ABSTRACT
The association of osteoporosis with COPD is well established, but the relationship between systemic inflammatory mediators and bone metabolism has not been explored. Plasma samples from 40 COPD patients awaiting lung transplantation were analyzed for 27 inflammatory mediators using a multiplex protein array. C-telopeptide type I collagen (CTx), a marker of bone resorption, was measured with ELISA, and N-terminal procollagen propeptide (P1NP), a marker of bone formation, was ascertained with a radioimmunoassay. Associations between inflammatory mediators versus CTx and P1NP with adjustments for steroid and bisphosphonate use were determined. Mean age was 59 years (± 6) and FEV1 was 23.5% (± 8.3%) predicted. Ninety-five percent of the subjects had low bone mineral density measured by dual x-ray absorptiometry (DXA). Tumor necrosis factor alpha and interleukin 4 were positively associated with CTx and P1NP. RANTES and eotaxin were inversely associated with CTx and P1NP. Interleukin 2 and interferon gamma were also directly associated with P1NP. Biologically plausible systemic mediators are associated with bone metabolism in patients with severe COPD, offering potential insight into risk factors and underlying mechanisms of bone disease. Furthermore, they may be useful in monitoring disease activity, and serve as targets for biological therapy.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), while defined by its respiratory impairment, is associated with numerous systemic consequences, including abnormalities of skeletal muscle, body composition and bone mineral density (BMD) (Citation1–3). Whereas studies have firmly established an epidemiologic link between COPD and osteoporosis (Citation4–6), the study of these disease relationships are confounded by complex, interactive risk factors. For instance, patients with severe obstructive lung disease also have decreased physical activity, frequently use inhaled and oral corticosteroids, and typically have decreased muscle mass, all of which are additional, potential contributing factors for loss of BMD (Citation7). Nonetheless, studies have alluded to an increased prevalence of low BMD in COPD patients that is independent of steroid use (Citation6) and early in the course of their pulmonary disease (Citation8), suggesting that independent systemic factors may play a synchronous role in the etiologic link between lung and bone processes.
Systemic inflammation, now well recognized in obstructive lung disease (Citation9, 10), may be an important factor linking the development of osteoporosis to COPD. Numerous cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6, have been implicated in both disease processes (Citation9, Citation11, Citation12). Osteoporosis can result from disorders that increase bone resorption, for instance cystic fibrosis (Citation13), or other factors that decrease bone formation, as in corticosteroid use (Citation14). Inflammatory diseases, including rheumatoid arthritis (Citation15), may result in both increased resorption and formation, but with a net imbalance favoring degradation.
However, studies have not explored the precise mechanisms involved in the association between COPD and osteoporosis. Investigation of systemic inflammatory mediators mutual to both disease processes may identify molecular targets for early osteoporosis screening and monitoring of disease activity. Ultimately, identification and interruption of these processes may lead to novel therapies that could have an important impact on the care of patients with COPD who have systemic manifestations of their lung disease. Therefore, we examined the relationship between peripheral inflammatory biomarkers and markers of bone metabolism in a group of patients with severe COPD.
MATERIALS AND METHODS
Subject selection
The pulmonary transplant registry at the University of Pittsburgh was queried for patients undergoing lung transplantation from 2004 to 2007 with a pre-transplant diagnosis of either chronic obstructive pulmonary disease (COPD) or emphysema. Information pertaining to subject demographics, smoking history, medication usage, pulmonary function testing, and dual x-ray absorptiometry (DXA) results was abstracted from the database. Each subject had blood obtained on the day of transplantation immediately prior to surgery. All had provided written consent for the research use of their clinical information, radiographic studies, and stored blood samples, and the University of Pittsburgh's Institutional Review Board approved the study. Subjects with a T-score (the World Health Organization classification of BMD in terms of the number of standard deviations from values of a gender-matched young adult at peak bone mass (Citation16)) of −1.0 to −2.5 at either the hip or lumbar spine were classified as low bone mass and those with a T-score of at or below −2.5 at either of these sites were classified as osteoporosis.
