ABSTRACT
Chronic obstructive pulmonary disease is now considered as a systemic disease originating in the lungs. The natural history of this disease reveals numerous extrapulmonary manifestations and co-morbidity factors that complicate the evolution of COPD. Recent publications have documented these systemic manifestations and co-morbidities and clarified somewhat the role of muscle dysfunction, nutritional anomalies, endocrine dysfunction, anaemia, osteoporosis and cardiovascular and metabolic disorders as well as lung cancer and psychological elements in this complex disease. Importantly, recent studies have shown that effort intolerance, exertional desaturation, loss of autonomy and reduced physical activity, loss of muscle mass and quadriceps strength as well as dyspnoea and impaired quality of life can be considered as independent predictive factors for survival in COPD. Use of these data may advance understanding of mechanisms; improve evaluation and thereby patient management in COPD.
Keywords: :
INTRODUCTION
Although the data vary in relation to the definition used, COPD is recognised as a major cause of increased mortality and morbidity. Despite being a treatable and preventable disease, the prevalence of COPD continues to rise because of the worldwide epidemic of smoking. COPD is projected the third most important cause of mortality in the world by 2020 (Citation1). It is largely evidenced that a specific therapy for COPD should start with cessation of exposure to the most important risk factor: tobacco smoke. It is now established that COPD can not simply be considered as a simple pulmonary disease.
Although this disease is evidently of respiratory origin, many extra pulmonary manifestations exist and a number of common co-morbidities complicate the natural history of COPD, thereby altering the prognosis and the quality of life of patients (Citation2–4) (). Among those are muscular dysfunction, nutritional and metabolic abnormalities, hormonal deficit, anaemia, and increased prevalence of osteoporosis, lung cancer, cardiovascular diseases, diabetes, anxiety and depression.
Figure 1. While COPD is evidently of respiratory origin, many extra pulmonary manifestations exist and a number of common comorbidities complicate the natural history of this disease, thereby altering the prognosis and the quality of life of patients.
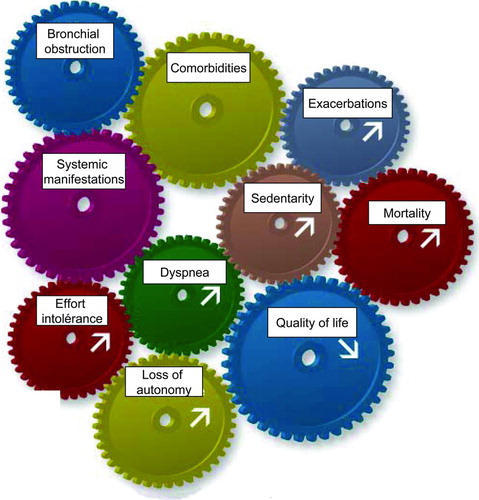
For many years the level of airflow obstruction has been considered the single prognostic factor in COPD. However, recent publications have shown that many other factors, even non pulmonary, play an important role in the natural evolution of this disease. Thus, effort intolerance, exertional desaturation, loss of autonomy, the level of regular physical activity, the level of loss of muscle strength in the quadriceps, breathlessness and quality of life status are now recognized as independent predictive factors for survival in this disease. More recently a constellation of these factors has been proposed as an integrated predictor of survival in the BODE (Body-mass index, airflow obstruction, dyspnoea and exercise) index that predicts five year survival in COPD (Citation5). It is thus important that we look beyond the lungs in this disease and address the patient in global terms. Use of these new data may advance understanding of mechanisms; improve evaluation and thereby patient management in COPD.
EXTRAPULMONARY MANIFESTATIONS AND CO-MORBIDITIES
Peripheral muscle dysfunction
In patients with chronic obstructive pulmonary disease, peripheral muscle dysfunction is clearly demonstrated by the significant reduction in both strength and endurance in the muscles of locomotion in comparison with healthy subjects. Indeed, patients with severe COPD have on average a loss of 50% in endurance function (Citation6–9) and an increased fatigability of peripheral muscles (Citation10). These abnormalities are related to alterations in aerobic metabolism, essentially a drastic reduction in the proportion of type I muscle fibers observed within quadriceps muscles (Citation11). Patients with severe stable COPD also have on average a loss of 30% in strength in the quadriceps muscles, mainly explained by a loss in muscle mass (Citation12, 13).
These muscle effects are seen predominantly in the lower limb muscles in COPD patients while upper limb muscles tend to be preserved (Citation14). This peripheral muscle dysfunction, which is now recognized as one of the main systemic effects of COPD, contributes greatly to exercise intolerance and reduced quality of life of COPD patients. Disuse is almost certainly an important factor in the skeletal muscle dysfunction of COPD. However, the most recent data indicate that disuse only partly explains the severe morphological, metabolic and functional abnormalities observed in peripheral muscle in COPD. Instead, the data support the concept of an intrinsic myopathy (Citation15).
The physiopathological mechanisms (i.e., inflammation, oxidant stress, and hypoxemia or pharmacological interventions such as use of corticosteroids…) of this myopathy have not been completely clarified. From this perspective, physical training, perhaps in association with other therapeutic strategies, may be useful. This possibility, as well as defining the best training modality for these patients, needs to be further investigated.
