour rupture of membranes (PROM) at term”. J Perinat Med 2013;41:651–2
- Eschenbach D. Reply to: Ismail AQT, Lahiri S. Management of prelabor rupture of membranes (PROM) at term. J Perinat Med 2013;41:653–5
- Ramsauer B, Vidaeff AC, Hosli I, et al. The diagnosis of rupture of fetal membranes (ROM): a meta-analysis. J Perinat Med 2013;41:233–40
- Pintucci A, Meregalli V, Colombo P, et al. Premature rupture of membranes at term in low risk women: how long should we wait in the “latent phase”? J Perinat Med 2014;42:189–96
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog 2006;113:17–42
- Romero R, Lockwood CJ. Pathogenesis of spontaneous preterm labor. In: Creasy RK, Resnik R, Iams JD, eds. Creasy and Resnik's maternal-fetal medicine: principles and practice. Philadelphia: Elsevier;2009:521–3
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5
- Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol 1982;59:539–45
- Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol 1984;63:38–43
- Zlatnik FJ, Cruikshank DP, Petzold CR, et al. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med 1984;29:656–60
- Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66:316–21
- Feinstein SJ, Vintzileos AM, Lodeiro JG, et al. Amniocentesis with premature rupture of membranes. Obstet Gynecol 1986;68:147–52
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6
- Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol 1991;31:331–6
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Font GE, Gauthier DW, Meyer WJ, et al. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol 1995;85:656–8
- Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40
- Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol 1999;11:541–7
- Romero R, Espinoza J, Chaiworapongsa T, et al. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–74
- Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39
- Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2010;203:211. e1–8
- Lee SE, Romero R, Lee SM, et al. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med 2010;38:39–44
- Cobo T, Palacio M, Martinez-Terron M, et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol 2011;205:126. e1–8
- Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–19
- Kacerovsky M, Andrys C, Hornychova H, et al. Amniotic fluid soluble toll-like receptor 4 in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2012;25:1148–55
- Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 2012;17:12–19
- Kacerovsky M, Andrys C, Drahosova M, et al. Soluble toll-like receptor 1 family members in the amniotic fluid of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2012;25:1699–1704
- Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2013;26:795–801
- Romero R, Chaemsaithong P, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med 2015. 2015 Jul 25:1-8. [Epub ahead of print]
- Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125. e1–15
- DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57
- Kacerovsky M, Pliskova L, Bolehovska R, et al. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol 2011;205:213. e1–7
- Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409
- Kacerovsky M, Menon R, Drahosova M, et al. Amniotic fluid nucleosome in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2014;27:155–61
- Fortner KB, Grotegut CA, Ransom CE, et al. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PLoS One 2014;9:e83338
- Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014;210:325. e1–10
- Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23
- Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014. Sep 24: 1-17. [Epub ahead of print]
- Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74
- Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58
- Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36
- Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003;101:178–93
- Mercer BM, Goldenberg RL, Das AF, et al. What we have learned regarding antibiotic therapy for the reduction of infant morbidity after preterm premature rupture of the membranes. Semin Perinatol 2003;27:217–30
- Simhan HN, Canavan TP. Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG 2005;112:32–7
- Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005;32:411–28
- Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol 2009;201:230–40
