ABSTRACT
The Towards a Revolution in COPD Health (TORCH) trial was an international clinical trial of chronic obstructive pulmonary disease (COPD) patients where cause of death was assigned by an independent committee. Comparison of death certificate data and adjudicated cause of death allows a unique opportunity to determine death certificate accuracy and frequency of COPD listing on death certificates of COPD patients. In this analysis, the authors determine the concordance between adjudicated cause of death and primary and secondary cause of death from death certificates. In 317 (80%) of informative deaths, the primary or secondary cause of death from certificates agreed with adjudicated cause of death. Only 229 (58%) of death certificates in these COPD patients listed COPD on the certificate. COPD was not listed on the death certificate in 21% of deaths adjudicated to be caused by COPD exacerbation. Compared with pulmonary causes, the listing of COPD on certificates occurred with less frequency than cardiovascular, cancer and other categories of death. The combined primary and secondary listing on death certificates has good concordance with actual cause of death. COPD is under-reported on death certificates, and this under-reporting is more frequent when the primary cause of death is not pulmonary.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and worldwide (Citation1, 2). Mortality estimates for COPD are obtained by extracting the underlying cause of death from death certificates (Citation3, 4). COPD-related mortality is underestimated in the United States and internationally (Citation5–10). Using data from the US National Center for Health Statistics from 1979 to 1993, Mannino and colleagues found that of 2.6 million individuals with a diagnosis of obstructive lung disease, only 43.4% had obstructive lung disease listed as an underlying cause of death on death certificates (Citation5). Additionally, Hughes and colleagues reported on mortality data from a one-year period in Kentucky (Citation6). These authors found that when including death certificates listing COPD as a contributory rather than cause of death, the COPD age-adjusted mortality estimate increased by 39%. Jensen and colleagues observed that among Danish subjects with severe baseline disease, COPD was listed as a cause of death only 25% of the time (Citation7). Similar magnitudes of underreporting have been described in France, Italy and Poland (Citation8–10).
Although studies provide strong evidence of the underreporting of COPD deaths, they are unable to present an estimate of the degree of concordance between death certificates and actual cause of death. To provide this estimate, an independent adjudication of the cause of death needs to be determined for comparison to the listed causes. Smyth and colleagues reviewed medical records to adjudicate an accurate cause of death in 176 patients in Northern Ireland for whom asthma or COPD was listed on the death certificate (Citation11). These authors determined the sensitivity and specificity of death certificate cause of death in predicting actual cause of death for COPD as 69% and 70%, respectively.
Beyond this single-country report of selected cases, no other data exist describing the accuracy of death certificates when compared to an adjudicated cause of death. The Towards a Revolution in COPD Health (TORCH) (Clinicaltrials.gov NTC00268216) trial was the first international trial of COPD therapy that used all-cause mortality as a primary endpoint(Citation12). In TORCH, the cause of death was determined by a three-member masked clinical endpoint committee (CEC), that adjudicated category and specific cause of death using a standardized, consistent format (Citation13). As part of this process, death certificates were obtained and reviewed when available. The presence of death certificate information and rigorously adjudicated cause of death offers a unique opportunity to characterize the accuracy of death certificates in patients with an established diagnosis of COPD.
In this report, we use death certificate and adjudicated category and cause of death data from the TORCH trial to determine the concordance of cause of death from death certificates with category and cause of death in patients with COPD defined using a standardized CEC. Additionally, we describe the frequency with which COPD is listed as cause of death or significant condition on death certificates in patients with a confirmed diagnosis of COPD.
MATERIALS AND METHODS
TORCH study design
The TORCH trial design (SCO30003, NCT00268216) has been described previously(Citation12, Citation14). Briefly, the trial was a randomized, double masked, parallel group, controlled clinical trial comparing inhaled salmeterol 50 microgram twice daily, inhaled fluticasone propionate 500 microgram twice daily, combined salmeterol 50 microgram and fluticasone propionate 500 microgram inhaled twice daily and placebo. Eligible participants included current or former smokers with moderate-to-severe COPD. The primary outcome measure was all-cause mortality. Other outcome measures included cause-specific mortality and deaths related to COPD. TORCH was an international study, with 6184 participants enrolled in the overall safety population at 444 centers in 42 countries. A total of 911 deaths occurred in randomized participants.