Abnormalities in body composition
The first studies in this domain show an increased prevalence of weight loss in COPD that can be up to 25 to 50% of patients according to the level of severity of the disease (Citation16). Conventional wisdom says that COPD patients lose weight because of malnutrition (i.e., a mismatch between energy intake and energy use). However, these patients have increased metabolism at rest, probably explained by increased work of respiratory muscles, the treatments use and generalised inflammation (Citation17). Furthermore, patients often have depression and breathlessness at rest which spontaneously limit daily caloric intake (Citation18). However, recent studies have shown that COPD patients have more a problem of muscle loss (amyotrophy) than a general problem of weight loss.
It is now recognized that a simple calculation of body mass index (BMI) is insufficient for correct evaluation of body mass and in particular malnutrition in COPD patients. Indeed, the COPD patients may have a raised or reduced body mass index when stable, while others may have manifest nutritional problems within the normal limits of BMI. Studies have shown that a normal body weight might hide a significant loss of muscle mass in COPD patients (Citation19, 20). This amyotrophia is associated with a cachexic process: predominantly an accelerated loss of muscle mass. This may be related to a specific nutritional deficit in protein metabolism, perhaps with systemic inflammation as part of the disease process, or hormonal deficit or the effects of hypoxemia or oxidant stress (Citation21).
Usually the diagnosis of malnutrition is established by a body mass index <21 Kg.m2 and/or a fat free mass <15 Kg for women and <16 Kg for men. Impedancimetry is preferable to a simple calculation of BMI for the diagnostic of body composition abnormalities in COPD. One study has shown that 7% of COPD patients have greater than 10% weight loss in a year while the prevalence of malnutrition evaluated by body composition was 54% (Citation22).
Endocrine anomalies
Despite certain clinical indicators, no study has clearly shown endocrine anomalies in COPD patients, apart from the most severe and hypoxemic cases (Citation23). Indeed, the concentrations in the serum of thyroid hormones and testosterone are abnormally low and the level of reduction correlates with PaO2 in this population (Citation24, 25). As in other chronic diseases, the levels of circulating cortisol, DHEA and growth hormone mediators (Insulin like growth factors) also tend to be abnormally low in COPD patients (Citation26).
On the other hand, other studies have reported an increase in the concentrations of growth hormone in the most severe cases of COPD, possibly related to physiological stress combined with hypoxemia and bronchoconstriction (Citation27). However, these data are scarce and controversial. Further studies are required to clarify the cause of these endocrine anomalies in COPD, to justify the recommendation of hormonal supplementation on a solid scientific basis.
Anaemia
Recent studies have shown a large number of COPD patients to be anaemic. This may be from 15 to 30% of COPD patients and predominantly the more severe cases, while the prevalence of erythrocytosis is estimated at 6% (Citation28–30). The mechanisms of this anaemia are not completely clarified but may be related to resistance to the effects of erythropoietin since her erythropoietin concentrations are abnormally rised in this population (Citation31). Consequently, the treatment of anaemia by erythropoietin or by iron supplements may be inadapted, and blood transfusion may be more effective (Citation32). Anaemia is a recently described manifestation of systemic disease in COPD that should be sought and evaluated in the management of these patients.
Osteoporosis
In recent years, studies have shown a marked prevalence of osteoporosis and loss of bone mineral density in COPD, which may be present in 9 to 75% of cases depending on the studies and independent of gender. This prevalence is much higher in COPD compared to normal subjects and compared to patients with other chronic diseases (Citation33–35). The risk factors for osteoporosis and loss of bone mineral density in the COPD population may include systemic inflammation, increased levels of certain catabolic proteins, malnutrition, disease severity, long-term corticosteroids, age (>Citation55 years), vitamin D deficit and physical inactivity (Citation36–38). It is necessary to improve diagnosis and use of adapted treatments to limit the risk of fractures in this population. Fractures such as vertebral collapse are relatively frequent and may lead to scoliosis which further limits pulmonary function in this population.
Anxiety and depression
These two disorders are closely linked and well recognized in COPD (Citation39). Nevertheless the prevalence varies according to the methods of the studies, for example size of sample, characteristics of the population, questionnaires used … (Citation40). Overall the prevalence of anxiety is variably estimated between 2% and 96% in COPD patients. Generally we can distinguish a generalised illness associated with chronic anxiety, whose prevalence can be estimated between 10% and 33% of the COPD population, from repeated transient episodes of intense anxiety whose prevalence is estimated between 8% and 67% according to different studies (Citation41).
It seems that an anxiety state is more frequent in the female population, in smokers and in those with dissatisfaction in personal relationships (Citation42, Citation43). Apart from these known risk factors, it would seem that the feeling of a lack of control of disease symptoms, in particular dyspnoea, plays the greatest part in the patient's anxiety (Citation44). Statistics regarding depression are also extremely varied. The overall estimation of the prevalence of depression in COPD varies from 6% to 80%, with a mean value in the most rigorous studies of around 40% (Citation45).