- Singh K, Mercer B. Antibiotics after preterm premature rupture of the membranes. Clin Obstet Gynecol 2011;54:344–50
- Faksh A, Wax JR, Lucas FL, et al. Preterm premature rupture of membranes ≥32 weeks' gestation: impact of revised practice guidelines. Am J Obstet Gynecol 2011;205:340. e1–5
- Di Renzo GC, Roura LC, Facchinetti F, et al. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Matern Fetal Neonatal Med 2011;24:659–67
- van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol 2012;207:276. e1–10
- Rutanen EM. Comment on: guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes and preventive tools for preterm birth. J Matern Fetal Neonatal Med 2012;25:546–9. author reply 547-548
- van Teeffelen AS, van der Ham DP, Willekes C, et al. Midtrimester preterm prelabour rupture of membranes (PPROM): expectant management or amnioinfusion for improving perinatal outcomes (PPROMEXIL - III trial). BMC Pregnancy Childbirth 2014;14:128
- Lee J, Kang M, Kim E, et al. Effect of triple antibiotics on amniotic fluid infection/inflammation: an inter-era comparison of 20 years in patients with preterm PROM (Abstract 475). Am J Obstet Gynecol 2014;210:S238
- Amon E, Lewis SV, Sibai BM, et al. Ampicillin prophylaxis in preterm premature rupture of the membranes: a prospective randomized study. Am J Obstet Gynecol 1988;159:539–43
- Johnston MM, Sanchez-Ramos L, Vaughn AJ, et al. Antibiotic therapy in preterm premature rupture of membranes: a randomized, prospective, double-blind trial. Am J Obstet Gynecol 1990;163:743–7
- McGregor JA, French JI, Seo K. Antimicrobial therapy in preterm premature rupture of membranes: results of a prospective, double-blind, placebo-controlled trial of erythromycin. Am J Obstet Gynecol 1991;165:632–40
- Christmas JT, Cox SM, Andrews W, et al. Expectant management of preterm ruptured membranes: effects of antimicrobial therapy. Obstet Gynecol 1992;80:759–62
- Kurki T, Hallman M, Zilliacus R, et al. Premature rupture of the membranes: effect of penicillin prophylaxis and long-term outcome of the children. Am J Perinatol 1992;9:11–16
- Mercer BM, Moretti ML, Prevost RR, et al. Erythromycin therapy in preterm premature rupture of the membranes: a prospective, randomized trial of 220 patients. Am J Obstet Gynecol 1992;166:794–802
- Lockwood CJ, Costigan K, Ghidini A, et al. Double-blind; placebo-controlled trial of piperacillin prophylaxis in preterm membrane rupture. Am J Obstet Gynecol 1993;169:970–6
- Owen J, Groome LJ, Hauth JC. Randomized trial of prophylactic antibiotic therapy after preterm amnion rupture. Am J Obstet Gynecol 1993;169:976–81
- Ernest JM, Givner LB. A prospective, randomized, placebo-controlled trial of penicillin in preterm premature rupture of membranes. Am J Obstet Gynecol 1994;170:516–21
- Grable IA, Garcia PM, Perry D, et al. Group B Streptococcus and preterm premature rupture of membranes: a randomized, double-blind clinical trial of antepartum ampicillin. Am J Obstet Gynecol 1996;175:1036–42
- Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National institute of child health and human development maternal-fetal medicine units network. JAMA 1997;278:989–95
- Magwali TL, Chipato T, Majoko F, et al. Prophylactic augmentin in prelabor preterm rupture of the membranes. Int J Gynaecol Obstet 1999;65:261–5
- Kenyon SL, Taylor DJ, Tarnow-Mordi W, et al. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001;357:979–88
- Lewis DF, Adair CD, Robichaux AG, et al. Antibiotic therapy in preterm premature rupture of membranes: Are seven days necessary? A preliminary, randomized clinical trial. Am J Obstet Gynecol 2003;188:1413–6. discussion 1416-1417
- Kwak HM, Shin MY, Cha HH, et al. The efficacy of cefazolin plus macrolide (erythromycin or clarithromycin) versus cefazolin alone in neonatal morbidity and placental inflammation for women with preterm premature rupture of membranes. Placenta 2013;34:346–52
- August Fuhr N, Becker C, van Baalen A, et al. Antibiotic therapy for preterm premature rupture of membranes – results of a multicenter study. J Perinat Med 2006;34:203–6
- Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol 2008;199:620. e1–8
- Lamont RF. Antibiotics used in women at risk of preterm birth. Am J Obstet Gynecol 2008;199:583–4