Blood specimen analysis
Stored plasma samples were analyzed for C-telopeptides of type I collagen (CTx), a marker of bone resorption, by ELISA (Immunodiagnostic Systems, Fountain Hills, AZ). N-terminal procollagen propeptide (P1NP), a marker of bone formation, was measured by radioimmunoassay (Immunodiagnostic Systems). Samples were also analyzed for twenty-seven plasma chemokines and growth factors using a bead-based cytometric immunoassay system (Bio-Rad Laboratories, Hercules, CA). All assays were performed once on each plasma sample. To control for assay consistency, standards were performed in duplicate. Cytokines and growth factors measured included IL-1b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, fibroblast growth factor basic (FGF-basic), granulocyte-colony stimulating factor (g-csf), granulocyte monocyte-colony stimulating factor (gm-csf), interferon gamma (IFN-γ), interferon-inducible protein 10 (IP-10), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 alpha and 1-beta (MIP-1α, MIP-1β), platelet derived growth factor (PDGF), regulated upon activation normal T Cell expressed and secreted (RANTES), TNF-α, and vascular endothelial growth factor (VEGF). A description of this multiplex assay has been previously detailed (Citation17). Standard curves were generated for each cytokine following the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA) and the concentrations of unknown samples were calculated using a 5 parametric curve-fitting program with logistic regression.
Statistical analysis
Continuous data were summarized as mean ± standard deviation and range and categorical data were expressed as percentages. Serum biomarkers with concentrations above or below the detection threshold of the assay were respectively assigned the highest or lowest extrapolated value for that given marker and included in the final analysis. Those biomarkers with greater than 40% out of range values were excluded from the final analysis. Because the biomarker data was not normally distributed, these values were log-transformed. The correlation between CTx and P1NP was assessed with the Pearson's correlation coefficient. The associations of CTx and P1NP with log-transformed plasma biomarker levels were analyzed with linear regression analysis adjusted for bisphosphonate, oral steroid, and inhaled steroid use. All statistical procedures were performed using SAS version 9.1.
RESULTS
Forty subjects had lung transplantations for end-stage COPD or emphysema between December 2004 and June 2007 and had complete clinical information and stored blood samples (). The majority of the subjects was severely obstructed and used either inhaled and/or oral steroids. There was a remarkably high prevalence of abnormal bone mineral density with only 2 subjects having normal BMD and almost half of the subjects meeting criteria for osteoporosis at either the hip or lumbar spine. The average T-score of the hip was −1.9 with 18 subjects meeting criteria for low bone mass and 8 meeting criteria for osteoporosis. The average T-score of the lumbar spine was −2.1 with 22 subjects and 11 subjects meeting criteria for low bone mass and osteoporosis, respectively. More than half of the subjects were using oral bisphosphonate therapy.
Table 1. Subject demographics N = 40
Measured levels of IL-15, IL-17, FGF basic, and MCP-1 were undetectable in greater than 60% of the subjects and thus were excluded from the final analysis. The co-efficient of variation was less than 15% for all remaining biomarkers included in the final analysis. CTx levels were detectable in 34 of the 40 subjects and P1NP levels were detectable in all. CTx levels were highly correlated with P1NP levels (; r = 0.84, p < 0.0001), reflecting high turnover with a coupling of bone resorption and bone formation. Both CTx and P1NP levels were directly associated with IL-4 and TNF-α levels (, , ) but inversely related to RANTES and eotaxin levels.
Figure 1. Type 1 collagen C-telopeptide (CTx), a marker of bone resorption, is positively associated with amino-terminal propeptide of type 1 procollagen (P1NP), a marker of bone formation. (r = 0.84, p < 0.0001).
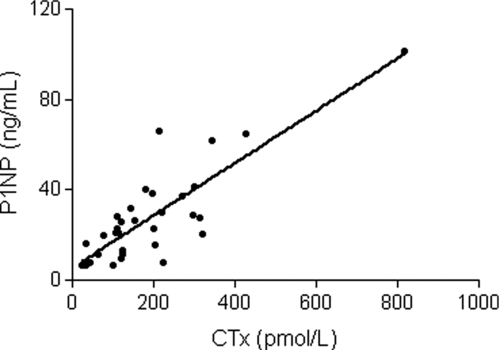
Table 2. Plasma markers associated with markers of bone formation and resorption⊥
In addition, P1NP levels increased with increasing IL-2 and IFN-γ levels. These relationships remained significant after adjustment for steroid and bisphosphonate use. There were no significant associations found between markers of bone formation or resorption and the remaining 16 inflammatory mediators. There were also no associations between the above markers of current inflammation or active bone turnover and the DXA T-score of the hip or spine.