It is important to distinguish severe depression requiring medical intervention that is estimated at a prevalence of 19 to 42% in stable COPD patients (Citation44, Citation46). Recent studies indicate that the underlying cause of depression in COPD involves several components including genetic predisposition, the psychological stress generated by the disease itself (loss of function, loss of autonomy, loss of self-esteem…) and the neuropsychological effects of chronic illness (hypoxia, inflammation, long term treatment, smoking…) (Citation45; Citation47). In practice, therapeutic education and help with smoking cessation are of capital importance in the management of COPD patients and necessary for the treatment of anxiety and depression in this population.
Diabetes and metabolic syndrome
Longitudinal studies on thousands of patients have shown that COPD is an important risk factor (× 1,5-1,8) for the development of type II diabetes, even in moderate disease (Citation48). Long-term steroids do not seem to be the prime cause of this co-morbidity and chronic inflammation may play an important role. Thus, it has been shown that certain pro-inflammatory cytokines that are specifically elevated in COPD (such as TNF alpha, and interleukin 6) favor insulin resistance and thus the development of diabetes (Citation49). This increased insulin resistance, related to inflammatory processes is characteristic of the metabolic syndrome.
Although definitions vary between countries and health care systems, there are two diagnostic systems in use (1999- WHO amended and 2001 – American National cholesterol educational program). Thus, the metabolic syndrome is defined clinically by the presence of at least three of the following risk factors: abdominal obesity (girth >88 cm females, >102 cm in men), raised fasting triglycerides (≥1.5 g/L), arterial hypertension (>130 mmHg systolic/ ≥85 mmHg diastolic), low fasting HDL cholesterol (<0.5 g/L for females and <0.4 g/L for men) and raised fasting blood sugar (≥1 g/L). Recent studies have shown that the prevalence of metabolic syndrome may be twice as common in COPD patients compared to healthy subjects matched for age and sex (Citation50, 51). In COPD, the metabolic syndrome is associated with an increased level of circulating inflammatory markers and a significant reduction in physical activity; this is more commonly found in overweight and obese patients compared to patients with normal weight (Citation52). In practice, it is necessary to identify patients at high risk of developing metabolic syndrome and to adapt their management since it has been well demonstrated that patients with metabolic syndrome are at increased risk of developing diabetes, cardiac disease and strokes.
Cardiac diseases
The anatomical and functional relationship between heart and lung is such that dysfunction of one has consequences for the other. These interactions are important in COPD and these patients have an elevated risk of cardiovascular events which, in fact, are one of the principal causes of death in this population (Citation53). The main cardiovascular co-morbidities in COPD are cardiac failure, coronary atherosclerosis, cerebrovascular accidents, cardiac arrhythmias and pulmonary artery hypertension.
Heart failure is difficult to diagnose in COPD because of overlapping symptoms and clinical signs in both disorders and the difficulties and under use of echocardiography in this population. One study has shown that over 20% of COPD patients had undiagnosed heart failure (Citation54). The prevalence of heart failure has been estimated at 20–30% in COPD patients and this comorbidity may be the cause of 5% of the overall mortality in this population (Citation53). The prevalence of coronary artery disease in COPD patients varies according to studies from 15 to 25%, in relation to population heterogeneity. Patients with emphysema who are candidates for surgical lung volume reduction seem particularly susceptible (Citation55).
Cardiac arrhythmias are frequent in COPD patients, less by night than by day. Overall supraventricular dysrhythmias are more frequent in patients in respiratory distress while ventricular dysrhythmias are often seen in stable patients. However the prevalence of dysrrythmia varies from study to study, because of different definitions, different grades of severity, different levels of clinical stability and different diagnostic methods (Citation56).
Thus, up to 84% of stable COPD patients show some cardiac arrhythmias on ECG monitoring while only 20% have arrhythmias on standard ECG at rest (Citation57). Pulmonary arterial hypertension (PAH) is another well known complication in COPD. When present, PAH is usually mild to moderate in severity during stable periods of the illness. Thus, the possibility of evolution towards cardiac failure is limited. However the pulmonary artery pressure may increase during exercise, in certain phases of sleep and during disease exacerbations (Citation58).
PAH marks the beginning of the effects of COPD on the pulmonary circulation and thereby on the right side of the heart. Thus the increased work load on the heart induced by PAH may eventually lead to right heart failure with right ventricular hypertrophy and dilation of right heart chambers, eventually affecting the left side of the heart. This is cor pulmonale (Citation59). Thus, nearly 50% of patients with severe COPD that were evaluated for volume reduction surgery or transplantation were found to have moderate to severe PAH (Citation60). Besides this specific population the prevalence of PAH is estimated at 5% in advanced stages of the disease.
Lung cancer
Even if smoking is the major cause of lung cancer, certain non-smoking patients also develop the disease. In view of this, it has been postulated that certain non cancerous pulmonary diseases may increase susceptibility to lung cancer (Citation61). Thus, various longitudinal studies containing tens of thousands of individuals have shown that the presence of COPD significantly increase the risk of developing lung cancer (Citation62–66). A meta-analysis (Citation67) based on longitudinal studies of over 5000 subjects matched for smoking reported that the presence of bronchial obstruction multiplied the risk of developing lung cancer by 2.23 in men and by 3.97 in women.
