ABSTRACT
Background: The slope of phase III (single breath nitrogen test), an index of ventilation inhomogeneity, has been used for early detection of COPD. Tidal airway closure (cyclic opening and closure of the peripheral airways during tidal breathing; ACT) and expiratory flow limitation (attainment of maximal expiratory flow during tidal expiration; EFLT) cause small airways disease (SAD). The relationships of these indices with COPD severity may reflect the progress from SAD to overt COPD. Methods: In this cross-sectional study we have assessed for the first time the phase III slope, ACT and EFLT in 10 smokers with normal spirometry (group O) and 40 COPD patients with GOLD scores from I to IV. Results: In most group O smokers the phase III slope was increased, and further increased with GOLD severity (up to 800%pred in GOLD IV). A close correlation was found of slope with GOLD (r = 0.77). ACT was absent in smokers with normal spirometry and in most patients with mild COPD. EFLT first appeared in GOLD II patients and its prevalence progressively increased in GOLD III and IV patients. Conclusions: Most group O smokers exhibit increased phase III. With overt COPD there is a progressive increase in phase III and reduction of FEV1/FVC ratio from GOLD I to IV. A reduction of FEV1 occurs from GOLD stage II. As the disease progresses from moderate to severe, there is an increasing presence of ACT. Tidal EFL, with dynamic hyperinflation and severe dyspnea is present only in GOLD III and IV.
INTRODUCTION
Most of the early studies dealing with the slope of phase III of the single-breath nitrogen test (SBN2T) were centered on early detection of small airway disease (SAD) in smokers (Citation1–5), but no systematic data were provided of the relationship of phase III slope to the severity of chronic obstructive pulmonary disease (COPD). In a recent cross-sectional study (Citation6) in patients with Z a1-antitrypsin deficient emphysema the phase III slope, an overall index of ventilation inhomogeneity, did not correlate significantly with FEV1 (range 73–29%pred). In contrast, the phase III slope, correlated significantly with pulmonary diffusion capacity for carbon monoxide (DLCO). Fregonese et al. (Citation6) concluded that in patients with Z a1-antitrypsin deficient emphysema the phase III slope provides complementary information to spirometry. No such cross-sectional studies have been reported in COPD patients.
According to a recent hypothesis (Citation7) the transition from SAD to overt COPD in smokers is characterized by three stages in which tidal airway closure (ACT) and tidal expiratory flow limitation (EFLT) play a central role: Stage I in which ACT eventually occurs; Stage II, in which tidal EFL eventually develops; and Stage III characterized by pulmonary hyperinflation, severe dyspnea and severe exercise limitation. The ACT (namely cyclic opening and closure of the peripheral airways during tidal breathing) (Citation8,9) and EFLT (namely attainment of maximal expiratory flow during tidal expiration) (Citation10) occur when the end-expiratory lung volume is below the closing volume i.e., the lung volume at which small airway closure starts.
Table 1. Anthropometric characteristics and respiratory function data of: 10 normal never-smokers, 10 smokers with normal spirometry (group O), and 40 COPD patients stratified according to GOLD12 (GOLD I to GOLD IV).
Both ACT and EFLT imply inhomogeneity of ventilation distribution with concurrent impairment of gas exchange within the lung (cf 8) and unevenly distributed stress and strain within the lung, which is amplified by tissue interdependence (Citation11) and may lead to small airway injury (Citation7–10). The latter is histologically characterized by denuded epithelium, rupture of alveolar-airway attachments, and increased number of polymorphonuclear leucocytes (Citation8,9). Tidal EFL also promotes dynamic pulmonary hyperinflation with concurrent dyspnea and exercise limitation (Citation10). This scenario was suggested in 2004 in this journal (Citation7) and has been since supported by the results of several experimental studies (cf 8). Despite these potentially adverse consequences of ACT and EFLT, their prevalence has not been studied in the same population of smokers.