DISCUSSION
COPD is a systemic illness with disease manifestations that extend beyond the lung to impact multiple organ systems (Citation1, 2). Evidence suggests that the complex relationship between obstructive lung disease and decreased BMD occurs independently of the known risk factors for osteoporosis that are frequently present in individuals with severe airflow limitation (Citation6, Citation8). Decreased BMD can be due to either a primary increase in bone resorption through augmentation of osteoclast formation and activity, as seen in the inflammatory arthritities and estrogen deficiency (Citation18, 19), or result from a primary suppression of bone formation through inhibition or apoptosis of osteoblasts, as seen in chronic steroid use (Citation14).
Figure 2. Interleukin-4 levels increase with increasing levels of type 1 collagen C-telopeptide (CTx) and amino-terminal propeptide of type 1 procollagen (P1NP).
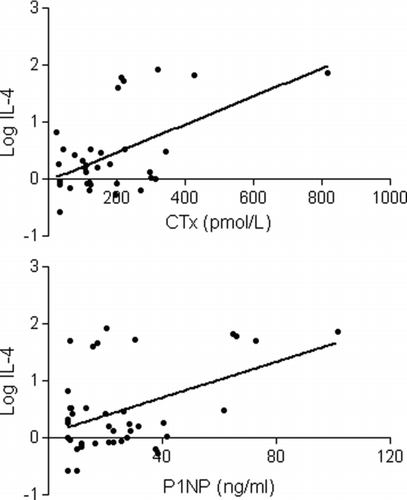
Figure 3. Tumor necrosis factor alpha (TNF-α) levels increase with increasing levels of type 1 collage C-telopeptide (CTx) and amino-terminal propeptide of type 1 procollagen (P1NP).
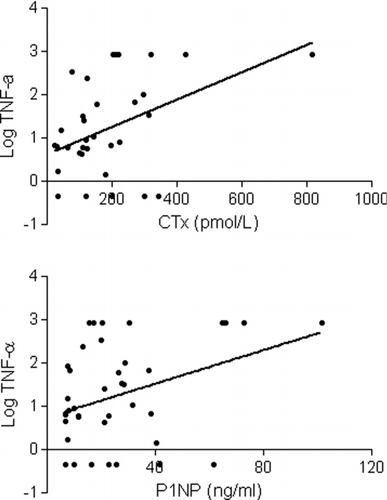
The present data demonstrates a direct relationship between CTx, a marker of resorption, and P1NP, a marker of formation, thus supporting a mechanistic model of coupling of bone formation and resorption with increased bone turnover in these patients. We have also shown a relationship between biologically plausible systemic inflammatory mediators and markers of bone metabolism that provides insight into the mechanisms linking COPD and osteoporosis. In addition to implicating key peripheral cytokines and growth factors involved in the connection between the pulmonary and skeletal systems, we have identified potential molecular candidates, notably TNF-α, for future, targeted therapies.
Although few studies have directly investigated the role that systemic inflammation plays in the relationship between COPD and osteoporosis, the literature evaluating the pathogenesis of both diseases involves inflammatory models with common mediators. The imbalance between bone formation and resorption, which leads to accelerated BMD loss and osteoporosis, is orchestrated by an elaborate network of inflammatory cells and cytokines (Citation11, 12), many of which are also intimately involved in the pathogenesis of COPD (Citation20, 21). Early studies searching for a single “osteoclast activating factor” uncovered an extensive list of pro-inflammatory mediators that were capable of directly stimulating or regulating osteoclastic bone resorption (Citation22).
Many of these mediators, including TNF-α (Citation20, Citation23), have also been implicated in obstructive lung disease. Furthermore, some of the strongest evidence supporting the importance of inflammation in the pathogenesis of bone resorption that provides a theoretical framework for the role of systemic inflammation in COPD-related osteoporosis comes from studies examining the prevalence of decreased BMD in other analogous disease states (Citation24, 25). The frequency of osteoporosis in patients with cystic fibrosis is of particular relevance given the similarities between COPD and cystic fibrosis, such as propensities for infectious exacerbations, and characteristic low body weights and decreased muscle mass (Citation26).
Other reports have shown that increased levels or production of proinflammatory cytokines (e.g., IL-6 and TNF-α) are associated with BMD and markers of bone resorption in cystic fibrosis patients. These levels have been shown to subsequently decrease with antibiotic treatment of acute infection (Citation27, 28). Chronic microbial colonization and frequent infectious exacerbations also likely influence the systemic inflammatory milieu in COPD, as in cystic fibrosis (Citation27, 28), and predispose a subset of patients to accelerated loss of BMD and osteoporosis.