It should be emphasised that this risk remains elevated even in patients with mild airflow obstruction (Citation62, Citation64) and in those who had quit smoking (Citation66). Exacerbation of the disease and a rapid decline in respiratory function significantly increases the risk of lung cancer in COPD patients (Citation64, 65). Finally, several studies have shown that the risk was greater in women with COPD than in men with this disorder (Citation63, Citation66). The mechanisms for this increased susceptibility of lung cancer in COPD patients and particularly in women remain to be elucidated.
Altogether associated co-morbidities in COPD induce the patients into a real vicious circle dominated by effort intolerance, increased breathlessness and sedentarity, with consequent social isolation and loss of autonomy. This evolution degrades the quality of life punctuated with frequent hospitalisations and raised mortality.
PREDICTIVE FACTORS FOR SURVIVAL IN COPD
Loss of autonomy, effort intolerance and exertional desaturation
It has been shown for over 35 years that even mildly affected COPD patients have reduced effort tolerance compared to healthy subjects. This exercise intolerance has two components: firstly respiratory, related to dynamic hyperinflation and hyperventilation and secondly related to peripheral muscles with deconditioning and myopathy. COPD patients are limited as regards increasing effort but also, according to disease severity in activities of daily life. Thus, some patients when severely affected have such a degree of breathlessness and muscle wasting that they become partially or totally dependent and require help for such simple activities as washing, dressing and walking.
One study using the Barthel index examined the changes in autonomy over a period of hospitalisation for an episode of respiratory failure (Citation68). The results showed that by the second day of hospitalisation, these COPD patients had lost 50% of their autonomy compared to 15 days prior to hospitalisation. After a mean of 9 days of hospitalisation the level of autonomy improved gradually but remained at 33% of the base level. 60% of patients did not completely recover the level of autonomy by 3 months and remained with a loss of autonomy of 12% compared to prehospital values.
The level of functional dependence is recognised as a factor predictive of mortality during or after a decompensation episode (Citation69–71). Thus, mild dependency (Barthel index 85 and 40), moderate dependency (Barthel index 39-20) or total dependency (Barthel index 19-0) at the time of admission multiplies the risk of mortality respectively by 3; 3.5; 3.5 during hospitalisation. The chances of survival at 1 year for patients who survive a decompensation episode are closely related to the level of autonomy on leaving the hospital.
Effort intolerance and exertional desaturation are also recognized as predictive of survival in patients with COPD. Several studies have shown a relationship between aerobic effort capacity (72), 6-minute walk distance (6MWD) (Citation73–75), oxygen desaturation during 6-min walk test (6MWT) (Citation74, 75) and 5-year survival in COPD patients (). Casanova et al. (Citation75) have enrolled 576 stable patients with COPD and observed them for at least 3 years (median 60 months). They measured baseline FEV, body mass index, PaO2 at rest, comorbidities, 6MWD and oxygen saturation during the 6-min walk test (desaturation was defined as a fall in SpO2 ≥4% or SpO2 <90%).
Figure 2. Two year mortality progressively decreases as the 6-min walking distance (6MWD) increases. For distances < 100m (n = 19), for 101–200 m (n = 61); for 201 et 300 m (n = 57); for 301-400 m (n = 46) and for >400 m (n = 15). [Ref. 73]
![Figure 2. Two year mortality progressively decreases as the 6-min walking distance (6MWD) increases. For distances < 100m (n = 19), for 101–200 m (n = 61); for 201 et 300 m (n = 57); for 301-400 m (n = 46) and for >400 m (n = 15). [Ref. 73]](/cms/asset/18e01e7c-197b-4a9e-ae7a-99c5363cf765/icop_a_482115_f0002_b.gif)
Their results showed that the 6 MWD was a good predictor of all-cause and respiratory mortality primarily in patients with FEV <50% prédicted (p< 0.001) after adjusting for all covariates. This study provides more evidence to support the implementation of 6-min walk test (6MWT) in the routine evaluation in COPD, primarily in those patients with FEV <50% of predicted. The authors also reported that approximately 50% of the patients with severe COPD and PaO2 >60 mmHg had a fall in SpO2 <90% during the 6MWT.
Figure 3. Percentages of time spent in each of the activities or body positions in healthy subjects and patients with chronic obstructive pulmonary disease during the day. Others = cycling or undeterrmined activity (2% in healthy elderly subjects and 3% in patients with COPD). [Ref. 79]
![Figure 3. Percentages of time spent in each of the activities or body positions in healthy subjects and patients with chronic obstructive pulmonary disease during the day. Others = cycling or undeterrmined activity (2% in healthy elderly subjects and 3% in patients with COPD). [Ref. 79]](/cms/asset/6da213dd-9d18-4015-bb90-f18cd292c8e2/icop_a_482115_f0003_b.gif)
They observed that the patients with fall in SpO2 ≥ 4%, and in particular those with SpO2 <90%, had decreased survival independent of the walked distance, thus suggesting that monitoring of oxygenation could add some information to help predict which patients are more likely to have a poor outcome independent from the distance walked. The 8-year mortality rate of COPD patients with desaturation to this level was 67%, compared with 38% for patients without desaturation (p < 0.001). In this study, oxygen desaturation predicted mortality (relative risk 2.63; 95% confidence interval 1.53 to 4.51; p < 0,001) but with less power than PaO2 at rest. The authors discussed that the resting PaO2 is likely to more precisely reflect differences in disease severity compared with the flatter response of SpO2 levels measured in patients with normal or mild hypoxemia. Thus, changes in PaO2 at the flat portion of the oxygen-hemoglobin dissociation curve may have a clinical significance that is not detectable by the small changes in simultaneoulsy measured oximetry.