The aim of the study was to investigate whether the concurrent measurement of phase III slope, ACT, and EFLT may provide useful insight into the evolution from small airway disease to overt COPD. Accordingly, in the present cross-sectional investigation, using the SBN2T, we have assessed the phase III slope and “routine” respiratory function in 10 smokers with normal spirometry and 40 smokers with different severity of COPD staged according to GOLD (Citation12). Measurements also included: chronic mMRC dyspnea, “open capacity” (part of the SBN2T) with which tidal airway closure (ACT) can be assessed (Citation8), and tidal expiratory flow limitation (EFLT), assessed with the negative expiratory pressure (NEP) test (Citation13). A secondary aim was to investigate which test may be “the best” for evaluating the status and progress of COPD.
METHODS
Subjects
The study was performed in 50 smokers, 40 with COPD and 10 with normal spirometry (group O). Three of the latter were asymptomatic while 7 had simple chronic bronchitis (cough and sputum production for at least 3 months in 2 consecutive years) (Citation12). In the 40 smokers with COPD its severity was classified using post-bronchodilatation spirometric values according to the GOLD guidelines (Citation12). Severity ranged from GOLD I to IV (). At study time, the clinical and functional state of all patients had been stable for at least 4 weeks. Patients were excluded if obese (BMI ≥ 30 Kg/m2) or had a history of other pulmonary, neuromuscular and cardiovascular disorders, and/or any comorbidities. Our group O differs from the GOLD O in the original GOLD classification (Citation14) because in our case presence of simple chronic bronchitis was not required. It was absent in 3/10. The study had the approval of the local ethics committee. All subjects gave informed consent.
Dyspnea and respiratory function
Chronic dyspnoea was rated according to the modified Medical Research Council (mMRC) 6-point scale (Citation15). The spirometric indices were measured (Vmax Encore: SensorMedics, Yorba Linda, USA) using the “fast maneuver” (Citation16). The functional residual capacity (FRC) was determined (Vmax Encore) by multiple nitrogen washout (Citation17) and the total lung capacity (TLC) was calculated as sum of FRC and inspiratory capacity (IC). The latter was measured during stable resting breathing before the performance of the SBN2T. The DLCO was also determined with the Vmax Encore apparatus (SensorMedics). Predicted values of spirometry, static lung volumes and DLCO were from the European Community for Coal and Steel (Citation18). Predicted IC was obtained as difference between predicted TLC and predicted FRC. The arterial PO2 and PCO2 were measured with the 288 Blood gas system (Ciba-Corning, MA, USA).
Tidal expirory flow-limitation (EFLT) was assessed using the NEP technique (Citation13). Subjects breathed through a pneumotachograph (Screenmate-Box; Erich Jaeger Gimp & Co, Germany). At onset of tidal expiration a NEP of -5 cmH2O was applied at the mouth by a vacuum cleaner. The control tidal expiratory flow-volume curve was superimposed on the subsequent expiration with NEP: patients in whom the expiratory flow was not increased during part or all of the control expiration were considered to have EFLT, while subjects in whom flow increased with NEP throughout the volume range of the control tidal expiration were considered as non flow-limited (NEFLT) (Citation13).
The single breath N2 test (SBN2T) was performed with the Vmax Encore apparatus. Subjects were asked to breathe to residual volume (RV) and immediately perform a slow maximal inspiration of oxygen followed by a slow (∼0.5 l/s) expiration to RV (Citation19). All subjects successfully performed this test. Most subjects of group O, GOLD I and GOLD II exhibited an abrupt upward increase in expired N2 concentration (onset of phase IV) during the slow vital capacity expiration ( and B). “Open capacity” (OC) was computed as the volume subtended between TLC and the onset of phase IV (A) (Citation7). “OC” is a useful alternative of closing capacity (CC) because it is easier to perform and requires less equipment (Citation8). If OC < IC (hence OC-IC<0), tidal airway closure (ACT) was present. Patients without an apparent phase IV () were labeled “no OC” (Citation8).