The redundancy of the inflammatory mediators implicated in the pathogenesis of both COPD and osteoporosis suggests that systemic inflammation may orchestrate the link between the lung and skeletal system. To our knowledge, the present study is the first to demonstrate a direct relationship between biologically plausible systemic inflammatory proteins and markers of bone metabolism in COPD. We have previously shown more frequent production of IFN-γ and IL-4 from isolated CD8+ T cells and increased CD4 T cell production of IL-2 was directly correlated with obstruction severity in patients with COPD (Citation10). Others have demonstrated an elevation of TNF-α in COPD patients with stable disease (Citation9). Both IL-2 and TNF-α levels have also been shown to be systemically elevated in patients with high-turnover postmenopausal osteoporosis (Citation29), and TNF-α has been well established in both basic and translational experiments as a key mediator involved in osteoclastogenesis (Citation19, Citation30, Citation31).
Furthermore, immunologic studies have described osteoblast expression of IL-4 and IFN-γ (Citation32) and increased CD8+ T cell expression of TNF-α and IFN-γ in patients with osteoporotic fractures (Citation33). With the demonstration of a direct correlation between IL-2, IL-4, TNF-α, and IFN-γ and markers of bone metabolism, our data supports a high bone turnover model induced by systemic proteins relevant to the pathogenesis of both obstructive lung disease and osteoporosis, and thus provides novel insights into potential mechanisms linking these two disease processes.
Although we found several significant, biologically plausible associations between inflammatory mediators and markers of bone resorption and formation, we did not see a significant relationship between peripheral mediators or bone markers and T scores at the hip or spine. Because systemic levels of bone markers and inflammatory proteins reflect a cross-sectional sampling at a single point in time, whereas DXA measurement of BMD represents an integration of longitudinal bone remodeling influenced by potentially intermittent activity, this is not a surprise. Indeed, CTx and P1NP levels do not always accurately depict the severity of BMD loss (Citation34), and serial measurement of these markers appear to be far more sensitive for identifying those patients at risk for fracture, as well as for monitoring clinical responses to anabolic and anti-resorptive therapies (Citation35). The magnitude of CTx levels present in our patient cohort are also likely influenced by the prevalence of bisphosphonate use (Citation36) as well as the inherent overlap in CTx levels between healthy and osteoporotic individuals.
Future studies in treatment naïve subjects may reveal a greater differential in CTx levels based on the severity of bone density abnormalities. Although the multiple measurements enabled by Luminex technology increases the probability of type I error, we did not correct for multiple comparisons in our study. A formal adjustment would minimize the number of false positive findings but would also increase the probability of missing clinically meaningful associations. In this exploratory study, we did not want to overlook possible relationships and instead focused on those markers that demonstrated both biological plausibility and consistency in their associations with both markers of bone metabolism. For instance, all significant biomarkers, with the exception of IFN-γ, demonstrated significant associations with both CTX and P1NP.
CONCLUSIONS
COPD-related osteoporosis, with the associated increase in fracture risk, adversely impacts quality of life, necessitating a more thorough understanding of the pathogenic mechanisms linking these two disease processes. Whereas shared risk factors partially explain the increased occurrence of osteoporosis in patients with COPD, we have demonstrated an association between systemic inflammatory proteins and markers of bone metabolism that may explain the prevalence of osteoporosis in steroid naïve, less severely obstructed individuals. Our findings not only offer insight into the mechanistic relationship between COPD and osteoporosis, but also provide potential markers for initial osteoporosis screening and monitoring, as well as identify targets for novel, molecular-based therapies.
Declaration of interest
This work was supported by the National Institute of Health through the University of Pittsburgh SCCOR in COPD [NHLBI 1P50 HL084948 and P50-CA90440] and by the Snee-Reinhardt Foundation. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wouters EFM. Chronic obstructive pulmonary disease: Systemic effects of COPD. Thorax 2002; 57:1067–1070.
- Agusti AGN. Systemic effects of chronic obstructive pulmonary disease. Proc Amer Thorac Soc 2005; 2:367–370.
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences—Clinical impact, mechanisms, and potential for early intervention. COPD 2008; 5:235–256.
- Ionescu AA, Schoon E. Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J 2003; 22:64S–75.
- Sin DD, Man JP, Man SF. The risk of osteoporosis in caucasian men and women with obstructive airways disease. Am J Med 2003; 114:10–14.