Figure 4. Transplant-free survival for patients with normal and reduced quadriceps strength, as defined by quadriceps maximal voluntary contraction force >120% or <120% of body mass index. The curves are significantly different, p = 0.017 [Ref.12].
![Figure 4. Transplant-free survival for patients with normal and reduced quadriceps strength, as defined by quadriceps maximal voluntary contraction force >120% or <120% of body mass index. The curves are significantly different, p = 0.017 [Ref.12].](/cms/asset/37e108a6-eb1c-4b49-9246-946844a739a0/icop_a_482115_f0004_b.gif)
Finally, it is important to consider that there is no evidence that maintenance of normoxemia during exercise improves the survival of patients with COPD (Citation75). Further studies are needed to determine the efficacy of oxygen supplementation during exercise on survival in patients with COPD and clarify the criteria for oxygen prescription in this population.
Activities of daily living: In recent years, there has been increasing interest in evaluating the clinical impact of physical activity in COPD patients. A study designed to evaluate the level of physical activity by subjective and objective methods showed that COPD patients underestimate time spent sitting down, and overestimated time walking (Citation77). The increasing use of actimetry or accelerometry has shown that the daily level of activity is markedly diminished in COPD patients compared to age matched healthy subjects (Citation78, 79) (). Thus, it has been shown that time walking is reduced by half, time standing-up was also diminished while time spent sitting or lying was greatly increased (Citation79).
Recent studies have emphasised the importance of regular physical activity in the prognosis of COPD patients. One study has shown that regular physical activity may improve alveolo-capillary gas diffusion; thus demonstrating a possible improvement in gas exchange and oxygen transport in peripheral tissues. Physical activity also increased expiratory muscle strength and reduced the raised levels of circulating proinflammatory cytokines (Citation80). A study of 2,386 patients showed that a risk of respiratory decompensation and death was significantly raised in patients who performed little or no physical activity compared to those who performed some activity on a regular basis (Citation81). Thus it has been shown that a daily walk of at least 30 minutes may significantly reduce the number of exacerbations and improve outcome in these patients (Citation82).
Quadriceps
Peripheral muscle in COPD and in particular the quadriceps is now recognized as a real independent prognostic factor in the evaluation of the disease. Thus, 2 studies have shown that independently of bronchial obstruction, life expectancy at 5 years was increased in relation to muscle atrophy or loss of strength in the lower limbs (Citation12, Citation83) (). Thus, for the same level of obstruction, the presence of amyotrophy or muscle weakness doubles the mortality risk.
Quality of life
Evaluation of health related quality of life in COPD has been widely studied. All these studies show that patients have a perception of a poor quality-of-life as regards relationships with others and daily activities. Since the 2000s, concepts have advanced beyond simple quality of life evaluation and studies have shown that health related quality of life scores are independent predictors of survival in COPD. Thus, it has been established that a total score of a specific health-related quality of life tool such as the St. Georges respiratory questionnaire (SGRQ) is related (independently of the level of airflow obstruction) to increased mortality in COPD patients at 5 years (Citation69, Citation72, Citation84–87) (). It has also been shown that an improvement of 4 points in the total score in the SGRQ (considered as clinically significant improvement in quality of life) is associated with an improvement in mortality risk of 12.9% at 3 years in COPD patients (Citation88).
Dyspnoea
It has been shown that the level breathlessness is more closely related to the level of quality of life score than is the level of bronchial obstruction in COPD patients (Citation88). Subsequent studies have shown that the level of dyspnoea as measured by the (Medical Research Council) MRC score was significantly correlated with 5-year mortality in COPD (Citation90–92) (). There is increased risk of mortality with increased level of MRC dyspnoea. Compared to stage II dyspnoea, there is a 2.21-fold increased 5-year mortality for stage III and a 8.31-fold increased for stage IV and finally 6.61-fold increased mortality for stage V (Citation90).