Figure 1. Relationship of fractional expired concentration of nitrogen to expired volume during SBN2 test in a non smoker (A), in a GOLD II COPD patient (B) and in a GOLD IV COPD patient (C) who did not exhibit a phase IV (please see text). Perpendicular arrow indicates closing volume (CC). The volume expired from total lung capacity (0 volume) to this arrow is “open capacity” (“OC”). The four phases (I-IV) are indicated. Broken lines: slope of phase III. Note: (1) presence of cardiac oscillations in A but not B and C, and (2) marked increase of slope of phase III in B and C. FEN2%: fractional expired concentration of nitrogen.
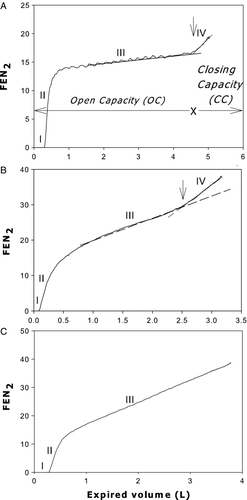
It should be stressed, however, that “no OC” does not mean that airway closure was absent but rather that it could not be detected with the SBN2T because of absence of a phase IV (). In normal subjects and smokers with mild changes in lung function the peripheral airways close as a “cluster” when the critical closing pressure (Pc) is reached during expiration because Pc is relatively uniform within the lung (Citation8). As a result of the “cluster” airway closure there is a well-defined phase IV.
Table 2. Slope of phase III, tidal airway closure, and tidal expiratory flow limitation in seated position in: 10 normal never-smokers, 10 smokers with normal spirometry (group O), and 40 COPD patients stratified according to GOLD12 (GOLD I to GOLD IV).
In contrast, in smokers with severe lung function impairment (GOLD III and IV) there is marked interregional inhomogeneity of Pc within the lung and hence peripheral airway closure occurs diffusely throughout the vital capacity expiration, resulting in an increase of the slope of phase III and disappearance of phase IV (see discussion). Phase III slope was calculated as best-fit line through phase III (Citation20). The reported values of “OC” and phase III slope are means of two or more acceptable tracings, i.e., records obtained with identical inspiratory and expiratory vital capacities at the desired flow rate (∼0.5 l/s). Predictions of phase III slope were made according to Buist and Ross (Citation20). Predicted “OC” values were computed as difference between predicted TLC and predicted closing capacity (Citation21). We validated this method for predicting “OC” in 10 seated normal never-smokers (3 males), whose age and BMI (mean ± SD) were 38 ± 12 yrs and 24 ± 3 Kg/m2, respectively. No significant differences were found between predicted and measured values of “OC” or phase III slope.
Statistical analysis
Data are expressed as mean ± SD, unless otherwise stated. For comparisons between groups Student's one-way ANOVA with Bonferroni's correction or x2-test used where appropriate. Linear regression analysis was used to obtain correlations. Statistics were performed using SigmaStat (V3.5) and SigmaPlot (V10.0) (Jandel Scientific, CA, USA) statistical software.
RESULTS
provides anthropometrics and respiratory function data of the 50 smokers stratified according to GOLD (Citation12). There were no significant differences in age (except for never-smokers), height, BMI and blood gases test results among groups. The mMRC increased with GOLD stage (from an average of 1.3 in group O to 3.9 in group IV), while all spirometric parameters and DLCO decreased. In the control group of never-smokers the respiratory data were within normal limits.
The SBN2T and NEP data, together with related parameters, are given in . The phase III slope correlated closely with the GOLD stages (). Significant correlations between the slope and FEV1(%pred), and other spirometric indices shown in Tables and were also found, the best correlation was with FEV1, while poor correlations were found with DLCO, mMRC, and blood gases ().
Figure 2. Relationship of slope of phase III of single-breath N2 test to GOLD stage of 50 seated smokers. Solid line: regression line. Broken line: 100%pred of phase III. Closed circles: mean values. Bars: ± SE. N = normal subjects.