Adjudication of cause of death in the TORCH trial
After a participant death occurred in TORCH, an investigation into the cause of death was carried out by the site investigator that included review of available death certificate, medical records, radiograph and laboratory information, operative reports, results of biopsy specimens and necropsy reports. For non-medical facility deaths, witness interviews were sought. Medical records were translated to English when necessary. This information was then compiled and forwarded to the CEC for adjudication. As previously described (Citation13), the CEC comprised three physicians from different countries with clinical and research expertise in internal medicine and pulmonary and critical care medicine (MJ, RW, LM), none of whom were site investigators. Using the available information, the CEC categorized the cause of death and the relationship of cause of death to COPD in a systematic, masked and independent manner. Although the CEC did have access to the death certificates to assist in determining date of death, the CEC cause of death was based on the clinical narrative provided by site investigators. Each CEC member rendered an independent opinion as to the cause of death. If a unanimous decision was not reached, the case was discussed to reach a consensus decision. Cause-specific mortality was first attributed to a specific cause of death, and then grouped into one of four general pathophysiologic categories (pulmonary, cardiovascular, cancer, other). If the cause of death could not be determined, it was classified as unknown.
Comparison of death certificate data to adjudicated cause of death
For the analysis presented here, all death certificates obtained in the course of the TORCH study were reviewed. Death certificates deemed non-informative (e.g., illegible or left blank) or cases where the CEC determined the cause of death to be unknown were excluded from analysis. From death certificates, data regarding listed primary and secondary cause of death and significant conditions were extracted. The CEC adjudicated category and cause of death were also collected. In this analysis, using a modified Delphi approach (Citation15), 3 reviewers (RW, MJ, LM) independently compared the death certificate data and the previously adjudicated CEC cause and category of death, determining concordance between them. In cases where cause of death was listed as “respiratory failure” or “cor pulmonale,” the reviewers considered this equivalent to death due to COPD. Because death certificate completion customs may vary by geographic reason, CEC cause of death was compared to primary, secondary and pooled primary/secondary death certificate data.
Cases where the 3 reviewers did not agree on concordance were independently re-reviewed. Remaining cases lacking complete consensus on death certificate-CEC cause of death concordance were discussed in person by reviewers. This process led to complete consensus in all cases. To measure intra-reviewer consistency, a random sample of 40 cases was selected and sent to each reviewer separately for re-review and comparison to the first comparison of death certificate data and CEC cause of death. For analyses by geographic region, countries were grouped into 5 regions using the World Health Organization organizational system (Citation16).
Statistical analysis
Baseline clinical and demographic data are presented as mean (standard deviation) or n(%). In 2-way comparisons, continuous variables were compared using t-tests while categorical variables were compared with Pearson's chi-squared. When testing across multiple categories of continuous variables, analysis of variance was performed to test differences in means. A Bonferroni corrected p-value was used to assess for statistical significance in subsequent two-way comparisons using t-tests. When testing across multiple categories of categorical variables, overall chi squared analysis was calculated. The kappa statistic was calculated to assess the intrareviewer and interreviewer agreement (Citation17). This is a measure of agreement observed beyond simple chance. The kappa statistic ranges from 0 to 1.0, with 0 indicating no agreement better than chance. Kappa statistics were calculated after the first round of the review process. Stata version 10.0 (Stata Corp, College Station, TX), was used for statistical calculations.
RESULTS
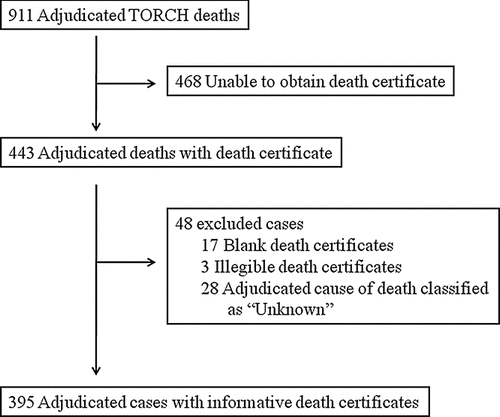
Case selection and adjudication ()
For inclusion in this analysis, the 911 adjudicated deaths from the initial TORCH study were considered. In 468 (51.4%) cases, death certificate information was not able to be obtained. Of the 443 adjudicated deaths with death certificate data, 48 were excluded due to non-informative death certificate data such as being illegible or left blank (n = 20) or the cause of death was adjudicated as “Unknown” (n = 28). The remaining 395 cases were examined by the reviewers for comparison of adjudicated cause of death and death certificate data.