Figure 6. Five-year survival according to the level of dyspnea as evaluated by the modified 5- point grading system. [Ref. 90]
![Figure 6. Five-year survival according to the level of dyspnea as evaluated by the modified 5- point grading system. [Ref. 90]](/cms/asset/08277a39-2b66-4357-967a-3f7a41667d10/icop_a_482115_f0006_b.jpg)
Figure 7. Kaplan-Meier survival curves for the four quartiles of the BODE index. Quartile 1 is a score of 0 to 2, quartile 2 is a score of 3 to 4; quartile 3 a score of 5 to 6, and quartile 4 a score of 7 to 10. Survival differed significantly among the four groups (p < 0,001 by the log-rank test). [Ref. 5]
![Figure 7. Kaplan-Meier survival curves for the four quartiles of the BODE index. Quartile 1 is a score of 0 to 2, quartile 2 is a score of 3 to 4; quartile 3 a score of 5 to 6, and quartile 4 a score of 7 to 10. Survival differed significantly among the four groups (p < 0,001 by the log-rank test). [Ref. 5]](/cms/asset/0ff6f675-0af4-485d-a3c0-8e2c17606571/icop_a_482115_f0007_b.gif)
BODE index
Perhaps the most important advance in evaluating prognosis in COPD patients in recent years has been the development of a composite index based on combination of single predictive factors. This has led to the BODE index which integrates the level of airflow obstruction, of breathlessness and 6-minute walking distance and level of BMI (Citation5). Thus, the BODE index integrates systemic manifestations of the disease into prognostic evaluation by using factors such as malnutrition, loss of muscle and change in ventilatory mechanics that give breathlessness and reduce effort tolerance.
This score allows improved evaluation of prognostic outcome in COPD and also examines pertinent factors for determining disease severity. Thus, the BODE index is much more discriminating that simple airflow obstruction for predicting 5-year mortality (Citation93). The BODE index score notes zero to 10 and 5-year survival correlates closely with BODE score. When separating by quartiles, lowest scores (between 0 and 2) have 5-year survival of 80% while the second quartile of lower score (between 3 and 4) have a 70% survival, the third quartile (between 5 and 6) have 60% and the fourth quartile (between 7 and 10) have 20% survival at 5 years (). Recently, these quartiles have also been shown to relate to the number and severity of exacerbations in COPD patients (Citation94). Finally, the BODE index may correlate with quality of life scores (Citation95), and levels of anxiety and depression (Citation96) in COPD patients.
CONCLUSION
Nowadays, COPD should be considered as a systemic disease of respiratory origin, given the number of extra pulmonary manifestations and co-morbidities that play a major role in the natural evolution and prognosis of this disease. Thus, diagnostic, test, evaluation and management of COPD patients require an integrated approach. This approach must not only consider the lungs but also peripheral muscles, associated co-morbidities, body composition, hormone balance, effort intolerance, exertional desaturation, regular physical activity, breathlessness, anxiety, depression and quality-of-life score. A global vision of “the patient and the disease” ensures correct diagnosis and management that is effective and adapted. Passing from theory to practice, there now exists useful validated tools such as the BODE index and its subsequent modifications (BODEx, ADO…)! These are simple to use but underused in clinical practice!
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chapman KR, Mannino DM, Soriano JB, Epidemiology and cost of COPD. Eur Respir J 2006; 27:188–207.
- Agusti A, Soriano JB. COPD as a systemic disease. COPD 2008; 5:133–138.
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences, clinical impacts, mechanisms and potential for early intervention. COPD 2008; 5:235–256.
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33:1165–1185.
- Celli BR, Cote CG, Marin J, Casanova C, Montes de Oca M, Mendez R, Pinto-Plata V, Cabral H. The body mass-index, airflow obstruction, dyspnea and exercise capacity index in COPD. N Engl J Med 2004; 350:1005–1012.
- Coronell C, Orozco-Levi M, Méndez R, Ramírez-Sarmiento A, Gáldiz JB, Gea J. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J 2004; 24:129–136.
- Allaire J, Maltais F, Doyon JF, Noel M, Leblanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004; 59:673–678.
- Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut Ch. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:1–7.
- Van't hul A, Harllar J, Gosselink R, Hollander P, Postmus P, Kwakkel G. Quadriceps muscle endurance in patients with chronic obstructive pulmonary disease. Muscle Nerve 2004; 29:267–274.
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of pre-induced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol 2009 Jul 2. [Epub ahead of print).
- Gosker H, Zeegers M, Wouters E, Schols A. Muscle fiber type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 2007; 62:944–949.
- Swallow E, Reyes D, Hopkinson N, Man W, Porcher R, Cetti E, Moore A, Moxham J, Polkey M. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62:115–120.
- Vilaro J, Rabinovich R, Gonzalez-deSuso JM, Troosters T, Rodríguez D, Barberà JA, Roca J. Clinical assessment of peripheral muscle function in patients with chronic obstructive pulmonary disease. Am J Phys Med Rehabil 2009; 88:39–46.
- Man WD, Soliman MG, Nikoletou D, Harris ML, Rafferty GF, Mustfa N, Polkey MI, Moxham J. Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax 2003; 58:665–669.
- Couillard A, Prefaut C. From muscle disuse to myopathy in patients with COPD: potential contribution of oxidative stress. Eur Respir J 2005; 26:703–719.
- Schols AMWJ, Soeters PB, Dingemans MC, Mostert R, Frantzen PJ, Wouters EFM. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147:1151–1156.
- Creutzberg EC, Schols AM, Bothmer-Quaedvlieg FC, Wouters EF. Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr 1998; 52:396–401.
- Schols AMWJ, Mostert R, Soeters PB, Saris WHM, Wouters EFM. Energy balance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 143:1248–1252.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 2006; 173:79–83.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007; 132:164–169.
- Remels AH, Gosker HR, Van Der Velden J, Langen RC, Schols AM. Systemic inflammation and skeletal muscle dysfunction in chronic obstructive pulmonary disease: state of the art and novel insights in regulation of muscle plasticity. Clin Chest Med 2007; 28:537–552.