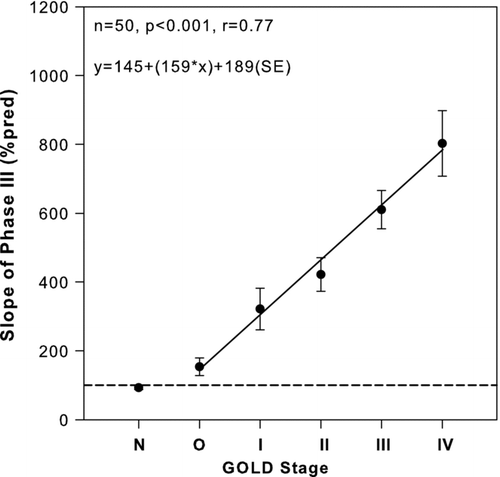
Table 3. Pearson correlation coefficient (r) and coefficients of determination (r2) of slope of phase III (%pred) significant to various respiratory variables shown in Tables 1 and 3
In all normal never-smokers the phase III slope, “OC”, and IC were within normal limits. In all GOLD II-IV patients, as well as in 7 GOLD I and 6 group O smokers (all 6 with simple chronic bronchitis), the phase III slope was >120%pred. The IC was below the normal limits only in GOLD III and IV patients (). The “OC” decreased progressively from group O to GOLD II, and was within normal limits in all group O, in 9 out of 10 GOLD I and 4 out of 10 GOLD II patients. In contrast, the “OC” was below the normal limit (<80% pred), in 1 out of 10 GOLD I and 5 out of 9 GOLD II patients.
In 8 out of 10 GOLD III and 10 out of 10 GOLD IV patients there was no apparent phase IV (“no OC”), as shown in the example in C. None of the normal never-smokers and group O patients exhibited tidal airway closure (OC-IC<0), which was present in 1 out of 10 GOLD I and 3 out of 9 GOLD II patients in whom “OC” could be determined. In contrast tidal EFL was absent in all smokers, except in 3 GOLD III and 5 GOLD IV patients. Patients with tidal EFL exhibited severe dyspnoea and hyperinflation (reflected by reduced IC).
DISCUSSION
The main findings of this cross-sectional investigation are that in all GOLD I-IV smokers as well as in most smokers without airways obstruction (group O): (a) the slope of phase III increased (up to 800%pred in GOLD IV) and correlated closely with the severity of airways obstruction graded according to GOLD; (b) with increasing severity of COPD, the phase IV became progressively less pronounced and eventually disappeared in most GOLD III and IV patients rendering the assessment of “OC” or CC impossible with the SBN2T. Several GOLD III and IV patients exhibited tidal EFL with concurrent severe dyspnea and hyperinflation (reflected by reduced IC).
Relationship of phase III slope to GOLD stages
might reflect the natural history of COPD in smokers. Most (6/10) smokers of group O exhibited an increase in phase III slope, although spirometric data were still within normal limits. These patients are said to have SAD (Citation8). At the next severity stage (GOLD I) the smokers exhibited a reduction in FEV1/FVC ratio below 70% while FEV1 was still within normal limits. Finally, starting from GOLD II, the FEV1 fell below 80% pred. The classification of COPD is in contrast to pre-GOLD criteria whereby the diagnosis of COPD required a reduction in both FEV1 and FEV1/FVC ratio (Citation22). In the pre-GOLD classification the GOLD I patients were merged with the O group ones.
As a result the number of smokers with overt COPD was lower than with GOLD, a fact that needs to be taken into account when comparing prevalence of COPD obtained in pre-GOLD to post-GOLD surveys. In this context, the fact that the GOLD classification is also based on post-dilatation vales in contrast to pre-GOLD data should also be taken into account. The different pre-GOLD criteria for COPD diagnosis also rendered the longitudinal analyses of the transition of smokers with increased phase III slope but normal spirometry into overt COPD confusing to the extent that the initial study group of smokers (group O) included subjects with overt COPD according to GOLD.