When comparing the clinical characteristics of the 516 subjects without informative or obtainable death certificates to the 395 included in this analysis, no differences existed in age, gender, baseline FEV1 % predicted, smoking history or total exacerbations in the year prior to enrollment (). Availability of informative death certificates did differ by geographic region, with the Americas constituting the most informative death certificates (43%) while Europe made up the most deaths without informative/available death certificates (57%). A higher proportion of pulmonary deaths were present with informative death certificates compared to non-informative/unobtainable certificates (43% vs. 30%; p<0.01).
Table 1. Demographic and Clinical Characteristics of 911 Torch Deaths
Table 2. Demographic and clinical characteristics
After the first round of independent review, the reviewers reached unanimous consensus on level of agreement between adjudicated cause of death and death certificate data in 74.6% of cases. After the second round of review, consensus was achieved in 92.5% of cases. After discussion on the remaining cases, 100% consensus on level of agreement between adjudicated cause of death and death certificate data was attained. Intra-reviewer agreement was measured by repeat adjudication of a random sample of 40 cases, comparing these results with the first review. Overall, each reviewer had excellent consistency on repeat subset analysis with kappas of 0.87 (MJ), 0.88 (RW) and 0.83 (LM). Substantial agreement was observed among the three members of the review committee (kappa 0.68).
Baseline characteristics ()
Of the 395 deaths included in this analysis, 168 (43%) were adjudicated as pulmonary deaths, 111 (28%) cardiovascular, 83 (21%) cancer and 33 (8%) as other causes of death. Overall, the study population consisted of older, male former smokers with severe obstructive lung disease (mean forced expiratory volume in 1 sec (FEV1) of 39.4% predicted). When comparing the cohort's characteristics at study entry by category of death, no differences were observed in age, gender distribution, current smoking status, pack-years smoked or mean exacerbations in the last year.
The mean % predicted FEV1 differed by categories of death. The FEV1 % predicted was lower for individuals who died of pulmonary causes compared to cardiovascular causes (35.9 (13.3) vs. 43.7 (12.8)% predicted) and cancer causes (35.9 (13.3) vs. 40.8 (12.9)% predicted). Individuals who died of pulmonary causes also had a higher total St. George's Respiratory Questionnaire score (worse functioning) compared to the three other categories, although only the cancer-related category was statistically lower.
Death certificate concordance with adjudicated category of death ()
The primary cause of death, secondary cause of death and significant conditions listed on all 395 death certificates were compared to the cause of death adjudicated by the CEC in order to assess concordance. Overall, the primary or secondary cause of death agreed with the CEC adjudicated cause of death in 317 (80%) cases. In 69 (18%) deaths, the death certificate had no concordance with the adjudicated cause of death. There was no difference in concordance between adjudicated and listed cause of death when comparing different categories of death (P = 0.15). In cases where the CEC assigned the category of death as pulmonary, the death certificate matched on the primary or secondary cause of death in 138 (82%) of cases. Similar rates of agreement were observed for cardiovascular (77%), cancer (80%) and other (85%) categories of death. The fraction of death certificates having no concordance with adjudicated cause of death was also similar across categories of death: pulmonary (15%), cardiovascular (23%), cancer (16%) and other (15%).
Table 3. Death certificate concordance with adjudicated category of death, n(%)
Table 4. Death certificate concordance with adjudicated category of death by geographic region
Death certificate concordance by geographic region ()
To determine if the degree of concordance between death certificates and adjudicated cause of death differed by regions of the world, deaths were grouped by geographic regions as delineated by the World Health Organization. The enrolled sites were categorized into five regions: the Americas, Europe, Western Pacific, Africa, Southeast Asia. The highest degree of concordance between the adjudicated cause of death and the combined primary and secondary cause of death was observed in Europe (87%), the Americas (83%) and the Western Pacific (83%). Differences in the proportion of concordant cases did exist by geographic region (overall P < 0.01). Africa had significantly lower concordance between the adjudicated cause of death and combined primary/secondary death certificate cause compared to Europe (33% vs. 87%; p < 0.01), the Americas (33% vs. 83%; p < 0.01), the Western Pacific (33% vs. 83%; p < 0.01) and Southeast Asia (33% vs. 70%; p = 0.04).