- Cano NJ, Roth H, Court-Ortuné I, Cynober L, Gérard-Boncompain M, Cuvelier A, Laaban JP, Melchior JC, Pichard C, Raphaël JC, Pison CM. Nutritional depletion in patients on long-term oxygen therapy and/or home mechanical ventilation. Eur Respir J 2002; 20:30–37.
- Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003; 46:76s–80s.
- Dimopoulou I, Ilias I, Mastorakos G, Mantzos E, Roussos C, Koutras DA. Effects of severity of chronic obstructive pulmonary disease on thyroid function. Metabolism 2001; 50:1397–1401.
- Kamischke A, Kemper DE, Castel MA, Luthke M, Rolf C, Behre HM, Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J 1998; 11:41–45.
- Debigare R, Marquis K, Cote CH, Tremblay RR, Michaud A, Leblanc P, Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 2003; 124:83–89.
- Scalvini S, Volterrani M, Vitacca M, Clark AL, Solfrini R, Panzali AM, Ferrari R, Levi GF. Plasma hormone levels and haemodynamics in patients with chronic obstructive lung disease. Monaldi Arch Chest Dis 1996; 51:380–386.
- John M, Lange A, Hoernig S, Witt C, Anker SD. Prevalence of anemia in chronic obstructive pulmonary disease: comparison to other chronic diseases. Int J Cardiol 2006; 111:365–370.
- Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J 2007; 29:923–929.
- Chambellan A, Chailleux E, Similowski T; ANTADIR Observatory Group. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest 2005; 128:1201–1208.
- John M, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest 2005; 127:825–829.
- Schönhofer B, Wenzel M, Geibel M, Köhler D. Blood transfusion and lung function in chronically anemic patients with severe chronic obstructive pulmonary disease. Crit Care Med 1998; 26:1824–1828.
- Graat-Verboom L, Wouters EF, Smeenk FW, Van Den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 2009; 34:209–218.
- Jørgensen NR, Schwarz P. Osteoporosis in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2008; 14:122–127.
- Vrieze A, de Greef MH, Wijkstra PJ, Wempe JB. Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int 2007; 18:1197–1202.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009; 179:630–636.
- Graat-Verboom L, Spruit MA, Van Den Borne BE, Smeenk FW, Martens EJ, Lunde R, Wouters EF, CIRO Network. Correlates of osteoporosis in chronic obstructive pulmonary disease: An underestimated systemic component. Respir Med 2009; 103:1143–1151.
- Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, Yates JC, Vestbo J, Celli B. Prevalence and progression of osteoporosis in patients with COPD. Results from TORCH Chest 2009 Jul 6. [Epub ahead of print]
- Putman-Casdorph H, McCrone S. Chronic obstructive pulmonary disease, anxiety, and depression: state of the science. Heart Lung 2009; 38:34–47.
- Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, Hanania NA; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest 2008; 134:43S–56S.
- Hynninen KM, Breitve MH, Wiborg AB, Pallesen S, Nordhus IH. Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res 2005; 59:429–443.
- Gudmundsson G, Gislason T, Janson C, Lindberg E, Suppli Ulrik C, Brøndum E, Nieminen MM, Aine T, Hallin R, Bakke P. Depression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countries. Respir Med 2006; 100:87–93.
- McCathie HC, Spence SH, Tate RL. Adjustment to chronic obstructive pulmonary disease: the importance of psychological factors. Eur Respir J 2002; 19:47–53.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J 2008; 31:667–677.
- Norwood RJ. A review of etiologies of depression in COPD. Int J Chron Obstruct Pulmon Dis 2007; 2:485–491.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly patients with chronic obstructive pulmonary disease. Age Ageing 2006; 35:457–459.
- Al-Shair K, Dockry R, Mallia-Milanes B, Kolsum U, Singh D, Vestbo J. Depression and its relationship with poor exercise capacity, BODE index and muscle wasting in COPD. Respir Med 2009 Jun 25 [Epub ahead of print].
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–969.
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003; 52:812–817.
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, Poirier P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005; 25:226–232.
- Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J 2008; 31:204–212.
- Watz H, Waschki B, Kirsten A, Müller KC, Kretschmar G, Meyer T, Holz O, Magnussen H. The Metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009 Jun 19. [Epub ahead of print].
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005; 128:2640–2646.
- Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J Heart Fail 2006; 8:706–711.
- Thurnheer R, Muntwyler J, Stammberger U, Bloch KE, Zollinger A, Weder W, Russi EW. Coronary artery disease in patients undergoing lung volume reduction surgery for emphysema. Chest 1997; 112:122–118.
- Marquis K, Maltais F, Poirier P. Cardiovascular manifestations in patients with COPD. Rev Mal Respir 2008; 25:663–673.
- Kleiger RE, Senior RM. Longterm electrocardiographic monitoring of ambulatory patients with chronic airway obstruction. Chest 1974;65:483–487.
- Weitzenblum E, Kessler R, Oswald M, Fraisse P. Medical treatment of pulmonary hypertension in chronic lung disease. Eur Respir J 1994; 7:148–152.