Since according to the GOLD scale () GOLD I is a mandatory stage in the worsening of COPD it is not surprising that in the pre-GOLD longitudinal studies on the progression from an increased slope of phase III (which was deemed an early test of small airway disease and herald of COPD), it was concluded that “only the association of high phase III slope and decreased FEV1/FVC ratio predicts a low FEV1 in smokers (Citation22,23,Citation24). Our cross-sectional results are consistent with the notion that in smokers the evolution of small airway disease into COPD (GOLD II-IV), characterized by reduction of FEV1 and FEV1/FVC ratio, involves an obligatory passage through GOLD I (FEV1/FVC<0.7, FEV1≥80% pred).
Verbanck et al. (Citation5) have performed a cross-sectional study in smokers using a multiple breath washout test (MBWT). Their results suggest that MBWT, as well as DLCO (%pred), detect small airway disease earlier than FEV1 (%pred) and FEV1/FVC ratio. Unfortunately, they did not measure the slope of phase III. Interestingly, they showed that after smoking for more than 40 pack-years there is a rapid deterioration in lung function that may reflect in part the increased prevalence of tidal AC and tidal EFL found in our seated GOLD III and IV smokers (). In this connection it should be noted that in the supine position the prevalence of tidal AC and EFL increases (Citation25).
Relation of phase III slope to Respiratory Function
The close correlation (r = −0.76) of phase III slope to FEV1 in our COPD patients is in contrast with the results in Z a1-antitrypsin deficit emphysema (AATD) patients, in whom no significant correlation was found (Citation6). According to stepwise linear regression analysis, among the respiratory variables in , the FEV1(%pred) was the only significant contributor to the phase III slope.
In our smokers there were also a significant correlations between phase III slope and many other spirometric variables studied, though the coefficients of determination (r2) were lower than for GOLD and FEV1.
In line with previous results, most (6/10) group O smokers exhibited a phase III slope >120%pred, while the “OC” was within normal limits, supporting the notion that phase III slope is a sensitive index to detect small airway disease.
Nature of phase III slope
The phase III slope of the SBN2T has been studied extensively (Citation2,Citation26,Citation27), though its precise nature remains controversial. Since the contribution of pulmonary gas exchange is very small (Citation26), it follows that the slope reflects the combined effect of (a) intrapulmonary inhomogeneity of ventilation distribution at the end of the slow VC inspiration of oxygen, and (b) sequential emptying during the subsequent slow VC expiration (Citation28). As a result of gravity, at the end of the VC inspiration of oxygen, the alveolar N2 concentration (PAN2) in the upper lung regions is normally substantially higher than that in the dependent zones, reflecting higher RV/TLC ratio in the upper lung zones (Citation28).
Such interregional PAN2 inhomogeneity is a necessary precondition for the occurrence of a positive slope of phase III. A positive slope of phase III slope occurs if the better ventilated units contribute preferentially to the early part of expiration (Citation26,Citation29). The slope is surprisingly small in healthy non-smokers, indicating that normally the degree of sequential lung emptying is small, and it has been suggested that it is mainly due to “intraregional” ventilation inhomogeneity (Citation26,27). In smokers, however, there is a marked increase in phase III slope (up to 800%pred in GOLD IV), which is unlikely to reflect solely “intraregional” ventilation inhomogeneity. Sequential airway closure during expiration occurring in the volume domain of phase III may explain, at least in part, the marked increase of phase III slope in smokers (Citation30).
AC and EFL
In our group O smokers, “OC” was within normal limits and tidal airway closure was absent. In most previous studies, however, some smokers with normal spirometry exhibited tidal airway closure (Citation3,Citation31). The discrepancy with the present results may be due to methodological factors (e.g., bolus vs resident gas SBT). Another explanation is that in pre-GOLD era the group O would include also GOLD I patients (see above). In fact one of our GOLD I patients exhibited tidal airway closure. A detailed discussion of tidal AC is beyond the scope of this report as its role in COPD and other respiratory disorders has been recently reviewed in detail (Citation8). Briefly, tidal AC promotes impaired ventilation distribution and peripheral airway injury which results from a cyclic opening and closing of the terminal bronchioles (Citation8,9).