- de Vries F, van Staa TP, Bracke MSGM, Cooper C, Leufkens HGM, Lammers JWJ. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J 2005; 25:879–884.
- Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: A cross sectional study. Respir Med 2007; 101:177–185.
- Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, Nixon LS, Evans WD, Griffiths TL, Shale DJ. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2004; 170:1286–1293.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR. Peripheral t cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol 2009; 182:3270–3277.
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. New Engl J Med 1995; 332:305–311.
- Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest 2005; 115:3318–3325.
- Aris RM, Ontjes DA, Buell HE, Blackwood AD, Lark RK, Caminiti M, Brown SA, Renner JB, Chalermskulrat W, Lester GE. Abnormal bone turnover in cystic fibrosis adults. Osteopor Int 2002; 13:151–157.
- Canalis E, Mazziotti G, Giustina A, Bilezikian J. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteopor Inter 2007; 18:1319–1328.
- Cortet B, Flipo RM, Pigny P, Duquesnoy B, Boersma A, Marchandise X, Delcambre B. Is bone turnover a determinant of bone mass in rheumatoid arthritis? J Rheumatol 1998; 25:2339–2344.
- WHO (1994). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: WHO.
- Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:981–987.
- Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology 2002; 41:1232– 1239.
- Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clinical Invest 2006; 116:1186–1194.
- Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-α is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med 2002; 166:849–854.
- Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Mundy GR. Osteoporosis and inflammation. Nutr Rev 2007; 65:S147–151.
- D'Hulst AI, Bracke KR, Maes T, De Bleecker JL, Pauwels RA, Joos GF, Brusselle GG. Role of tumour necrosis factor-α receptor p75 in cigarette smoke-induced pulmonary inflammation and emphysema. Eur Respir J 2006; 28:102–112.
- Pazianas M, Rhim AD, Weinberg AM, Su C, Lichtenstein GR. The effect of anti-tnf-α therapy on spinal bone mineral density in patients with crohn's disease. Ann NY Acad Sci 2006; 1068:543–556.
- Vis M, Havaardsholm EA, Haugeberg G, Uhlig T, Voskuyl AE, van de Stadt RJ, Dijkmans BA, Woolf AD, Kvien TK, Lems WF. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the nfkappab ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:1495–1499.
- Conway SP, Morton AM, Oldroyd B, Truscott JG, White H, Smith AH, Haigh I. Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: Prevalence and associated factors. Thorax 2000; 55:798–804.
- Ionescu AA, Nixon LS, Evans WD, Stone MD, Lewis-Jenkins V, Chatham K, Shale DJ. Bone density, body composition, and inflammatory status in cystic fibrosis. Amer J Respir Crit Care Med 2000; 162:789–794.
- Aris RM, Stephens AR, Ontjes DA, Denene Blackwood A, Lark RK, Hensler MB, Neuringer IP, Lester GE. Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Amer J Respir Crit Care Med 2000; 162:1674–1678.
- Gur A, Denli A, Nas K, Cevik R, Karakoc M, Sarac AJ, Erdogan F. Possible pathogenetic role of new cytokines in postmenopausal osteoporosis and changes during calcitonin plus calcium therapy. Rheumatol Int 2002; 22:194–198.
- Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology 1995; 136:3054–3061.
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing t-cell production of tnf-alpha. J Clin Invest 2000; 106:1229–1237.
- Ruiz C, Perez E, Garcia-Martinez O, Diaz-Rodriguez L, Arroyo-Morales M, Reyes-Botella C. Expression of cytokines il-4, il-12, il-15, il-18, and ifngamma and modulation by different growth factors in cultured human osteoblast-like cells. J Bone Miner Metab 2007; 25:286–292.
- Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, Peterlik M. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Exper Gerontol 2001; 36:1749–1759.
- Trento LK, Pietropolli A, Ticconi C, Gravotta E, De Martino MU, Fabbri A, Piccione E. Role of type I collagen C-telopeptide, bone-specific alkaline phosphatase and osteocalcin in the assessment of bone status in postmenopausal women. J Obstet Gynaecol Res 2009; 35:152–159.
- Garnero P. Biomarkers for osteoporosis management: Utility in diagnosis, fracture risk prediction and therapy monitoring y1. Mole Diagn Ther 2008; 157.
- Greenspan SL, Rosen HN, Parker RA. Early changes in serum n-telopeptide and c-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women. J Clin Endocrinol Metab 2000; 85:3537–3540.