- Burger CD. Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD 2009; 6:137–144.
- Thabut G, Dauriat G, Stern JB, Logeart D, Lévy A, Marrash-Chahla R, Mal H. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127:1531–1536.
- Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst 2006; 98:691–699.
- Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003; 163:1475–1480.
- Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 1996; 313:711–715.
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Ventilatory function and chronic mucus hypersecretion as predictors of death from lung cancer. Am J Respir Crit Care Med 1990; 141:613–617.
- Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25 year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev 1994; 3:289–298.
- Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007; 176:285–290.
- Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005; 60:570–575.
- Formiga F, Lopez-Soto A, Masanes F, Chivite D, Sacanella E, Pujol R. Influence of acute exacerbation of chronic obstructive pulmonary disease or congestive heart failure on functional decline after hospitalization in nonagenarian patients. Eur J Intern Med 2005; 16:24–28.
- Almagro P, Calbo E, Ochoa de Echaguen A, Mortality after hospitalization for COPD. Chest 2002; 121:1441–1448.
- Rozzini R, Sabatini T, Cassinadri A, Boffelli S, Ferri M, Barbisoni P, Frisoni GB, Trabucchi M. Relationship between functional loss before hospital admission and mortality in elderly persons with medical illness. J Gerontol A Biol Sci Med Sci 2005; 60:1180–1183.
- Minicuci N, Maggi S, Noale M, Predicting mortality in older patients. The VELCA Study. Aging Clin Exp Res 2003; 15:328–335.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167:544–549.
- Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23:28–33.
- Takigawa N, Tada A, Soda R, Date H, Yamashita M, Endo S, Takahashi S, Kawata N, Shibayama T, Hamada N, Sakagucchi M, Hirano A, Kimura G, Okada C, Takahashi K. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med 2007; 1001:561–567.
- Casanova. C, Cote C, Marin JM, Pinto-Plata V, De Torres JP, Aguirre-Jaime A, Vassaux C, Celli BR. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008; 134:746–752.
- Panos RJ, Eschenbacher W. Exertional desaturation in patients with chronic obstructive pulmonary disease. COPD 2009; 6:478–487.
- Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2005; 86: 1979–1985.
- Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J 2009; 33:262–272.
- Pitta F, Troosters T, Spruit MA, Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:972–977.
- Garcia-Aymerich J, Serra I, Gómez FP, Farrero E, Balcells E, Rodríguez DA, de Batlle J, Gimeno E, Donaire-Gonzalez D, Orozco-Levi M, Sauleda J, Gea J, Rodriguez-Roisin R, Roca J, Agustí AG, Antó JM. Physical activity and clinical and functional status in COPD. Chest 2009; 136:62–70.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61:772–778.
- Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58:100–105.
- Marquis K, Debigaré R, Lacasse Y, Leblanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with COPD. Am J Resp Crit Care Med 2002; 166:809–813.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, España PP, Pérez-Izquierdo J, Capelastegui A. Predictors of mortality in patients with stable COPD. J J Gen Intern Med J 2008; 23:1829–1834.
- Antonelli-Incalzi R, Pedone C, Scarlata S, Battaglia S, Scichilone N, Forestiere F, Bellia V. Correlates of mortality in elderly COPD patients: focus on health-related quality of life. Respirology 2009; 14:98–104.
- Conte ME, Pedone C, Forastiere F, Bellia V, Antonelli-Incalzi R. Discriminative and predictive properties of disease-specific and generic health status indexes in elderly COPD patients. BMC Pulm Med 2008; 8:14.
- Halpin DM, Peterson S, Larsson TP, Calverley PM. Identifying COPD patients at increased risk of mortality: predictive value of clinical study baseline data. Respir Med 2008; 102:1615–1624.
- Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Félez M, Khalaf A, Marrades RM, Monsó E, Serra-Batlles J, Antó JM. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:680–685.
- Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest 1999; 116:1632–1637.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121:1434–1440.
- Schembri S, Anderson W, Morant S, Winter J, Thompson P, Pettitt DM, Macdonald TH, Winter J. A predictive model of hospitalisation and death from chronic obstructive pulmonary disease. Respir Med 2009; Jun 8. [Epub ahead of print].
- Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med 2009; Jul 14. [Epub ahead of print].
- Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med 2008; 102 Suppl 1:S27–S35.
- Marin JM, Carrizo SJ, Casanova C, Martinez-Camblor P, Soriano JB, Agusti AG, Celli BR. Prediction of risk of COPD exacerbations by the BODE index. Respir Med 2009; 103:373–378.
- Medinas Amorós M, Mas-Tous C, Renom-Sotorra F, Rubí-Ponseti M, Centeno-Flores MJ, Gorriz-Dolz MT. Health-related quality of life is associated with COPD severity: a comparison between the GOLD staging and the BODE index. Chron Respir Dis 2009; 6:75–80.
- Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—A cross-sectional study. Respir Res 2009; 10:1.

![Figure 5. Kaplan-Meier survival curves according to tertiles of Saint Georges Respiratory Questionnaire total score (n = 312). [Ref. 88]](/cms/asset/8617eeb5-5f02-4d95-87e5-db0c9edc497d/icop_a_482115_f0005_b.gif)