- Ehsanipoor RM, Chung JH, Clock CA, et al. A retrospective review of ampicillin-sulbactam and amoxicillin + clavulanate vs cefazolin/cephalexin and erythromycin in the setting of preterm premature rupture of membranes: maternal and neonatal outcomes. Am J Obstet Gynecol 2008;198:e54–6
- Lee J, Romero R, Kim SM, et al. A new antimicrobial combination prolongs the latency period, reduces acute histologic chorioamnionitis and funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2015. Sep 16:1-14. [Epub ahead of print]
- Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet 2008;372:1310–18
- Morales WJ, Angel JL, O'Brien WF, et al. Use of ampicillin and corticosteroids in premature rupture of membranes: a randomized study. Obstet Gynecol 1989;73:721–6
- Muller AE, DeJongh J, Oostvogel PM, et al. Amoxicillin pharmacokinetics in pregnant women with preterm premature rupture of the membranes. Am J Obstet Gynecol 2008;198:108. e1–6
- Pierson RC, Gordon SS, Haas DM. A retrospective comparison of antibiotic regimens for preterm premature rupture of membranes. Obstet Gynecol 2014;124:515–9
- Gelber S, Brent E, Varrey A, et al. Equivalence of erythromycin and azithromycin for treatment of PPROM (Abstract number 690). Am J Obstet Gynecol 2013;208:S291
- Dunlop PDM, Crowley PA, Lamont RF, et al. Preterm ruptured membranes, no contractions. J Obstet Gynaecol 1986;7:92–6
- Blanco J, Iams J, Artal J, et al. Multicenter double-blind prospective random trial of ceftizoxime vs placebo in women with preterm premature ruptured membranes. Am J Obstet Gynecol 1993;168:378
- Canzoneri BJ, Grotegut CA, Swamy GK, et al. Maternal serum interleukin-6 levels predict impending funisitis in preterm premature rupture of membranes after completion of antibiotics. J Matern Fetal Neonatal Med 2012;25:1329–32
- Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2003;82:423–31
- Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol 2004;191:1382–86
- Kacerovsky M, Pavlovsky M, Tosner J. Preterm premature rupture of the membranes and genital mycoplasmas. Acta Medica (Hradec Kralove) 2009;52:117–20
- Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 2013;26:231–40
- Kwak DW, Hwang HS, Kwon JY, et al. Co-infection with vaginal ureaplasma urealyticum and mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2014;27:333–7
- Allen-Daniels MJ, Serrano MG, Pflugner LP, et al. Identification of a gene in mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol 2015;212:779. e1–13
- Kechagia N, Bersimis S, Chatzipanagiotou S. Incidence and antimicrobial susceptibilities of genital mycoplasmas in outpatient women with clinical vaginitis in Athens, Greece. J Antimicrob Chemother 2008;62:122–5
- Dongya M, Wencheng X, Xiaobo M, et al. Transition mutations in 23S rRNA account for acquired resistance to macrolides in ureaplasma urealyticum. Microb Drug Resist 2008;14:183–6
- Bayraktar MR, Ozerol IH, Gucluer N, et al. Prevalence and antibiotic susceptibility of mycoplasma hominis and ureaplasma urealyticum in pregnant women. Int J Infect Dis 2010;14:e90–5
- Xiao L, Crabb DM, Duffy LB, et al. Mutations in ribosomal proteins and ribosomal RNA confer macrolide resistance in human Ureaplasma spp. Int J Antimicrob Agents 2011;37:377–9
- Redelinghuys MJ, Ehlers MM, Dreyer AW, et al. Antimicrobial susceptibility patterns of ureaplasma species and mycoplasma hominis in pregnant women. BMC Infect Dis 2014;14:171
- Park CW, Kim SA, Huh HJ, et al. Prevalence and antibiotics susceptibilities of the ureaplasma species isolated from asymptomatic pregnant women (Abstract 163). Am J Obstet Gynecol 2015;212:S97
- Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–7
- Lee J, Oh KJ, Yang HJ, et al. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23
- Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24
- Park CW, Yoon BH, Park JS, et al. A fetal and an intra-amniotic inflammatory response is more severe in preterm labor than in preterm PROM in the context of funisitis: unexpected observation in human gestations. PLoS One 2013;8:e62521
- Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–9
- Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol 2001;185:1156–61
- Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–6
- Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292. e1–5
- Lee SE, Romero R, Jung H, et al. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294. e1–6
- Park CW, Lee SM, Park JS, et al. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008;36:497–502