Tidal EFL is also said to contribute to airway injury (Citation10), as well as to concurrent dynamic hyperinflation, which is the main cause of dyspnoea in COPD (Citation10). Tidal EFL was absent in all group O and GOLD I patients, and was present in 2/10 GOLD II patients. In contrast, in GOLD III and IV tidal EFL was common and was associated with dynamic hyperinflation as reflected by decreased IC. The GOLD III and IV patients also exhibited the highest mMRC scores. In our 50 smokers, there was a close correlation (p < 0.001) between mMRC and IC (%pred).
Clinical implications
In line with previous results (Citation31, 32), in many group O smokers phase III slope was above the normal limits, indicating presence of small airway disease. Since in smokers an increased phase III slope heralds a decrease in FEV1/FVC ratio and in FEV1 (Citation32), the group O smokers should be regarded as patients “at particular risk” of developing COPD. Whether stopping smoking and/or anti-inflammatory drug treatment can reverse the phase III slope in group O smokers remains to be determined.
CONCLUSIONS
The slope of phase III is frequently abnormal in smokers with normal spirometry (group O). In smokers with and without airways obstruction, the phase III slope is closely related to GOLD stage. Our results suggest that in smokers there is sequencing from group O to GOLD I before there is a reduction of FEV1 (GOLD II-IV). In the early stages of COPD, a reduction of “open capacity” (“OC”) (with tidal airway closure) is less frequent than an abnormal slope of phase III. Tidal EFL occurs commonly in GOLD III and IV patients causing dynamic hyperinflation and severe dyspnoea. Phase III slope, “OC”, and detection of EFLT are easy to assess and may routinely be used for evaluating the status of COPD. Assessment of tidal airway closure is useful in GOLD I and II, while measurement of tidal EFL is useful in GOLD III and IV, the slope of phase III increases throughout the GOLD scale in close correlation with decreasing FEV1.
Acknowledgments
We thank Prof. J Milic-Emili for his constructive criticisms and technician Mr Stelios Vechlidis for his valued technical support.
Declaration of interest
The authors report no conflicts of interest. All authors made measurements on the subjects participating in the study, analysed the data and contributed in lengthy discussions during the writing of the paper. SA Gennimata and NG Koulouris wrote the paper.
REFERENCES
- Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298:1277–1281.
- Anthonisen NR. Closing volume. In: Regional differences in the lung, West JB. Academic Press, London 1977;451–482.
- Oxhoj H, Bake B, Wilhelmsen L. Ability of spirometry, flow-volume curves and the nitrogen closing volume test to detect smokers. A population study. Scand J Respir Dis 1977; 2:80–96.
- Buist SA, Vollmer WM, Johnson LR, Mccamant LE. Does the single-breath N2 test identify the smoker who will develop chronic airflow limitation? Am Rev Respir Dis 1988; 137:293–301.
- Verbanck S, Schuermans D, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers. Am J Respir Crit Care Med 2004; 170:414–419.
- Fregonese L, van Veen HPAA, Sterk PJ, Stolk J. Ventilation inhomogeneity in alpha1-antitrypsin-deficient emphysema. Eur Respir J 2006; 28:323–329.
- Milic-Emili J. Does mechanical injury of the peripheral airways play a role in the genesis of COPD in smokers? COPD 2004; 1:85–92.
- Milic-Emili J, Torchio R, D’Angelo E. Closing volume: a reappraisal. Eur J Appl Physiol 2007; 99:567–583.
- D’Angelo E, Koulouris NG, Della Valle P, Gentile G, Pecchiari M. The fall in exhaled nitric oxide with ventilation at low lung volumes in rabbits: An index of small airway injury. Respir Physiol Neurobiol 2008; 160:215–223.
- Calverley PMF, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J 2005; 25:186–199.
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596–608.