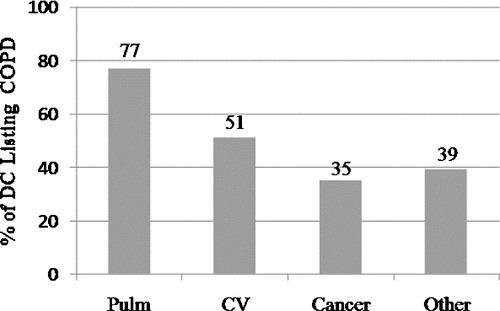
Frequency of COPD listed on death certificates (, )
Because all TORCH participants had spirometry-diagnosed COPD and were enrolled in a clinical trial of COPD therapy, evaluation of death certificates for the mention of COPD allows for an estimation of the frequency of COPD listing on death certificates of COPD patients. Of the 395 deaths, only 229 (58%) of death certificates listed COPD anywhere on the certificate. Of deaths categorized by the CEC to be due to pulmonary causes, 130 (77%) listed COPD anywhere on the death certificate. In 34% of deaths adjudicated to be directly caused by COPD exacerbation, the death certificate did not list COPD as the primary cause of death. Moreover, in 21% of deaths caused by COPD exacerbation, COPD was not mentioned anywhere on the death certificate. As seen in , listing COPD on the death certificate occurred with less frequency in cardiovascular (51%), cancer (35%) and other (39%) categories of death.
DISCUSSION
Using data from an international trial of COPD patients with rigorously determined cause of death, we have made several key findings regarding the accuracy of death certificate data in COPD deaths. First, while the primary cause of death listed for COPD patients was concordant with adjudicated cause of death only 60% of the time, the combined primary and secondary listing on death certificates accurately reported the cause of death 80% of the time, and this accuracy was independent of category of death. Second, COPD is underreported on death certificates. In approximately 1 out of 3 deaths adjudicated to be caused by COPD exacerbation, COPD was not listed as the primary cause of death. Moreover, in one out of five deaths caused by COPD exacerbation, COPD was not mentioned anywhere on the death certificate. Finally, death certificate accuracy differed among geographic regions of the world.
Table 5. Proportion of Death Certificates Listing COPD Anywhere
Our findings confirm the current body of literature regarding the under-reporting of COPD deaths. The rates of under-reporting of COPD deaths in this analysis are similar to prior reports (Citation6, 7). Multiple reasons exist for the underreporting of COPD-related deaths worldwide. Mortality statistics often rely upon the listed underlying cause of death, rather than contributory causes of death (Citation3, Citation6, Citation10) . This approach may lead to an underrepresentation of COPD deaths, especially when the final cause of death is unclear. Varying institutional and government practices regarding the approach to completion of death certificates may contribute to under-reporting. Errors in death certificates can also be attributed to unfamiliarity of the patient by physicians charged with completing certificates at the time of death (Citation18).
Nearly 1 out of 3 death certificates in this cohort of patients with established COPD did not mention COPD anywhere on the death certificate. Moreover, nearly 1 out of 5 deaths directly adjudicated by the CEC to be due to an exacerbation of COPD did not mention COPD anywhere on the death certificate. This observation highlights the underreporting of COPD by health care providers. No difference was observed in the concordance between the death certificate and adjudicated cause of death when stratifying by category of death. However, the frequency at which COPD was listed anywhere on the death certificate did differ by category of death, despite all patients being enrolled in a clinical trial of COPD.
Cancer and cardiovascular-related deaths were less likely to mention COPD anywhere on the death certificate when compared to pulmonary related deaths. This observation can be explained by the multiple possible causes of death in a patient with several co-morbid conditions. For instance, in a patient with multiple co-morbidities, the presence of cancer or coronary artery disease may overshadow the presence of COPD. The finding that underreporting of COPD increases as multiple co-morbidities are present has been suggested previously (Citation8). When multiple chronic illnesses are present, this decreases the likelihood of listing COPD as a primary cause of death, which ultimately further contributes to the underestimation of COPD deaths.