- Park CW, Yoon BH, Kim SM, et al. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet Gynecol Sci 2013;56:167–75
- Kim SM, Romero R, Park JW, et al. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med 2014;20:1–10
- Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70
- Redline RW, Heller D, Keating S, et al. Placental diagnostic criteria and clinical correlation-a workshop report. Placenta 2005;26:S114–17
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301
- Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213(4 Suppl):S29-52
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000 Nov;183(5):1124-9
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Yoon BH, Romero R, Shim JY, et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90
- Gomez R, Romero R, Nien JK, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med 2007;20:167–73
- Fidel PL, Jr Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75
- Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177:797–802
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110:124–7
- Redline RW, Minich N, Taylor HG, et al. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol 2007;10:282–92
- Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol 2008;198:466. e1–11
- Botet F, Figueras J, Carbonell-Estrany X, et al. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: a case-control study. J Perinat Med 2011;39:203–8
- Martinelli P, Sarno L, Maruotti GM, et al. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med 2012;25:29–31
- Korzeniewski SJ, Romero R, Cortez J, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med 2014;42:731–43
- Bendon RW, Faye-Petersen O, Pavlova Z, et al. Fetal membrane histology in preterm premature rupture of membranes: comparison to controls, and between antibiotic and placebo treatment. The National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network, Bethesda, MD, USA. Pediatr Dev Pathol 1999;2:552–8
- Mercer BM, Crouse DT, Goldenberg RL, et al. The antibiotic treatment of PPROM study: systemic maternal and fetal markers and perinatal outcomes. Am J Obstet Gynecol 012;206:145. e1–9
- Dando SJ, Nitsos I, Newnham JP, et al. Maternal administration of erythromycin fails to eradicate intrauterine ureaplasma infection in an ovine model. Biol Reprod 2010;83:616–22
- Kemp MW, Miura Y, Payne MS, et al. Repeated maternal intramuscular or intraamniotic erythromycin incompletely resolves intrauterine ureaplasma parvum infection in a sheep model of pregnancy. Am J Obstet Gynecol 2014;211:134. e1–9
- Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012;207:475. e1–14
- Acosta EP, Grigsby PL, Larson KB, et al. Transplacental transfer of azithromycin and its use for eradicating intra-amniotic ureaplasma infection in a primate model. J Infect Dis 2014;209:898–904
- Miura Y, Payne MS, Keelan JA, et al. Maternal intravenous treatment with either azithromycin or solithromycin clears ureaplasma parvum from the amniotic fluid in an ovine model of intrauterine infection. Antimicrob Agents Chemother 2014;58:5413–20
- Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol 2003;188:816–19
- Park HS, Ahn BJ, Kwan Jun J. Placental transfer of clarithromycin in human pregnancies with preterm premature rupture of membranes. J Perinat Med 2012;40:641–6
- Adu A, Armour CL. Drug utilisation review (DUR) of the third generation cephalosporins. Focus on ceftriaxone, ceftazidime and cefotaxime. Drugs 1995;50:423–39
- Klein NC, Cunha BA. Third-generation cephalosporins. Med Clin North Am 1995;79:705–19
- Lamb HM, Ormrod D, Scott LJ, et al. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 2002;62:1041–89
- Visser AA, Hundt HK. The pharmacokinetics of a single intravenous dose of metronidazole in pregnant patients. J Antimicrob Chemother 1984;13:279–83
- Amon I. Placental transfer of metronidazole. J Perinat Med 1985;13:97–8
- Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs 1997;54:679–708
- Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev 2013;26:526–46
- Philipson A, Sabath LD, Charles D. Transplacental passage of erythromycin and clindamycin. N Engl J Med 1973;288:1219–21
- Heikkinen T, Laine K, Neuvonen PJ, et al. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG 2000;107:770–5
- Lee SM, Romero R, Park JS, et al. A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes. J Perinat Med 2015;43:381–9