- 2006 Revision: GOLD Report, Global Strategy for Diagnosis, Management, and Prevention of COPD. Available from: http://www.goldcopd.org (November 2006).
- Koulouris NG, Valta P, Lavoie A, Corbeil C, Chassé M, Braidy J, Milic-Emili J. A simple method to detect expiratory flow limitation during spontaneous breathing. Eur Respir J 1995; 8:306–313.
- 2001 Original: Executive Summary, Global Strategy for the Diagnosis, Management, and Prevention of COPD. Available from: http://www.goldcopd.org
- Eltayara L, Becklake MR, Volta CA, Milic-Emili J. Relationship between chronic dyspnea and expiratory flow-limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 154:1726–1734.
- D’Angelo E, Prandi E, Marazzini L, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration in patients with chronic obstructive lung disease. Am J Respir Crit Care Med 1994; 150:1581–1586.
- Darling RC, Cournand A, Richards DW. Studies on the intrapulmonary mixture of gases. III. An open circuit method for measuring residual air. J Clin Invest 1940; 19:609–618.
- Quanjer PhH. (ed). Standardized lung function testing. Report Working Party “Standardization of Lung Function Tests”, European Community for Coal and Steel. Eur Respir J 1993; 6(Suppl. 16):1–100.
- Anthonisen NR, Danson J, Robertson PC, Ross WR. Airway closure as a function of age. Resp Physiol 1970; 8:58–65.
- Buist SA, Ross BB. Predicted value for closing volumes using a modified single breath nitrogen test. Am Rev Respir Dis 1979; 107:744–751.
- Torchio R, Gulotta C, Greco-Lucchina P, Perboni A, Montagna L, Guglielmo M, Milic-Emili J. Closing capacity and gas exchange in chronic heart failure. Chest 2006; 129:1330–1336.
- Standardization of Spirometry–1987 Update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987; 136:1285–1298.
- Stanescu D, Sanna A, Veriter C, Robert A. Identification of smokers susceptible to development of chronic airflow limitation. Chest 1998; 114: 416–425.
- Hogg JC. Identifying smokers at risk for developing airway obstruction. Chest 1998; 114: 355.
- Milic-Emili J, Koulouris NG, Tantucci C. Sprometric predictions of exercise limitation in patients with chronic obstructive pulmonary disease. In: Physiologic Basis of Respiratory Disease. Eds. Hamid Q, Shannon J, Martin J. BC Dekker Inc, Hamilton, Ontario, Canada, pp. 671–679, 2005.
- Engel LA. Intraregional mixing and distribution In: Gas mixing and distribution in the lung. Engel LA, Paiva M. Marcel Dekker, Inc., New York, 1985; 287–358.
- Verbanck S, Paiva M. Gas washout and aerosol bolus techniques: non-invasive measures of lung structure and ventilation heterogeneity. Mechanics of Breathing, Aliverti A, Brusasco V, Macklem PT, Pedotti A. Springer–Verlag, Milan, 2002; 129–145.
- Milic-Emili J, Henderson JA, Dolovich MB, Trop D, Kaneko KMB. Regional distribution of inspired gas in the lung. J Appl Physiol 1966; 21:749–759.
- Fowler WS. Lung function studies III. Uneven pulmonary ventilation in normal subjects and in patients with pulmonary disease. J Appl Physiol 1949; 2:283–300.
- Crawford ABH, Paiva M, Engel LA. Uneven ventilation, In: Crystal RG, West BJ, eds. The Lung: Scientific Foundations, New York, Raven Press; 1991; 1031–1041.
- McCarthy D, Spencer R, Greene R, Milic-Emili J. Measurement of “closing volume” as a simple and sensitive test for early detection of small airway disease. Am J Med 1972; 52:747–753.
- Olofsson J, Bake B, Svärdsudd K, Skoogh BE. The single breath N2-test predicts the rate of the decline in FEV1. The study of men born in 1913 and 1923. Eur J Resp Dis 1986; 69:46–56.