This study has some limitations. Informative death certificate data were only obtained in 395 of the 911 TORCH deaths. Although the baseline demographic and clinical characteristics did not differ between subjects included and excluded from this analysis, geographic and cause of death distributions were different between those with and without available/informative death certificates. These differences lead to a selection bias that limits the global generalizability of our observations. Subjects enrolled in clinical trials differ from the general population (Citation19), further limiting the applicability our findings to a larger population.
Local instructions and customs on death certificate completion may have differed from guidelines established by the CEC for adjudication. Because these patients were enrolled in a clinical trial of COPD, they may have been identified more readily as a COPD-related death than otherwise would have been the case. Thus, our estimates of COPD deaths may have been higher than in a non-study population. Despite these limitations, our analysis has several strengths. The global nature of TORCH, clear diagnosis of COPD by spirometry, and the consistent, reliable fashion that cause of death was adjudicated by the CEC permits valid estimates of death certificate accuracy on an international scope.
Using data from an international COPD trial, we have confirmed that death certificates accurately record cause of death in 80% of cases when considering both the primary and secondary listed causes of death. However, death certificates do not mention COPD in 42% of deaths in COPD patients. Moreover, in approximately 20% of deaths due to COPD, this disease was not mentioned anywhere on the death certificate. Finally, in 1 out of 3 deaths caused by COPD exacerbation, COPD was not listed as the primary cause of death. These findings highlight the underestimation of COPD-deaths using death certificates in patients enrolled in multinational clinical trials.
Declaration of interest
MBD reports no conflicts of interest. RAW, MJ and LG were paid consultants to GlaxoSmithKline for participation in the TORCH mortality review committee. MZ is an employee of GlaxoSmithKline which sponsored the TORCH study. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Jemal A, Trends in the leading causes of death in the United States, 1970-2002. JAMA 2005; 294(10):1255–1259.
- WHO. The Global Burden of Disease: 2004 Update, A.f. Retrieved from www.who.int/healthinfo/global_burden disease/2004_report_update.
- Kochanek KD, Deaths: Final data for 2002. Natl Vital Stat Rep, 2004; 53(5):1–115.
- Statistics, NCFH. Vital Statistics, instructions for classifying the underlying causes of death. NCHS instruction manual; Part 2a. Retrieved February 6, 2009, from http://www.cdc.gov/nchs/data/dvs/2a2005a.pdf.
- Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997; 156(3 Pt 1):814–818.
- Hughes TS, Muldoon SB, Tollerud DJ. Underestimation of mortality due to chronic obstructive pulmonary disease (COPD) in Kentucky. J Ky Med Assoc 2006; 104:331–339.
- Jensen HH, Potential misclassification of causes of death from COPD. Eur Respir J 2006; 28:781–785.
- Fuhrman C, , Deaths from chronic obstructive pulmonary disease in France, 1979–2002: A multiple cause analysis. Thorax 2006; 61(11):930–934.
- Faustini A, The concurrent COPD mortality doubles the mortality estimate from COPD as underlying cause in Lazio, Italy. Respir Med 2007; 101:1988–1993.
- May KL. Death certificates in asthma and COPD patients (survey of statistical data in Warsaw). Monaldi Arch Chest Dis 2002; 57:253–257.
- Smyth ET, Death from airways obstruction: accuracy of certification in Northern Ireland. Thorax 1996; 51:293–297.
- Calverley PM, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- McGarvey LP, Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007;62:411–415.
- Vestbo, J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J 2004; 24:206–210.
- Adler MZE, ed. Gazing into the Oracle: The Delphi method and its application to social policy and public health. 1996, London: Jessica Kingsley Publishers.
- World Health Organization. Global Health Risk: Mortality and Burden of Disease Attributable to Selected Major Risks. Retrieved December 7, 2009, from http://who.int/about/regions.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174.
- Pritt BS, Death certification errors at an academic institution. Arch Pathol Lab Med 2005; 129:1476–1479.
- Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med 2008; 168:136–140.